Abstract
Nocardia species are aerobic, gram positive filamentous branching bacteria that have the potential to cause localized or disseminated infection. Nocardiosis is a rare disease that usually affects immunocompromised patients and presents as either pulmonary, cutaneous or disseminated nocardiosis. Forty-two year-old hispanic male presented to our care with bilateral lower extremity weakness, frontal headache, subjective fever, nausea, and vomiting. Brain computed tomography (CT) revealed multiple hyperdense lesions with vasogenic edema in the frontal, parietal and left temporal lobes. Chest CT demonstrated bilateral cavitary nodules in the lung and right hilar lymphadenopathy. Brain magnetic resonance imaging revealed multiple bilateral supratentorial and infratentorial rim enhancing lesions involving the subcortical gray-white matter interface with vasogenic edema. Patient was started on empiric therapy for unknown infectious etiology with no response. He eventually expired and autopsy findings revealed a right hilar lung abscess and multiple brain abscesses. Microscopic and culture findings from tissue sample during autopsy revealed nocardia wallacei species with multidrug resistance. The cause of death was stated as systemic nocadiosis (nocardia pneumonitis and encephalitis). The presence of simultaneous lung and brain abscesses is a reliable indication of an underlying Nocardia infection. An increased awareness of the various presentations of nocardiosis and a high index of clinical suspicion can help in a rapid diagnosis and improve survival in an otherwise fatal disease. This case highlights the importance of obtaining a tissue biopsy for definitive diagnosis on the initial presentation when an infectious process is considered in the differential diagnosis and early treatment can be initiated.
Key words: nocardia, lung abscess, brain abscess, septic emboli
Introduction
Nocardia species are aerobic, gram positive filamentous branching bacteria that have the potential to cause localized or disseminated infection. Nocardia species are ubiquitous in the environment as saprophytic components in dust, soil, water, decaying vegetation and stagnant matter.1 Nocardiosis mainly affects immunocompromised patients, although rarely immunocompetent individuals could also be affected. The predominant form of nocardiosis is dependent on the species and the geographical location.2 The majority of nocardiosis cases are caused by the N. asteroides complex and N. brasiliensis. In the tropical climate cutaneous and lymphocutaneous infections caused by N. brasiliensis (mycetoma) predominate. In the temperate climate zone, pulmonary infections with N. asteroides complex will be more prevalent.2 The major risk factor for nocardia infection is being immunocomprised, specifically those with cell-mediated immunity defects.3 The main causes of immunocompromise are hematopoietic stem cell or solid organ transplantation, HIV infection, chemotherapy, malignancy or glucocorticoid therapy. Risks factors for pulmonary nocardiosis are alcoholism and chronic lung disease.
Nocardiosis is a rare disease that usually causes one of three clinical manifestations. Pulmonary nocardiosis is the most common manifestation nocardiosis. Pulmonary infection is acquired through the inhalation of the organism from dust or soil. Other sites of nocardial dissemination include skin, subcutaneous tissues, and the central nervous system.4 Disseminated disease is defined by the presence of two or more organs infected by nocardia. Disseminated nocardiosis is characterized by hematogenous spread of microbes into brain, eye, bone, joint, heart, kidney, skin or other organs and tissues.5 Nocardiosis has the ability to disseminate any organ, most commonly the central nervous system (CNS) and has a tendency to relapse or progress despite being given the appropriate therapy.6
We are presenting a case of an immunocompetent patient with risk factors of chronic alcohol abuse and environmental exposure as a construction worker that developed disseminated nocardiosis. This case was further complicated by the inability to obtain a tissue sample to characterize the nocardia species and give the appropriate therapy.
Case Report
Forty-two year-old Hispanic male presented with severe and constant bilateral lower extremity pain for 3 weeks, located at the upper thighs with radiation down the legs. He also complained of a non-radiating severe, frontal, throbbing headache for one week, associated with occasional episodes of diplopia, without a stiff neck or photophobia. Four days prior he developed bilateral lower extremity weakness and could not walk or stand on his own. Other complaints included nausea, vomiting and a low-grade fever of 37.7°C. No recent travel abroad or sick contacts. He had no medical problems or previous surgical interventions. He admitted to a 20 pack year history of smoking and chronic alcohol intake of four 8 oz cans of beer every day for the last twenty years. He currently worked as a construction worker. All vital signs were stable on admission and within normal range. Physical examination demonstrated somnolent but arousable patient in no acute distress. The complete neurological and lung examination were unremarkable except hypereflexia of the right brachioradialis and right biceps and a decrease of muscle strength of bilateral lower extremities. All other body systems of physical examination were unremarkable.
Initial laboratory work up (Table 1) was remarkable for mild hyponatremia, mild transaminase elevation and hyperbilirubinemia. The ammonia level was in the upper normal range. Urinalysis, urine toxicology screen and alcohol level, hepatitis serology panel and HIV were negative. Chest x-ray on admission was unremarkable. Computer tomography (CT) of the brain (Figure 1A) on admission revealed multiple intraaxial hyperdense lesions with surrounding vasogenic edema in the frontal, parietal and left temporal lobes. Hematogenous spread was suggested by the presence of several lesions at the gray-white junction. Chest-CT (Figure 1B) on admission demonstrated bilateral cavitary nodules in the lung and right hilar lymphadenopathy. Our differential diagnosis included metastatic versus infectious etiology. We therefore started the patient on empiric antibiotics of vancomycin 1 gram every 12 hours, ceftriaxone 2 grams every 12 hours, and dexamethasone 6mg IV every 8 hours for cerebral edema. We then proceeded to obtain a MRI of the brain (Figure 2) which revealed multiple bilateral supratentorial and infratentorial rim enhancing lesions involving the subcortical gray-white matter interface, superficial and deep white matter tracts, brainstem and cerebellum associated with vasogenic edema. These findings were more likely associated with an infectious etiology causing septic emboli. The initial blood and urine cultures on admission were negative.
Table 1.
Initial laboratory work up.
| White blood cell count | 10.05×103 UL (4.5-11.0×103/UL) |
| Hemoglobin | 16.0 g/dL (12.0-15.0 g/dL) |
| Hematocrit | 43.4% (36.0-47.0%) |
| Platelet count | 233×103/UL (150-450×103/UL) |
| Sodium | 130 mmol/L (135-145 mmol/L) |
| Potassium | 4.4 mmol/L (3.5-5.1 mmol/L) |
| Serum glucose | 137 mg/dL (70-100 mg/dL) |
| BUN | 15 mg/dL (7-22 mg/dL) |
| Creatinine | 0.8 mg/dL (0.60-1.30 mg/dL) |
| AST | 66 IU/L (15-37 IU/L) |
| ALT | 66 IU/L (12-78 IU/L) |
| Alkaline Phosphatase | 104 IU/L (50-136 IU/L) |
| Ammonia | 45 umol/L (10-40 umol/L) |
Figure 1.
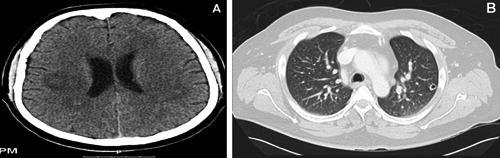
A) Computed tomography, brain: multiple intraaxial hyperdense lesions with surrounding vasogenic edema. B) Computed tomography, chest: bilateral cavitary nodules in the lung and right hilar lymphadenopathy.
Figure 2.
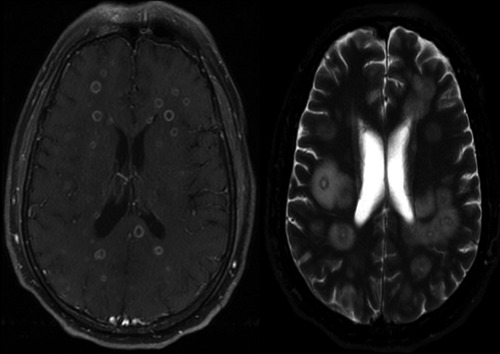
T1 and T2 magnetic resonance imaging, brain: multiple bilateral supratentorial and infratentorial rim enhancing lesions involving the subcortical gray-white matter interface associated with vasogenic edema.
The pulmonary service was consulted on the second hospital day for bronchoscopy for bronchoalveloar lavage (BAL). Bronchoscopy revealed normal airway anatomy with diffusely erythematous mucosa and clear secretions. BAL was obtained from the left upper lobe and cytology report had no significant findings. The BAL cultures were negative for fungal, bacteria and mycobacteria. AFB stain was performed on the BAL and was also negative. The infectious disease service was also consulted on the second hospital day and recommended a PPD, Quaniferon Gold, coccidiodies serology, aspergillus antigen, toxoplasma IgG antibody, serum crytococcal antigen and a lumbar puncture for cerebrospinal fluid (CSF) analysis. All of the stated lab tests were negative. Cerebrospinal fluid analysis (Table 2) was elevated WBC count, suggesting an infectious etiology. No evidence of vegetations was noted on echocardiogram. Due to the unclear etiology of the lung and brain lesions, neurosurgery was consulted for a brain biopsy. The neurosurgeon suggested that brain biopsy would not be possible considering the small size and depth of the lesions. By the tenth hospital day the mental status of the patient deteriorated to the point that he was now disoriented to time and place but oriented to person. By the twelfth hospital day the patient developed weakness of the left upper extremity and an unsteady antalgic gait. Then on the next day, he also developed right upper extremity weakness, ptosis and right lower facial weakness. Cardiothoracic surgery was consulted for video-assisted thoracoscopic surgery with biopsy of cavitary lung lesions but the location of the lesion was unsuitable for the surgical access. Eventually, the GCS of the patient decreased to 7, he was bradycardic and had complete right sided paralysis with a positive right side babinski.
Table 2.
Cerebrospinal fluid studies.
| Appearance | Clear and colorless |
|---|---|
| White blood cell count | 18/uL |
| Neutrophils | 64% |
| Lymphocytes | 25% |
| Monocytes | 11% |
| Red blood cell count | 1/uL |
| Protein | 37 mg/dL |
| Glucose | 55 mg/ dL |
| Corresponding serum glucose | 128 mg/ dL |
| Cerebrospinal fluid serology | |
| Cryptococcal antigen | Negative |
| Cysticerosis IgG antibody | Negative |
| Coccidioides antibody | Negative |
| Histoplasma antibody | Negative |
| CSF culture | Negative |
He was subsequently transferred to the medical intensive care unit (MICU) for further monitoring and cardiopulmonary support. The repeat head-CT with contrast revealed multiple ring enhancing lesions involving both cerebral, basal ganglia and brainstem. There was no evidence for acute intracranial hemorrhage, herniation, mass effect or midline shift. The infectious disease service decided to start the patient on amphotericin B 450 mg (5 mg/kg) IV every 24 hours and advised us to continue the same antibiotics and dexamethasone. On the nineteenth hospital day the patient became tachycardic (HR: 120-130’s) and desaturated to <80%, hence he was intubated and placed on mechanical ventilation support. The infectious disease consult service recommended albendazole 15 mg/kg/day through the naso-gastric-tube every 12 hours for probable neurocysticercosis, and then to initiate toxoplasmosis therapy despite negative test result. This therapy included pyrimethamine 200 mg PO (1 dose) then 75 mg/day plus sulfadiazine 1.5 g PO every 6 hours plus leukovorin 20 mg PO every 24 hours. Despite our best efforts, the patient remained tachycardic and begun to spike fevers ranging from 38.7°C to 39°C. Considering the worsening of his clinical status we continued to advocate the attempt for a tissue biopsy in order to help us guide in the diagnosis and treatment but to no avail. The patient continued to deteriorate, did not respond and subsequently expired on the twentieth hospital day after the family withdrew care.
Autopsy was subsequently performed two days later. Macroscopic findings of respiratory system included bilateral serosanguineous pleural effusions and a lung abscess at the right hilum. The microscopic examination of the right hilum abscess showed foci of necrosis with acute inflammation and microabscess formation. The macroscopic findings of the brain revealed multiple bilateral cavities present in the white matter throughout the entire cerebrum, and also present throughout the basal ganglia, midbrain, substantia nigra and right lobe of the cerebellum. Microscopic examination findings showed foci of acute inflammation and microabscess formation. Grocott methena mine silver (GMS), grain stain and Fite’s stain of the right hilum abscess and brain abscesses both revealed numerous gram positive branching rods, morphologically consistent with nocardia. Tissue samples from the brain abscess lesions and lung cavitary lesions were sent for cultures and subsequently grew nocardia species. PCR analysis revealed nocardia wallacei species with resistance to ceftriaxone, levofloxacin, aminoglycosides (amikacin) and to sulfonamides (trimethoprim-sulfamethoxazole – TMP-SMX). However, this species of nocardia was sensitive to imipenem. The final autopsy diagnosis revealed nocardia pneumonitis and encephalitis. The cause of death was stated as systemic nocadiosis (nocardia pneumonitis and encephalitis).
Discussion
The primary clinical presentation of nocardiosis is pulmonary involvement. Reported cases have been associated with pneumonia, empyema or lung abscesses. Our patient in particular presented with a major right hilar lung abscess and several microabscesses. A differential diagnosis for a lung abscess should include aspergillosis, actinomycosis, tuberculosis, bacterial lung abscess and malignancy. The mortality due to pulmonary nocardiosis is about 14-40% which increases significantly when there is dissemination to the central nervous system.7 Although 20% of extrapulmonary Nocardia infections occur in the absence of pulmonary disease, about 50% of all pulmonary cases disseminate extrapulmonary, most commonly the brain.8
Nocardia appears to have special tropism for neural tissue. Nocardia brain abscess are rare and accounts for only 1-2% of all cerebral abscesses.9 The mortality rate is 31% which is much greater than that of other agents that can cause cerebral abscesses being less than 10%.10 The presence of simultaneous lung and brain abscesses has been documented as a reliable indication of an underlying nocardia infection.11 Central nervous system (CNS) involvement with disseminated nocardiosis results in up to a 55% mortality rate in immunocompromised patients.12 If left untreated, disseminated nocardiosis has a mortality rate of greater than 85%.13
The main challenge with nocardiosis is the ability to arrive at a definitive diagnosis. Even though it has been reported in the medical literature, the relative rarity of this infection can lead to it being overlooked and misdiagnosed as other pathologies.14 The nonspecific presentation and imaging findings of the disease warrant culture and tissue diagnosis. Diagnosis of nocardia can only be confirmed by microbiological techniques for which adequate material must be obtained.15 Gram staining is the most sensitive method for visualizing and recognizing nocardia. Nocardia is partially acid-fast by conventional Ziehl-Nielsen staining and is reactive with Gomori methenamine silver.16 It is important to obtain cultures from tissue biopsies when disseminated infection is considered to allow for prompt organism identification and perhaps better patient care.17 Biochemical tests may be used for identification of a subset of nocardia species. It is important to distinguish nocardia species because of the resistance to certain antimicrobial agents and a higher risk of dissemination.18 This is important for epidemiological reasons due to the rising resistance of nocardia spp. to TMP-SMX.
Initial treatment for presumed nocardiosis must be empiric, due to the fact that it could take up to 3 weeks to speciate and determine sensitivities to nocardia.19 In general, the antibacterial agents most active against N. asteroides are the sulfonamides, amikacin, minocycline and imipenem.20 Sulfonamides such as trimethoprim-sulfamethoxazole are the treatment of choice for N. asteroides, with the best alternative being minocycline. The most effective intravenous regimen for N. asteroides is the combination of amikacin plus imipenem.21 In patients with a sulfa allergy or disseminated nocardiosis a combination of amikacin and ceftriaxone should be used.22 It is recommended that immunocompetent patients be treated for at least six months. Treatment for disseminated nocardia will depend upon the presence or absence of CNS involvement and/or multiple organ involvement.23 Treatment for 12 months is recommended if the central nervous system is involved. No randomized trials have been performed to determine the most effective antibiotic regimen for nocardiosis. However, TMP-SMX is considered the mainstay of therapy since most studies have shown a favorable suspectibility to this antibiotic.24
Conclusions
Nocardiosis is often misdiagnosed because of its rarity and nonspecific clinical picture. It is important to emphasize that an increased awareness of the various presentations of nocardiosis in combination with a high index of clinical suspicion can precipitate a rapid diagnosis and improve survival in an otherwise fatal disease. Nocardia should be included in the differential diagnosis when evaluating any patient with risk factors for immunosuppression and a possible opportunistic infection. Obtaining an accurate diagnosis and the initiation of early treatment are essential but can be very challenging. However, if a tissue biopsy can be obtained then nocardia can be quickly diagnosed by examination of pathological material with gram stain or a modified acid fast. This case highlights the importance of obtaining a tissue biopsy for microbiological and culture identification for organisms such as nocardia on the initial presentation when an infectious process is considered in the differential diagnosis and early treatment can be initiated. Another important point that this case presents is the existence of a less common nocardia species with multidrug resistance especially to aminoglycosides and sulfonamides. We stress the importance of determining the species of nocardia and antibiotic susceptibility to help guide the management of this devastating infectious disease.
References
- 1.Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ., Jr.Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev 2006;19:259-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smeets LC, van Agtmael MA, van der Vorm ER. Successful treatment of a disseminated Nocardia brasiliensis infection. Eur J Clin Microbiol Infect Dis 2005;24:350-1 [DOI] [PubMed] [Google Scholar]
- 3.Benes J, Viechova J, Picha D, et al. Disseminated Nocardia asteroides infection in an immunocompetent woman following an arm injury. Infection 2003;31: 112-4 [DOI] [PubMed] [Google Scholar]
- 4.Ramamoorthi K, Pruthvil BC, Rao NR. Pulmonary nocardiosis due to Nocardia otitidiscaviarum in an immunocompetent hosta rare case report. Asian Pac J Trop Med 2011;4;14-6 [DOI] [PubMed] [Google Scholar]
- 5.Cattaneo C, Antoniazzi F, Caira M, et al. Nocardia spp infections among hematological patients: results of a retrospective multi-center study. Int J Infect Dis 2013; 17:e610-4 [DOI] [PubMed] [Google Scholar]
- 6.Beaman BL, Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev 1994;7:213-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow E, Moore T, Deville J, Nielsen K. Nocardia asteroides brain abscesses and meningitis in an immunocompromized 10-year-old child. Scand J Infect Dis 2005; 37:511-3 [DOI] [PubMed] [Google Scholar]
- 8.Devi P, Malhotra S, Chadha A. Nocardia brasiliensis primary pulmonary nocardiosis with subcutaneous involvement in an immunocompetent patient. Indian J Med Microbiol 2011;29:68-70 [DOI] [PubMed] [Google Scholar]
- 9.Al Tawfiqa JA, Maymanb T, Memishc ZA. Nocardia abscessus brain abscess in an immunocompetent host. J Infect Publ Health 2013;6:158-61 [DOI] [PubMed] [Google Scholar]
- 10.Cassir N, Million M, Noudel R, et al. Sulfonamide resistance in a disseminated infection caused by Nocardia wallacei: a case report. J Med Case Rep 2013;7:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sirisena1 D, Swedan LA, Jayne D, Chakravarty K. A case of systemic nocardiosis in systemic vasculitis and a review of the literature. Singapore Med J 2013;54:e127-30 [DOI] [PubMed] [Google Scholar]
- 12.Montoya JP, Carpenter JL, Holmes GP, et al. Disseminated nocardia transvalensis Infection with osteomyelitis and multiple brain abscesses. Scand J Infect Dis 2003;35:189-212 [DOI] [PubMed] [Google Scholar]
- 13.Paramythiotou E, Papadomichelakis E, Vrioni G, et al. A life-threatening case of disseminated nocardiosis due to Nocardia brasiliensis. Indian J Crit Care Med 2012;16:234-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menkü A, Kurtsoy A, Tucer B, et al. Nocardia brain abscess mimicking brain tumour in immunocompetent patients: report of two cases and review of the literature. Acta Neurochir 2004;146:411-4 [DOI] [PubMed] [Google Scholar]
- 15.Höpler W, Laferl H, Szell M, et al. Blood culture positive Nocardia asteroides infection: a case report. Wien Med Wochenschr 2013;163:37-9 [DOI] [PubMed] [Google Scholar]
- 16.Budzik JM, Hosseini M, Mackinnon AC, Jr., Taxy JB. Disseminated nocardia farcinica: literature review and fatal outcome in an immunocompetent patient. Surg Infect 2012;13:163-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodiuk-Gad R1, Cohen E, Ziv M, Goldstein LH, et al. Cutaneous nocardiosis: report of two cases and review of the literature. Int J Dermatol 2010:49:1380-5 [DOI] [PubMed] [Google Scholar]
- 18.Brown-Elliott BA, Biehle J, Conville PS, et al. Sulfonamide resistance in isolates of Nocardia spp. from a US multicenter survey. J Clin Microbiol 2012;50:670-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosel D, Harris L, Fisher E, et al. Disseminated Nocardia infection presenting as hemorrhagic pustules and ecthyma in a woman with systemic lupus erythematosus and antiphospholipid antibody syndrome. J Derm Case Rep 2013;7:52-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uhde KB, Pathak S, McCullum I, Jr, et al. Antimicrobial-resistant nocardia isolates, United States, 1995-2004. Clin Infect Dis 2010;51:1445-8 [DOI] [PubMed] [Google Scholar]
- 21.Wakamatsu K, Nagata N, Kumazoe H, et al. Nocardia transvalensis pulmonary infection in an immunocompetent patient with radiographic findings consistent with nontuberculous mycobacterial infections. J Infect Chemother 2011;17:716-9 [DOI] [PubMed] [Google Scholar]
- 22.Sethi PK, Khandewal D, Sethi NK, et al. Neuroimage: disseminated nocardiosis. Clin Neurol Neurosurg 2008;100:97-100 [DOI] [PubMed] [Google Scholar]
- 23.Arora G, Friedman M, MacDermott RP. Disseminated nocardia nova infection. South Med J 2010;103:1269-71 [DOI] [PubMed] [Google Scholar]
- 24.Rosman Y, Grossman E, Keller N, et al. Nocardiosis: a 15-year experience in a tertiary medical center in Israel. Eur J Intern Med 2013;24:552-7 [DOI] [PubMed] [Google Scholar]


