Abstract
We report the case of a 51-year-old African woman with longstanding well-controlled HIV who developed relapsing systemic antineutrophil cytoplasm antibody (ANCA)-associated vasculitis and glomerulonephritis. She presented with an acute kidney injury and heavy proteinuria in the setting of a prolonged history of diffuse arthralgias and transient ocular symptoms. Antimyeloperoxidase (MPO) antibody titre was >100 IU/mL. Subsequent renal biopsy demonstrated a focal necrotising pauci-immune glomerulonephritis and a diagnosis of MPO-ANCA-associated microscopic polyangiitis was made. The patient was treated with tapering glucocorticoids and rituximab with resolution of her acute kidney injury and reduction in her proteinuria. Treatment was complicated by the development of steroid-induced diabetes and one mild clinical relapse, but was otherwise well tolerated without infectious complications or deterioration in her HIV disease.
Background
Antineutrophil cytoplasm antibody (ANCA) positivity is closely associated with primary small vessel systemic vasculitis. Detection of ANCA by indirect immunofluorescence (IIF) alone is sensitive but not highly specific for the diagnosis of small vessel vasculitis. Confirmation of reactivity by ELISA to myeloperoxidase (MPO) or proteinase-3 significantly increases the positive predictive value of the test from 45% to 88%.1 Additionally, ANCA has been reported to occur in a wide range of different infectious, inflammatory and malignant conditions, with or without underlying vasculitis.2 Since some of these conditions may present with features identical to those of small vessel vasculitis, it is imperative to differentiate false-positive ANCA (positive by immunofluorescence but not by ELISA and not associated with disease, possibly arising from a polyclonal gammopathy as can be often found in HIV or hepatitis C disease), secondary ANCA (arising as a result of chronic infectious stimulation and associated with disease, as may occur in bacterial endocarditis) and a true idiopathic primary ANCA-associated small vessel vasculitis.
Several studies have reported the occurrence of ANCA in HIV-positive patients, ranging from 13% to 42%, with the majority positive by IIF and only a small percentage positive by ELISA, with very few associated with clinical small vessel vasculitis.3–5 It has been proposed that ANCA in these patients is secondary to a marked polyclonal B-cell response to HIV infection. While autoantibodies may represent false positives in the context of active HIV with polyclonal gammopathy, this case emphasises that true systemic autoimmune disease should be considered in patients with HIV infection presenting with consistent clinical features. Thus, as we increasingly find patients with reconstituted immune systems, due to effective antiretroviral therapy, autoimmune phenomena may be more common and therefore represent another important form of kidney injury that can be found in patients with HIV infection. ANCA-associated small vessel vasculitis should therefore now enter the differential diagnosis of renal impairment in patients with HIV disease.
Case presentation
The patient's HIV was diagnosed 20 years previously during routine antenatal screening but she only started antiretrovirals 2 years later, when diagnosed with pneumocystis jiroveci pneumonia. Her nadir CD4 count was 20 cells/mm3 but with combination antiretroviral therapy (most recently Ritonavir and Darunavir) she had well-controlled HIV with undetectable viral loads and persistent CD4 counts of greater than 400 cells/mm3. In addition to her HIV diagnosis, the patient had osteoporosis.
She was born in East Africa, but moved to the UK 23 years ago. She did not smoke, drink alcohol and had never taken or injected recreational drugs. Her sister died from complications of HIV but there was no family history of renal disease.
Her symptoms started 3 years prior to her current presentation when she developed severe intermittent arthralgias of small and large joints with marked morning stiffness and coincident reduced vision in her left eye. MRI of her orbits was suggestive of lymphomatoid infiltration of the preseptal soft tissues and lacrimal glands bilaterally, while lacrimal gland biopsy demonstrated only acute on chronic inflammation. She had raised inflammatory markers (C reactive protein 82 mg/L, erythrocyte sedimentation rate 174 mm/h) with negative anti-nuclear antibody, rheumatoid factor and anti-CCP antibodies, with normal serum protein electrophoresis, and normal levels of immunoglobulins, complement proteins C3 and C4, creatine kinase, urate, vitamin D and parathyroid hormone. At this stage, the patient had a positive ANCA with a perinuclear staining pattern and an anti-MPO titre of 62 IU/mL (normal range <10), which was, erroneously, thought to be a false-positive ANCA in the setting of HIV disease. Ophthalmological review suggested a trial of oral steroids, with some relief from her ocular symptoms, but her arthralgias persisted, and she was treated with additional azathioprine, then methotrexate, without particular benefit, prompting the patient to stop these.
Three years following, she was noted during routine follow-up to have developed haematuria (3+) and proteinuria (4+) with an associated acute kidney injury.
Investigations
The patient's serum creatinine rose from 62–123 μmol/L with a urinary protein creatinine ratio of 802 mg/mmol. She had no other vasculitic symptoms of note, but had an ongoing inflammatory response and a repeat anti-MPO antibody titre of >100 IU/mL (no further anti-MPO titres had been measured in the intervening time period from initial presentation until this point).
Her renal ultrasound demonstrated normal-sized, unobstructed kidneys with no cortical thinning and she underwent a renal biopsy (figure 1) that demonstrated a pauci-immune, focal, necrotising glomerulonephritis (GN) with mild chronic damage and no features of HIV infection or complications of treatment.
Figure 1.
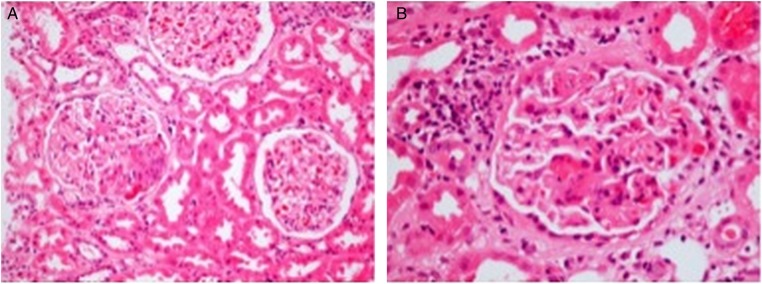
Photomicrograph of the renal biopsy at low (A) and high (B) power demonstrating a focal necrotising crescentic glomerulonephritis (H&E stain, ×100 and ×400). There was hardly any tubular atrophy found, but several surviving tubules showed acute damage and a few contained blood. Arteries and arterioles appeared virtually normal. Immunohistochemistry and electron microscopy (not shown) demonstrated no significant deposition of immunoproteins.
A diagnosis of MPO-ANCA-associated, microscopic polyangiitis was made.
Differential diagnosis
HIV-associated nephropathy (collapsing glomerulopathy)
Immune complex GN
Antiretroviral drug-associated kidney injury
Treatment
The patient was treated with pulsed methylprednisolone and rituximab, followed by a tapering course of oral prednisolone. She depleted her CD19 cells with a single dose of rituximab, to 0.002×109 cells/L at 1 month and 0.01×109 cells/L at 5 months (normal range of absolute CD19 number: 0.5–1.0×109 cells/L). She had an excellent clinical response with improvement in her arthralgic symptoms, a return of her serum creatinine to baseline over the next month, and a reduction in proteinuria over 6 months (figure 2).
Figure 2.
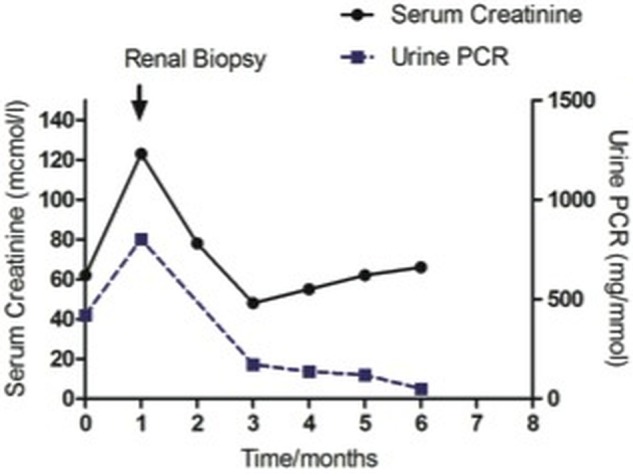
Graph depicting changes in renal function (serum creatinine (μmol/L)) and proteinuria (urine protein creatinine ratio (mg/mmoL)) over time. Diagnosis was made at the time of biopsy indicated by arrow, and the patient subsequently began treatment with steroids and rituximab.
Outcome and follow-up
The patient remained in remission despite persistently elevated anti-MPO antibody titres. However, following B lymphocyte repopulation at 6 months after the initial rituximab dose, she developed recurrent arthralgias, this time with stable renal function. She was retreated with a second dose of rituximab, and an increase in oral steroids, with good clinical response.
Her initial treatment was complicated by steroid-induced diabetes, treated with oral hypoglycaemics, but there were no other major complications, and specifically no infectious episodes. Her HIV remained well controlled, with unrecordable viral load and a CD4 count of 280 cells/mm3.
Discussion
ANCA positivity in HIV is reported in 13–42% of patients, and may be found at all stages of HIV disease from asymptomatic HIV, to AIDS-related complex. However, from a total of 94 patients with HIV infection who tested positive for ANCA in three separate series, only one was found to have clinical vasculitis.3–5 There have been sporadic reports of clinical vasculitis in patients with HIV infection, two with ANCA-negative eosinophilic granulomatosis with polyangiitis (formely Churg Strauss Syndrome),6 7 one of which was in association with a new diagnosis of HIV7; however, the first probable report of ANCA-positive vasculitis in HIV was of a patient with a clinical diagnosis of granulomatosis with polyangiitis (formerly Wegener's) but lacked histological confirmation of vasculitis.5 The authors of this case subsequently reviewed patients from a Dutch HIV registry and identified only one patient with GN of unknown cause. Serum from this patient was ANCA positive by IIF but negative on antigen-specific ELISA. Further details on the renal histology were not given.
Histologically confirmed cases of pauci-immune GN in patients with HIV infection are rare. Peraldi et al8 reviewed 92 cases of rapidly progressive renal failure in patients with HIV infection presenting over a 10-year period. Glomerular lesions were found in only four patients and in none of these was the histological lesion pauci-immune (rather immune-complex pattern). A recent study from India of 27 renal biopsies from patients with HIV infection and renal impairment demonstrated three cases of crescentic GN, one of which was pauci-immune (but no information on ANCA status was given).9 An additional report has recently been published of a patient with clinical vasculitis, positive ANCA with anti-MPO specificity and crescentic GN, in the setting of a new diagnosis of HIV and evidence of active EBV disease.10 This patient responded to treatment with steroids and cyclophosphamide.
We now report a case of ANCA-associated small vessel vasculitis in a patient with longstanding quiescent HIV disease, with positive perinuclear ANCA staining on IIF, high anti-MPO antibody titres confirmed by ELISA, and both clinical and histological evidence of disease. The patient responded well to treatment with steroids and rituximab, with no evidence of HIV re-activation or infectious complications. Our case highlights that true ANCA-associated vasculitis may occur in patients with HIV infection and clinicians should be aware of not dismissing a positive ANCA in this setting as insignificant. Careful follow-up of such patients is required as ANCA positivity may precede clinical vasculitis by many years.2 In our cohort of 120 patients with HIV infection who underwent renal biopsy between January 1998 and December 2012, we have only one other patient with a vasculitic GN, in the context of IgA nephropathy. However, recent case reports have described the combination of HIV and vasculitis, and it is possible that this is due to better testing or changes in autoimmune phenomena in those with better reconstituted immune systems.
Learning points.
Anti-neutrophil cytoplasm antibodies (ANCA) may be present in HIV without evidence of clinical vasculitis.
True ANCA-associated vasculitis is rare in HIV but does occur and should be considered if the clinical findings support it.
Patients with positive ANCA should be followed up closely as ANCA positivity may precede clinical vasculitis by many years.
Footnotes
Contributors: All authors contributed equally to the preparation of the manuscript. RE made corrections post initial submission.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Stone JH, Talor M, Stebbing J, et al. Test characteristics of immunofluorescence and ELISA tests in 856 consecutive patients with possible ANCA-associated conditions. Arthritis Care Res 2000;13:424–34 [DOI] [PubMed] [Google Scholar]
- 2.McAdoo SP, Hall A, Levy J, et al. Proteinase-3 antineutrophil cytoplasm antibody positivity in patients without primary systemic vasculitis. J Clin Rheumatol 2012;18:336–40 [DOI] [PubMed] [Google Scholar]
- 3.Savige JA, Chang L, Crowe SM. Anti-neutrophil cytoplasm antibodies in HIV infection. Adv Exp Med Biol 1993;336:349–52 [DOI] [PubMed] [Google Scholar]
- 4.Cornely OA, Hauschild S, Weise C, et al. Seroprevalence and disease association of antineutrophil cytoplasmic autoantibodies and antigens in HIV infection. Infection 1999;27:92–6 [DOI] [PubMed] [Google Scholar]
- 5.Klaassen RJ, Goldschmeding R, Dolman KM, et al. Anti-neutrophil cytoplasmic autoantibodies in patients with symptomatic HIV infection. Clin Exp Immunol 1992;87:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper LM, Patterson JA. Allergic granulomatosis and angiitis of Churg-Strauss. Case report in a patient with antibodies to human immunodeficiency virus and hepatitis B virus. Int J Dermatol 1989;28:597–9 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen H, Ferentz K, Patel A, et al. Churg-Strauss syndrome associated with HIV infection. J Am Board Fam Pract 2005;18:140–2 [DOI] [PubMed] [Google Scholar]
- 8.Peraldi MN, Maslo C, Akposso K, et al. Acute renal failure in the course of HIV infection: a single-institution retrospective study of ninety-two patients and sixty renal biopsies. Nephrol Dialysis Transplant 1999;14:1578–85 [DOI] [PubMed] [Google Scholar]
- 9.Vali PS, Ismal K, Gowrishankar S, et al. Renal disease in human immunodeficiency virus—not just HIV-associated nephropathy. Indian J Nephrol 2012;22:98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirsaeidi M, Syed F, Jaffe ES. Antineutrophil cytoplasmic autoantibody associated systemic vasculitis is associated with Epstein-Barr virus in the setting of HIV infection. Infect Dis Clin Pract 2013;21:50–3 [DOI] [PMC free article] [PubMed] [Google Scholar]


