Abstract
A 47-year-old HIV-positive woman presented with fever and a painful swollen right forearm. Clinical presentation and MRI were suggestive for a necrotising fasciitis. Surgical exploration revealed small transparent cystic bodies resembling white caviar, which were identified by their typical morphological features as larval stages (cysticerci) of Taenia crassiceps. Molecular methods, using sequence analysis of the small subunit rRNA gene, definitively confirmed T crassiceps. T crassiceps (Cestodea: Taeniidae) is a tapeworm found in the intestines of red foxes and dogs in the Northern Hemisphere. Human infections are rare and appear to depend on the host's immunocompetence. The eight published cases could not clarify the mode of infection but discuss ingestion of teniid eggs or penetration through a cutaneous wound. The optimal treatment remains unclear. We describe a detailed and successful treatment strategy including extensive surgical interventions, prolonged anthelmintic and antiretroviral treatment.
Background
We consider this case of great interest since necrotising fasciitis in an HIV-positive patient is a severe infection with high morbidity and mortality. A parasite is a rare infectious agent but not uncommon. With this case report and the three images we allow the reader an insight in the diagnostic procedures. So far, the optimal treatment with favourable outcome is unclear. However, we highlight our successful strategy with surgical interventions and the anthelmintic therapy challenged by the HIV infection and its management as helpful information for further infections due to Taenia crassiceps in HIV-positive patients.
Case presentation
In February 2010, a 47-year-old HIV-positive woman presented to our emergency department with a swollen and painful right forearm since 2 weeks. She had suffered an injury to her right wrist during her work as a zoo-employee 5 months previously. Her HIV infection had been diagnosed in 1993 and she started her first antiretroviral treatment (ART) in 1997. Because of various drug side effects and poor compliance, the ART had to be changed several times during the following years. The last ART consisting of emtricitabine/tenofovir and atazanavir/ritonavir was started in February 2008 and was stopped some months later by the patient. At that time, her HIV infection was classified at a CDC stage B2. Since then she has been off treatment. A chronic hepatitis C virus infection, acquired by intravenous drug injection, was known since 1990. She stopped the intravenous drug misuse successfully in the early 1990s.
Physical examination was unremarkable except for fever (38.9°C) and the swollen right forearm. The slight haematoma in the elbow area was painful to palpation. Laboratory findings were unremarkable including normal C reactive protein (3.9 mg/L) and a normal complete blood count. MRI of the forearm showed a significant oedema of the subcutis extending to the muscle brachioradialis without clear evidence of a fasciitis.
Suspecting necrotising fasciitis antibiotics were started and surgical exploration undertaken. After incision and preparation of the fascia numerous small (4–5 mm) transparent cystic bodies, resembling white caviar (figures 1 and 2), discharged. Morphological characteristics of the white caviar-like lesions included a small ellipsoid cystic body, a retractable neck with a single, completely or partially invaginated scolex that carries four suckers and two rows of characteristic hooklets as well as budding daughter cysts. In histological sections, a tegumental surface with wart-like protuberances and fine hair-like processes (microtriches) as well as the presence of small oval calcareous corpuscles in the parenchymatous tissue were indicative for a larval cestode (figure 3). The region of the retracted neck was heavily folded and parts of the suckers were recognisable and T crassiceps was suspected. Sequence analysis of a fragment of the small subunit rRNA gene1 permitted definitive species identification in our patient.
Figure 1.
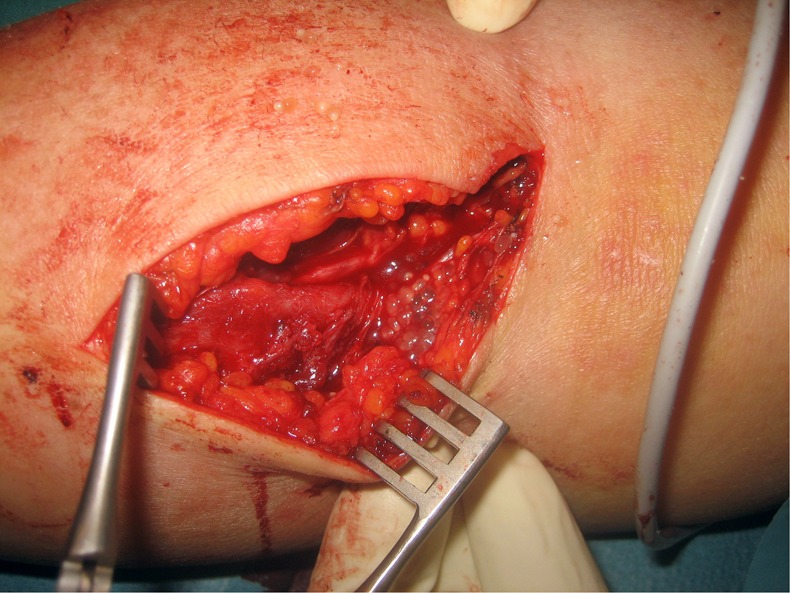
Incision of the skin revealed numerous transparent, ellipsoid vesicles, 4–5 mm in diameter.
Figure 2.
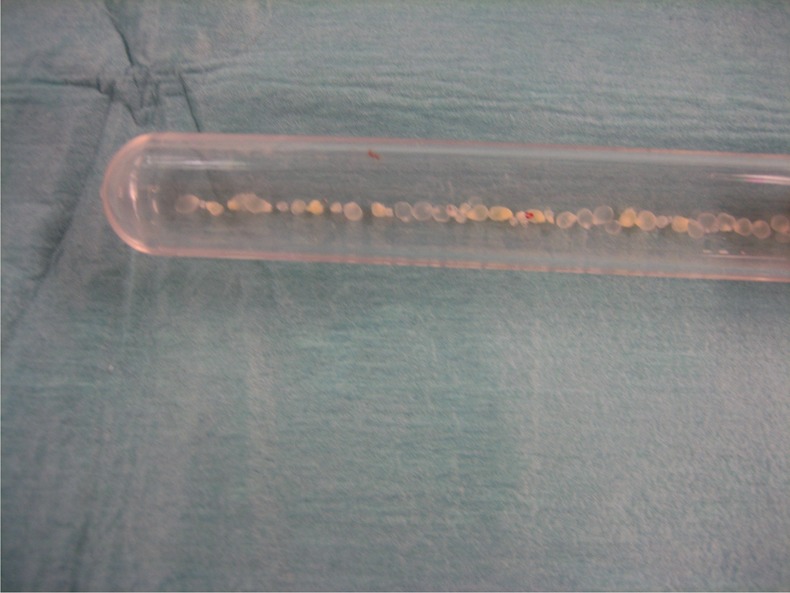
White caviar-like cystic bodies in the test tube taken during surgery.
Figure 3.
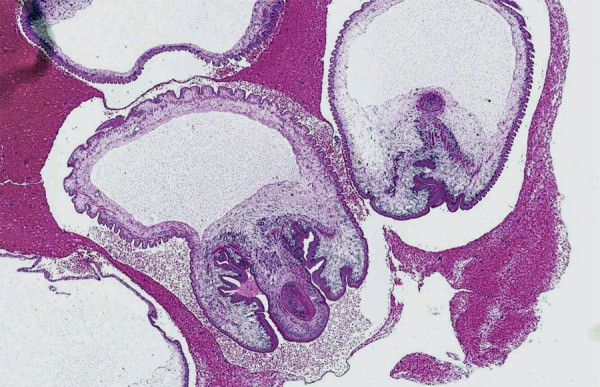
Section through one of the vesicles (H&E staining).
MRI excluded brain involvement and the ophthalmological examination was normal. Serology was negative for Taenia solium cysticercosis. The patient was severely immunodeficient with a CD4 cell count of 52 cells/μL and an HIV-1 RNA viral load of >4 million copies/mL.
Anthelmintic therapy with albendazole 400 mg twice daily combined with praziquantel 100 mg/kg body weight daily was initiated 2 weeks after the first operation together with a Pneumocystis jiroveci prophylaxis (trimethoprim/sulfamethoxazol (160 mg/800 mg) three times/week). However, 1 day later an extensive fasciitis of the right upper arm and forearm was found and a second surgical debridement and fasciectomy were necessary. At the surface of the fascia T crassiceps larvae were still detected. Combined anthelmintic treatment with albendazole and praziquantel were continued but praziquantel had to be stopped after 14 days because of severe neurological side effects. Albendazole induced a neutropenia. Therefore, neupogen was administered. ART was started 4 weeks after starting the anthelmintic treatment and consisted of emtricitabine/tenofovir, nevirapine and atazanavir/ritonavir. This regime was chosen based on the previous ART history and results of the HIV-resistance testing. The interaction profile (http://www.hiv-druginteractions.org) showed possible drug interactions for albendazole and ritonavir as well as for albendazole and nevirapine. Coadministration with ritonavir may decrease drug levels of albendazole,2 the interaction with nevirapine remains unclear. Therefore, drug levels of albendazole were monitored monthly and revealed two subtherapeutic (months 2 and 4) albendazole levels (<1 µmol/L).
One month after initiating ART 340 CD4 cells/μL were measured, and after 3 months 268 CD4 cells/μL and 1400 copies/mL of HIV-1 RNA. Five months later, still on albendazole the patient remained asymptomatic. Since the CD4 count was 290 cells/μL, and therefore had been >200 cells/μL for >6 months, albendazole was stopped.
Investigations
MRI for primary diagnosis of soft tissue infection, and exclusion of systemic spread. Serology, macrohistopathology and microhistopathology for detection of T crassiceps. Molecular methods (sequence analysis of a fragment of the small subunit rRNA gene) for parasite identification. Gram stains and bacterial cultures of the tissue were negative.
Differential diagnosis
Bacterial necrotising fasciitis.
Treatment
Repetitive surgical debridement and fasciectomy.
Anthelmintic drug treatment consisting of praziquantel 100 mg/kg body weight daily and albendazole 2×400 mg/day for 14 days, followed by albendazole monotherapy until CD4 cell count >200 cells/μL > 6 months.
Initiating ART after 2–4 weeks of anthelmintic treatment to reduce the risk of immune reconstitution inflammatory syndrome (IRIS).
Outcome and follow-up
After a first surgical exploration and initiating of the anthelmintic treatment the patient suffered from a relapse requiring further surgical debridement and fasciectomy. Four weeks later, the ART was started without serious side effects. One year after her first presentation while still on ART, the anthelmintic treatment was stopped since the CD4 cell count remained stable >6 month. The patient recovered completely and is still doing well. Her arm has regained normal functions.
Discussion
This severely immunocompromised HIV-positive patient presented with a fasciitis of her right arm due to T crassiceps.
T crassiceps (Cestodea: Taeniidae) is a tapeworm found in the intestines of red foxes and dogs in the Northern hemisphere. Rodents and moles act as intermediate hosts and may harbour larval stages under the skin and in body cavities. In humans infections are rare and depend on the host's immunocompetence. Among the eight published cases 3–10 only three individuals were immunocompetent and presented with intraocular infections.8–10 All these three cases were completely cured by surgical removal of the parasites. In the four cases occurring in immunocompromised patients, in particular in the individuals with advanced HIV infection, T crassiceps cysticerci showed a tropism for subcutaneous and muscular tissues, similar to cysticercosis caused by T solium, but without involvement of the central nervous system.3–7 Three of these four patients had previously experienced a trauma resulting in persisting haematomas prior to the infection.3–5 7 All patients were dog owners.
Our patient owned two dogs and worked with foxes at a zoo. However, T crassiceps was not detected in her dogs’ faeces. Fox faeces were not available for examination. The route of infection—oral uptake of eggs or inoculation of eggs into the patient's wound at the wrist with subsequent larval development and spread to the proximal forearm facilitated by the advanced HIV infection—remains unclear as it is the case for all published case reports.3–10
The optimal treatment remains unclear, especially in untreated HIV infection. The anthelmintic regimens consisting of albendazole and/or praziquantel (both with proven effects in vitro) have been used.3–5 7 However, surgery is considered to be as important as the anthelmintic treatment3 and can consist of a simple incision, debridement or fasciectomy.3–7 Nevertheless, 2 of the 3 published HIV-positive patients experienced relapses after months, despite combined surgical and prolonged (3–6 months) anthelmintic treatment.4 5 No details about the management of the HIV infection are available.4 5 7 Only in one case report ART was mentioned.6 Thus optimal treatment for HIV-positive patients remains unclear; in particular when to start ART, the benefits of surgical intervention, the question whether combined anthelmintic or monoanthelmintic therapy is superior and when anthelmintic treatment can be stopped without risking a relapse.
Our case report illustrates a sound treatment strategy. Since T crassiceps infections occur predominantly in severely immunocompromised individuals, it is considered an opportunistic infection. According to the general treatment guidelines for opportunistic infections in HIV-positive patients, we postponed ART start for 4 weeks after initiating anthelmintic treatment, to avoid IRIS. Our patient presented with an early relapse 2 weeks after her first surgical debridement. At that time, anthelmintic therapy had been installed for 1 day and ART had not even been started yet. Therefore, the relapse was not considered an IRIS. We conclude that early and repeated surgical debridement could be an advantage in the first weeks of the onset of infection since it reduces parasite load and stops the asexual budding of the cysticerci of T crassiceps at the site of infection. Surgical intervention is faster and more radical than anthelmintic treatment.
Praziquantel is thought to prevent budding in addition to its cestodicide nature by paralysing the parasitic muscles. However, its tissue penetration is unknown. The intake of the full dose of 100 mg/kg body weight daily is complicated by its bitter taste. Common side effects are malaise, headache, dizziness, abdominal discomfort and urticaria.
Albendazole is a cestodistatic drug as it interferes with the helmintic metabolism and provides a long-term suppression of the parasite. Side effects include headache, dizziness, abdominal discomfort and increase of liver enzymes. After long-term treatment bone marrow suppression resulting in pancytopenia may occur. Bioavailability is very low (5%) but can be improved by fatty food intake increasing bioavailability up to five times. Interactions between praziquantel and albendazole are possible resulting in increased albendazole levels. Our patient experienced a pronounced dizziness and somnolence under combination therapy, which disappeared immediately after stopping praziquantel. Because albendazole drug level was not in a toxic range at that time, we believe that the patient suffered of side effects from praziquantel.
In accordance with general principles of delaying ART in presence of opportunistic infection and because of the neutropenia due to albendazole we postponed ART for 4 weeks. The patient tolerated the new ART without side effects and an IRIS was not observed. Interactions are known to occur in all substrates of the CYP 450 enzymes. Albendazole is predominantly metabolised by CYP450 enzymes, including CYP3A4. Ritonavir, used as a protease booster, decreased albendazole and the active metabolite albendazole sulfoxide resulting in subtherapeutic levels (<1 μmol/L) as also observed twice in our patient.2 However, this questions the importance of albendazole therapy in patients with HIV on ART. In analogy to the treatment of other opportunistic infections we stopped albendazole successfully as soon as the CD4 cell count remained >200 cells/μL for >6 months. The patient, still on ART, is doing well without any further relapse.
We conclude that severe soft tissue infections caused by T crassiceps appear predominantly in severely immunocompromised individuals. Restoration of immunocompetence is essential to control the infection.6 In HIV-positive patients initiating ART is thus crucial. Until immunocompetence is obtained early surgical debridement seems to be more effective than anthelmintic treatment.
Learning points.
The tapeworm Taenia crassiceps causes human infections rarely.
Soft tissue infections are seen in immunocompromised individuals resulting in high morbidity and mortality; aggressive diagnostic workup is essential to ensure accurate microbiological diagnosis. Exclusion of central nervous system and eye involvement is advised.
A successful treatment strategy includes early extensive surgical debridement and antiretroviral treatment in HIV positive patients.
Anthelmintic therapy benefits remain unclear. When tolerated well and therapeutic levels are reached, the duration should be limited in analogy to the general treatment guidelines of opportunistic infections in HIV-positive individuals.
Patients infected with HIV should never stop or interrupt their treatment to avoid opportunistic infections.
Footnotes
Contributors: YFA and AZ were involved in the patient’s management, writing and literature search. FG was involved in the diagnostic procedures (identification of the parasite). NL was involved in the surgical procedures and collection of figures.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Trachsel D, Deplazes P, Mathis A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology 2009;134:911–20 [DOI] [PubMed] [Google Scholar]
- 2.Corti N, Heck A, Rentsch K, et al. Effect of ritonavir on the pharmacokinetics of the benzimidazoles albendazole and mebendazole: an interaction study in healthy volunteers. Eur J Clin Pharmacol 2009;65:999–1006 [DOI] [PubMed] [Google Scholar]
- 3.Heldwein K, Biedermann HG, Hamperl WD, et al. Subcutaneous Taenia crassiceps infection in a patient with non-Hodgkin's lymphoma. Am J Trop Med Hyg 2006;75:108–11 [PubMed] [Google Scholar]
- 4.Maillard H, Marionneau J, Prophette B, et al. Taenia crassiceps cysticercosis and AIDS. AIDS 1998;12:1551–2 [DOI] [PubMed] [Google Scholar]
- 5.Klinker H, Tintelnot K, Joeres R, et al. Taenia crassiceps infection in AIDS. Dtsch Med Wochenschr 1992;117:133–8 [DOI] [PubMed] [Google Scholar]
- 6.Francois A, Favennec L, Cambon-Michot C, et al. Taenia crassiceps invasive cysticercosis: a new human pathogen in AIDS? Am J Surg Pathol 1998;22:488–95 [DOI] [PubMed] [Google Scholar]
- 7.Goesseringer N, Lindenblatt N, Mihic-Probst D, et al. Taenia crassiceps upper limb fasciitis in a patient with untreated AIDS and chronic hepatitis C infection- The role of surgical debridement. J Plast, Reconstr Aesthet Surg 2011;64:e174–6 [DOI] [PubMed] [Google Scholar]
- 8.Chuck RS, Olk RJ, Weil GJ, et al. Surgical removal of a subretinal proliferating cysticercus of Taeniaformis crassiceps. Arch Ophthalmol 1997;115:562–3 [DOI] [PubMed] [Google Scholar]
- 9.Arocker-Mettinger E, Huber-Spitzy V, Auer H, et al. Taenia crassiceps in the anterior chamber of the human eye. A case report. Klin Monatsblatt Augenheilkunde 1992;201:34–7 [DOI] [PubMed] [Google Scholar]
- 10.Shea M, Maberly AL, Walters J, et al. Intraocular Taenia crassiceps. Trans AM Acad Ophthalmol Otolaryngol 1973;77:778–83 [PubMed] [Google Scholar]


