Abstract
Subareolar abscess of the male breast is a rare condition, which can be complicated by a fistula from the areolar skin into a lactiferous duct. In 1951, Zuska et al first characterised this entity in women. Literature on mammillary fistulas in men is scarce and therefore standardisation of treatment does not exist. We present two cases of recurrent subareolar abscesses with draining fistulas. Both patients were successfully treated by complete excision of the lactiferous duct fistula, and continue to do well with no evidence of disease recurrence. When male patients present with a draining subareolar abscess, one should have a high index of suspicion for a mammillary fistula. Failure to identify and surgically excise the fistula may lead to recurrence of the abscess and prolonged morbidity. The most effective management of this uncommon entity includes complete excision of the lactiferous duct fistula.
Background
Subareolar abscess in women is well recognised, but there is little literature in regard to this entity in men. Our experience included two cases involving men with a draining mammillary fistula from a subareolar abscess. After surgical excision, the disease was effectively cleared after 6 and 7 years follow-up. Publishing these cases and a discussion regarding this condition provides an opportunity to create awareness for this rare entity. Furthermore, its important to share that surgical resection is a successful treatment option.
Case presentation
Case 1
A 41-year-old Caucasian man presented with a six-month history of a subareolar mass and intermittent serosanguinous discharge from an areolar opening. His medical history was unremarkable, but he did have a 20-pack-year history of smoking. On physical examination, a 1 cm palpable nodule posterior to the right nipple-areolar complex was identified. A mammography demonstrated a hypoechoic nodule, questionable for a cyst versus solid mass. We chose to proceed to the operating room for excision of the mass. A lacrimal probe was used to penetrate an opening at the border of the areolar, which lead to a small mass. A circumareolar incision was made from the 11–4 o'clock position in normal tissue to access the subareolar space. By inverting the nipple, a clearly defined fistula tract was identified. Excision of the involved lactiferous duct fistula and surrounding fibrotic tissue was completed. Grossly, there was no pus or abscess identified. Microscopic examination of the tissue showed a lactiferous duct with an associated abscess. There was granulation tissue consistent with a keratin granuloma and squamous metaplasia (figure 1). The patient recovered well and 6 years later there was no evidence of recurrence.
Figure 1.
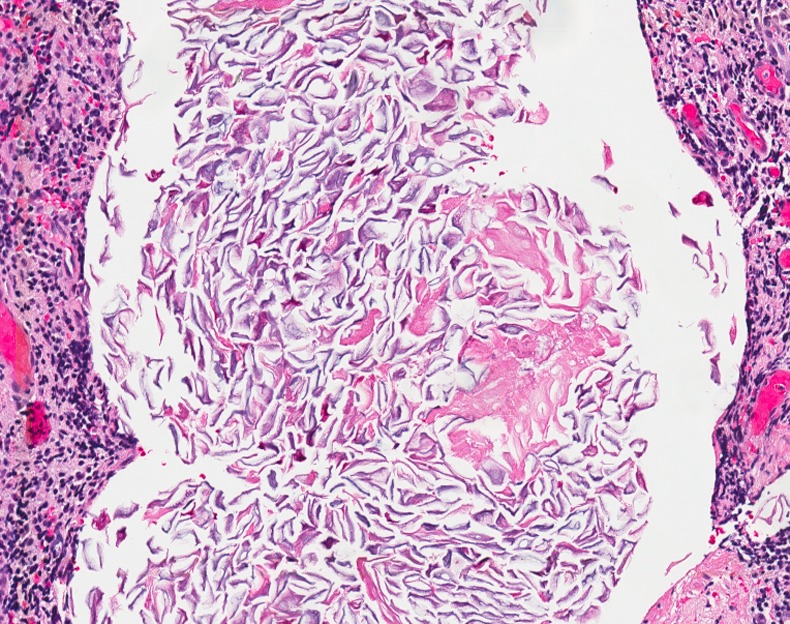
Squamous metaplasia of the cuboidal epithelium lining the lactiferous duct leads to hyperkeratinisation and plugging. The dilated lumen (white) of the subareolar duct contains anucleated cells.
Case 2
A 39-year-old Caucasian man, otherwise healthy, presented with an 8 mm open wound directly inferior and abutting the left areolar. Six months previously, the patient noticed a raised, tender nodule. The nodule ruptured, emitting a serous, non-purulent material. Since that time, the lesion became inflamed intermittently. On presentation, physical examination revealed a slightly erythematous but non-tender periareolar wound. There was healthy granulation tissue present. The nipple appeared slightly inverted. Mammography was non-specific, and an ultrasound examination demonstrated a small area of subcutaneous fluid. The patient received a five-day course of antibiotics and then returned to undergo complete excision of the lesion. Intraoperatively, a fistulous tract was easily probed from the lactiferous duct at the nipple and exiting at the inferior border of the areolar. The entire tract was removed with an elliptical excision extending radially from the nipple. Additionally, the nipple was released providing a better aesthetic outcome. Pathology revealed fibrofatty breast tissue with an abscess, dilated lactiferous duct and associated fistula. The patient recovered without complication. On 7-year follow-up, there was no evidence of recurrence.
Outcome and follow-up
This information is incorporated into the text of the case presentation. Patient 1 recovered well and 6 years later there was no evidence of recurrence. Patient 2 recovered without complication and on 7-year follow-up there was no recurrence.
Discussion
Subareolar abscess of the male breast, also known as mammillary fistula, is an uncommon entity that can lead to prolonged morbidity. The pathophysiology of the disease is unclear. Zuska et al1 postulated that fistulas of lactiferous ducts result as a complication of comedomastitis, or duct ectasia. Breast parenchyma consists of approximately 18 major lactiferous ducts that drain lobules made up of multiple alveoli. The ducts open into an ampulla and drain separately at the apex of the nipple. Lactiferous ducts are lined by a double layer of cuboidal epithelium up to the ampulla and distally by stratified squamous epithelium.2 Previous authors have postulated that squamous metaplasia of the cuboidal epithelium lining the lactiferous duct leads to keratinisation and plugging (figures 1 and 2).3–5 This obstruction causes dilation and rupture of the lactiferous sinus, which results in bacterial spillage and abscess formation. The cause of the squamous metaplasia is unclear, but smoking has been suggested, as it is a known risk factor. Gollapalli et al5 found that smokers are six times more likely to develop primary abscess when compared to non-smokers (OR of 6.15, 95% CI 2.65–14.29). Spontaneous drainage onto the border of the areola signifies the development of a fistulous tract.2 3 6 Other authors have demonstrated inconsistency with this theory. Hanavadi et al6 concluded that periductal mastitis is more commonly the culprit, as they were unable to consistently identify a keratin plug or duct ectasia. Likewise, in a study of 52 fistulas, Lambert et al7 drew similar conclusions.
Figure 2.

The white area demonstrates a cross-section of the lactiferous duct lumen. The intraluminal collection of cells resembling a snake is benign keratinised squamous epithelium.
Diagnosis of subareolar abscess of the male breast is suggested by clinical features and a purulent aspirate. Imaging modalities such as ultrasound and mammography can provide adjunctive information such as the size, depth and anatomical location of the lesion. The definitive diagnosis is made by fine needle aspiration with cytological examination or surgical excision.8 If a mass is present in male breast tissue, as in our cases, appropriate biopsies can be performed to rule out malignancy. The diagnostic cytological features of a subareolar abscess lesion, first described by Galblum and Oertel,8 are the presence of anucleated squamous cells, acute inflammatory cells (predominately neutrophils), granulation tissue and debris. Additionally, multinucleated giant cells are commonly seen as a foreign body response to keratinous material. The differential diagnosis of a subareolar mass includes gynaecomastia, lipoma, epidermal cyst, fat necrosis, pilomatrixoma and carcinoma.9
Various treatments have been suggested for subareolar abscess. First, one should determine if a mammillary fistula exists. Suspicion should be high in patients who present with recurrent inflammatory episodes.7 Zuska et al1 believed that recurrent abscess formation is a result of obstruction of the lactiferous duct, therefore, supported excision of the terminal (nipple) portion of the duct. A commonly acceptable approach to treatment of a mammillary fistula includes several principles: excision of the involved lactiferous duct as well as the ampulla and its abscess, a fistulectomy, reconstruction of the nipple-areola, and correction of the nipple inversion.2 10 Versluijs-Ossewaarde et al11 studied 204 subareolar abscesses in 85 patients. They found that the overall recurrence rate was 28% (11/39) after excision of the lactiferous ducts and 79% (128/163) after management without excision of the lactiferous ducts (p<0.001). Examples of lactiferous sparing procedures included incision and drainage, fistulectomy, and cone excision. Hanavadi et al, based on a retrospective study of 35 female patients, proposes that fistulectomy is more appropriate for simple fistulas, because total duct excision has unnecessary recurrence rate.11 Alternatively, some authors believe that these surgical options carry a significant risk of permanent nipple deformity, and thus have been proponents of repeated aspiration in combination with antibiotics.12 Antibiotic coverage should include both aerobic and anaerobic microorganisms. Gram-positive bacteria are more commonly found in primary subareolar abscesses, whereas anaerobic bacteria are more prevalent in recurrent abscesses.11
Mammillary fistulas should be suspected and treated appropriately in any male patient with a subareolar abscess. Failing to identify this important feature can lead to unnecessary recurrence, which will likely require further intervention. Despite the paucity of literature on this disease in men, it appears that the most successful outcomes are obtained with complete excision of the lactiferous duct fistula. In both our patients, excision of the abscess and lactiferous duct fistula has resulted in a successful treatment.
Learning points.
Subareolar abscess of the male breast is a rare condition, which can be complicated by a fistula from the areolar skin into a lactiferous duct.
When male patients present with a draining subareolar abscess, one should have a high index of suspicion for a mammillary fistula.
Failure to identify and surgically excise the fistula tract of Zuska's disease may lead to recurrence of the abscess and prolonged morbidity.
The most effective management of Zuska's disease includes complete excision of the lactiferous duct fistula.
Acknowledgments
The authors would like to thank Dr John Schaldenbrand (Department of Pathology, St. Joseph Mercy Health System, Ann Arbor, Michigan, USA) for providing and interpreting the histopathology images.
Footnotes
Contributors: GAS provided the care and management of the patient. SPJ and CK wrote the case reports with direction from GAS.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Zuska JJ, Crile G, Ayres WW. Fistulas of lactiferous ducts. Am J Surg 1951;8:312–17 [DOI] [PubMed] [Google Scholar]
- 2.Meguid MM, Oler A, Numann PJ, et al. Pathogenesis-based treatment of recurring subareolar breast abscesses. Surgery 1995;118:775–82 [DOI] [PubMed] [Google Scholar]
- 3.Patey DH, Thackray AC. Pathology and treatment of mammary-duct fistula. Lancet 1958;2:871–3 [DOI] [PubMed] [Google Scholar]
- 4.Habif DV, Perzin KH, Lipton R, et al. Subareolar abscess associated with squamous metaplasia of lactiferous ducts. Am J Surg 1970;119:523–6 [DOI] [PubMed] [Google Scholar]
- 5.Gollapalli V, Liao J, Dudakovic A, et al. Risk factors for development and recurrence of primary breast abscesses. J Am Coll Surg 2010;211:41–8 [DOI] [PubMed] [Google Scholar]
- 6.Hanavadi S, Pereira G, Mansel RE. How mammillary fistulas should be managed. Breast J 2005;11:254–6 [DOI] [PubMed] [Google Scholar]
- 7.Lambert ME, Betts CD, Sellwood RA. Mammillary fistula. Br J Surg 1986;73:367–8 [DOI] [PubMed] [Google Scholar]
- 8.Galblum LI, Oertel YC. Subareolar abscess of the breast: diagnosis by fine-needle aspiration. Am J Clin Pathol 1983;80:496–9 [DOI] [PubMed] [Google Scholar]
- 9.Silverman JF, Raso DS, Elsheikh TM, et al. Fine-needle aspiration cytology of a subareolar abscess of the male breast. Diagn Cytopathol 1998;18:441–4 [DOI] [PubMed] [Google Scholar]
- 10.Caswell AT, Burnett WE. Chronic recurrent breast abscesses secondary to inversion of the nipple. Surg Gynecol Obstet 1969;12:587–98 [PubMed] [Google Scholar]
- 11.Versluijs-Ossewaarde F, Roumen R, Goris R. Subareolar breast abscesses: characteristics and results of surgical treatment. Breast J 2005;11:179–82 [DOI] [PubMed] [Google Scholar]
- 12.Rosenthal LJ, Greenfield DS, Lesnick GJ. Breast abscess. New York State J Med 1981;81:182–3 [PubMed] [Google Scholar]


