Abstract
With better care and intensive insulin therapy, microvascular complications have reduced and longevity has increased in patients with type 1 diabetes (T1DM). Therefore, there is a need to change the focus from microvascular complications to cardiovascular disease and osteoporosis. Though number of studies from other parts of the world show that patients with T1DM are at increased risk of osteoporosis and fractures, there is a paucity of data from India. A number of factors and mechanisms affecting bone health in patients with T1DM have been proposed. The main defect in genesis of osteoporosis is osteoblastic function, rather than osteoclastic overfunction. Assessment of bone mineral density by dual X-ray absorptiometry and other risk factors for osteoporosis, as a part of diagnostic procedure can help to design tailored treatment plans. A physically active healthy lifestyle, prevention of diabetic complications and adequate calcium and vitamin D supplementation are the mainstay for prevention of osteoporosis. Treatment of osteoporosis is not evidence based but it is proposed to be similar to osteoporosis associated with other conditions. Bisphosphonates are the mainstay for treatment of osteoporosis in patients with T1DM. However, more studies are needed to make definitive guidelines on prevention and treatment of osteoporosis in patients with T1DM.
Keywords: Fracture risk, osteoporosis, type 1 diabetes
INTRODUCTION
Prevalence of type 1 diabetes (T1DM) is rising in India. There are only few epidemiological studies that estimated the prevalence of T1DM in India. The available published studies from different parts of India reveal the prevalence of T1DM in the range of 3.7 to 10.2 per 100,000 populations, with higher prevalence in urban area and in males.[1,2,3,4] With the advent of insulin in 1921-1922, there has been dramatic reduction in mortality due to acute complications in patients with T1DM. Furthermore, with the advancement in newer insulin analogues and technologies like continuous glucose monitors and insulin pumps, more patients with T1DM are able to achieve goal A1c without significant hypoglycemia. Additionally, there has been a significant decline in microvascular complications.[5,6] As a result of this, the life expectancy in patients with T1DM has increased remarkably[7] and more patients with T1DM are now older than 60 years. Hence, there is need to focus more on other age related complications which can be potentiated by diabetes, including osteoporosis, cardiovascular disorders and cognition in older individuals with T1DM. Evidence based management of type 2 diabetes (T2DM) in older patients with various comorbidities is well- known, but is not clear for TIDM.[8]
Localized involvement of skeleton like Charcot's arthropathy is a well recognized complication of diabetes. Though Albright recognized generalized involvement of skeletal in 1948, there was not much attention focused on this area.[9] In the past decade, a number of epidemiological studies showed that there is an increase in the incidence of osteopenia, osteoporosis and fracture risk in patients with TIDM. A number of mechanisms have been proposed but none of them have been proven. There is lack of data in the therapeutic area for patients with osteoporosis and T1DM. This review will highlight the epidemiology, mechanisms, preventive strategies, treatment and future perspective in this important area.
EPIDEMIOLOGY OF OSTEOPOROSIS IN PATIENTS WITH T1DM
Osteopenia was described in patients with T1DM even before the availability of bone mineral density (BMD) measurement technique.[9] Many studies, though not all, carried out with the older techniques to measure BMD, like single photon absorptiometry (SPA) or dual photon absorptiometry (DPA), demonstrated that BMD is lower in adolescents and adults with T1DM compared to control population.[10,11,12,13,14] Similar findings have been noted by the recent studies carried out using newer technologies to measure BMD like dual X-ray absorptiometry (DXA).[15,16,17,18] However, the data is not consistent among all the studies.[10,11,12,13,14,15,16,17,18,19,20]
Despite the lack of consistent evidence of reduced BMD in T1DM, combined study analysis have estimated that in T1DM fracture risk is increased by 1- to 2-fold at any skeletal site.[21] A recent meta-analysis revealed six-fold increased risk for hip fracture in patients with T1DM, which was higher than would have been expected on the basis of BMD.[22] Looking at the literature, it is quite clear that patients with T1DM are at high risk of osteoporosis and fracture.
There is relative paucity of data on prevalence of osteoporosis and fracture in patients with T1DM from India. The only published study from western India by Joshi et al., compared 86 patients of T1DM between 12- 45 years of age and mean disease duration of 14.6 years with age, sex and body mass index (BMI) matched controls. BMD of total body and lumbar spine was significantly lower in patients with T1DM compared to controls. Furthermore, patients with T1DM had 10% less bone mineral content (BMC) in comparison with controls.[23] This study suggests that Indian patients with T1DM are at higher risk for fracture.
FACTORS INFLUENCING THE BONE IN PATIENTS WITH T1DM
The decrease bone formation and inadequate accrual peak bone mass in children with prepubertal onset T1DM has been proposed as a major contributing factor for low bone strength and osteoporosis in later life.[24] Men with T1DM tend to be particularly prone to osteopenia or osteoporosis compared to women of similar ages.[19,25,26] Estrogen adequacy and/or use of estrogen-based oral contraceptive pills might be the reason for higher bone mass in women compared to men.[25,27] Furthermore, hypogonadism is quite common in men with T1DM and that may contribute to osteoporosis in males.[28,29] Another factor which may influence the BMD is body mass index (BMI). Studies have shown that lower BMI is associated with higher incidence of osteoporosis. The adipose tissue, apart from providing mechanical loading, also increases BMD through the activity of adipocytokines. Patients with T1DM have low BMI and hence, they are more prone to develop osteoporosis.[21] Patients with TIDM also have a negative calcium balance as a result of hypercalciuria during periods of hyperglycemia, functional hypoparathyroidism, vitamin D deficiency and alterations in vitamin D metabolism.[30,31,32]
A number of studies have shown that poor glycemic control in patients with T1DM is associated with osteopenia and osteoporosis.[22,23] Poor glycemic control for long period of time can result into microvascular complications and can aggravate the loss of bone density by various mechanisms. Retinopathy and neuropathy can predispose patients with diabetes to fall while nephropathy results into hypercalciuria and can alter the vitamin D metabolism causing vitamin D deficiency.[33,34,35] Furthermore, use of loop diuretics in patients with diabetic nephropathy is associated with low BMD as these drugs cause the renal excretion of calcium.[36]
Patients with T1DM have increased risk of other autoimmune disorders like autoimmune thyroid disease and celiac disease.[37] Clinical observation indicates that the clustering of three autoimmune diseases (Type 1 diabetes, celiac disease and thyroiditis) significantly increases the occurrence of osteopenia. It is possible that bone impairment might be considered not only a complication due to endocrine or nutritional mechanisms, but also a consequence of an immunoregulatory imbalance.[38]
In a study of 260 patients of T1DM from South India, Turner's syndrome was associated with 3.5% patients and Klinefelter's syndrome with 1.9% patients.[39] These associated syndromes in patients with T1DM can pose higher risk for osteoporosis.[40,41]
POTENTIAL MECHANISMS FOR OSTEOPOROSIS IN T1DM
The growth of bone during early age and puberty is by a process called “modeling”, which is different than remodeling that occurs in adults. In modeling, the osteoblast functions to lay down the bone which is being shaped by osteoclast.[42] The number of hormones like gonadal steroids, insulin, growth hormones, growth factors and cytokines play vital a role in this process of modeling resulting in the achievement of peak bone mass. Any alteration in these hormones is associated with orchestrated bone modeling process which can result in osteopenia and osteoporosis. Many theories have been proposed for the development of osteopenia or osteoporosis in patients with T1DM. Most of the work has been carried out in animal models or in vitro analysis and none has been proven. Here, we summarize the possible detrimental consequences of insulin deficiency and hyperglycemia on bone development.
T1DM is characterized by autoimmune destruction of beta cells resulting in near complete deficiency of insulin causing hyperglycemia.[6] Hyperglycemia affects the bone development in various ways; (1) it damages osteoblast either by osmotic damage or by suppressing gene expression responsible for osteoblast maturation.[43](2) it increases PPARγ that promote adipogenesis from mesenchymal stem cells at the expense of bone formation thus reducing bone accrual and peak bone mass.[44,45] Glitazones, like pioglitazone, are agonists of PPARγ and they are linked to more fractures and low bone mass.[46,47](3) The mice model and in vitro studies showed that hyperglycemia directly inhibits the bone formation as shown by expression of transcription factor RUNX2, biochemical markers and histomorphometric analysis.[48,49](4) hyperglycemia also induces the expression of proinflammatory cytokines like TNFα which inhibits osteoblast differentiation and activity, thus increasing osteoblastic apoptosis.[50,51](5) hyperglycemia may results in the generation of increase reactive oxygen species which in turn can increase osteoclast formation and activity.[52](6) Chronic hyperglycemia leads to development of microvascular complication like retinopathy, neuropathy and nephropathy.[53] Advance retinopathy increases the risk of fall by impairing vision which is further aggravated by the presence of diabetic neuropathy. Additionally, nephropathy can result in increase in protein loss which aggravates osteoporosis in patients with diabetes.[21,54,55][Figure 1]
Figure 1.
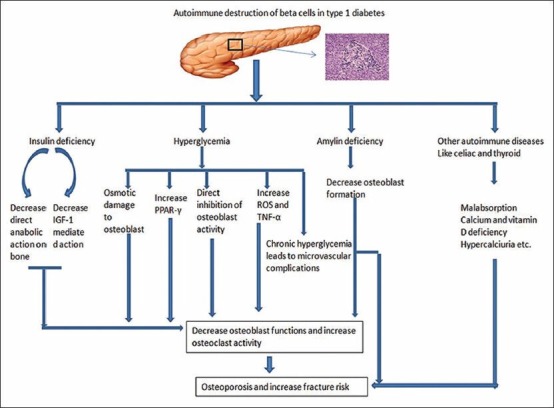
Potential mechanisms leading to osteoporosis in patients with T1DM
The distinct reduction of peak bone mass in some patients with T1DM has led to the hypothesis that insulin has anabolic effects on bone.[56] Hence, patients with TIDM who are deficient in insulin are at risk of osteoporosis. This hypothesis is being substantiated by the fact that BMD is higher in patients with T2DM who are hyperinsulinemic. The mechanism underlying the anabolic effect of insulin may be either by direct stimulation of osteoblast or indirectly by increasing the transcription of RUNX2.[57,58] Furthermore, it is known many of the effects of insulin are mediated by Insulin- like Growth Factor-1 (IGF).[59] In patients with uncontrolled T1DM, the levels of free IGF-1 are low due to increase in IGF- binding proteins particularly IGFBP3.[60] Hence, low IGF-1, as a result of insulin deficiency may result in low accrual of peak bone mass. Furthermore, intensive insulin therapy has been shown to stabilize the BMD in patients with T1DM.[21][Figure 1].
In addition to insulin, patients with T1DM are deficient in amylin (IAPP); a hormone co-secreted with insulin by pancreatic beta cells. In rodent models of T1DM, amylin has been shown to increase osteoblastic and chondrocyte proliferation activity, while suppressing osteoclastic proliferation and activity.[61] [Figure 1]
T1DM is associated with other autoimmune syndromes like celiac disease. In a study from North India, the prevalence of celiac disease in patients with T1DM is 11.1%.[62] Celiac disease via various mechanisms like vitamin D deficiency, malabsorption, weight loss and reduction in IGF-1 results into low BMD and osteoporosis in children.[63]
DIAGNOSIS OF OSTEOPOROSIS IN T1DM
Although diagnostic modalities for osteoporosis are similar in individuals with or without diabetes, some additional considerations should be applied to patients with diabetes.[21] DXA is the standard bone imaging method for children, adolescents and adults because of its availability, reproducibility, speed and low exposure to ionizing radiation.[64,65] There are no guidelines available to clinicians on how and when to measure the BMD in children with T1DM. The International Society of Clinical Densitometry (ISCD) recommends that bone mass measured by DXA should be reported as BMC or areal BMD which can be compared with reference values from children of similar age, gender and if possible race/ethnicity to calculate Z-score.[66] However, this recommendation is based mainly on expert opinion rather than evidence. The challenges currently for the measurement of BMD in children are; (1) there is no standardized reference BMD using DXA technology (2) there is no normative data across different ethnic children standardized as per age, race, BMI and height. However, attempts are going on towards making a normative curve for BMD in children.[67] Recently, American academy of pediatrics has published guidelines for the measurement of BMD in children and adolescent. These guidelines focus mainly on children with cystic fibrosis and cancer, but lack focus on T1DM.[68] One potential drawback of DXA when used for patients with T1DM is that it does not take into account the bone size and geometry because children with T1DM have small bones.[24] Another bone assessment technique, peripheral quantitative computed tomography (pQCT), might overcome this limitation. It measures volumetric apparent BMD, cross sectional area (CSA) and also differentiates between cortical and trabecular bone.[69] Finding from clinical assessment and BMD should be considered together to calculate the 10-year risk of sustaining an osteoporotic fracture. The FRAX® tool from the WHO is available to facilitate the calculation of fracture risk.[70] This tool was initially used in 12 countries, but recently data from Indians living in Singapore has also been added to it. Many Endocrinologists suggest the use of FRAX using the Singapore data to predict the fracture risk among Indians.[71] This tool has many limitations including its utility in children and adolescents with T1DM, which is currently unknown.[72]
PREVENTION OF OSTEOPOROSIS IN T1DM
Since the data indicates that bone accrual decreases from the diagnosis of T1DM, the preventive strategies should be considered from the beginning and incorporated along with the diabetes management. Physical activity is the best way to promote the bone accrual and bone strength during childhood and adolescence. The discussion of type of exercise, mechanism by which exercise helps, is beyond the scope of this review.[73] In one study, regular weight-bearing physical activity (180 min/wk, including ball games, jumping activities, and gymnastics) improved total and lumbar bone mineral accretion in children with T1DM, in a similar magnitude to healthy subjects.[74] Therefore, children with T1DM should be encouraged to have regular physical activity. As we discussed previously, vitamin D deficiency is rampant in these children. Replacement of vitamin D along with calcium has been found to improve the BMD in children with T1DM. Therefore, all children with T1DM should be recommended to have adequate calcium intake (1200 mg/day) and replacement of vitamin D, if they are deficient. Diabetic complications like retinopathy and nephropathy can increase the risk of osteoporosis and fracture. Prevention and early treatment of these complications benefit patients with T1DM. Diabetes Control and Complications Trial (DCCT) clearly showed that intensive insulin treatment reduces the risk of these microvascular complications by 35-60%.[75] Hence, the physicians should try to achieve the goal A1c without causing hypoglycemia in patients with T1DM. Certain medications like glitazone and diuretics should be avoided in high risk patients.[36,46,47] Furthermore, the treatment of associated autoimmune disease may prevent osteoporosis. For example, gluten free diet in patients with celiac disease increases the BMD.[76,77]
TREATMENT OF OSTEOPOROSIS IN PATIENTS WITH T1DM
The management of osteoporosis in patients with TIDM is not evidence based. Vestergaard and colleagues studied whether the reduction in bone turnover by use of antiresorptive drugs is detrimental in patients with diabetes. They studied a nationwide cohort from Denmark who used antiresorptive therapy and compared the benefit in terms of fracture prevention in those with diabetes and those without diabetes. They concluded that diabetes does not seem to affect the fracture-preventive potential of bisphosphonates or raloxifene.[78] In absence of randomized control trials, this study at least reassures clinicians to use antiresorptive therapy in patients with T1DM with osteoporosis. The newer agent, denosumab has been used in many osteoporotic conditions, but there is lack of data on its use in patients with T1DM.
The main defect in the genesis of osteoporosis in patients with TIDM is osteoblastic dysfunction rather than osteoclastic overfunction. Hence, it is logical that PTH therapy should be superior in comparison to antiresorptive therapy in patients with T1DM. Motyl and colleagues recently published the effect of different doses of PTH in the T1DM mice model and found that high dose of PTH significantly increased tibial trabecular bone density parameters in both control and diabetic mice models, and the lower dose elevated trabecular bone parameters in diabetic mice. The increased bone density was due to increased mineral apposition and osteoblast surface, all of which are defective in T1DM.[79] Therefore, PTH may be a more promising agent to treat osteoporosis in patients with T1DM; however it needs to be proven in the human study.
In recent years, it has been found that pancreas-kidney transplant improves adverse clinical outcomes and lowers fracture risk in patients with T1DM and end stage renal disease. Nikkel et al., showed that pancreas-kidney transplant was associated with 31% reduction of fracture risk in men.[80] Still long term studies are required in this field to implement this as one of the methods for fracture prevention in T1DM.
FUTURE DIRECTIONS
There are more questions than answers as far as osteoporosis is concerned in patients with T1DM. There is no clear epidemiological data from India on prevalence of osteoporosis in T1DM. There is no evidence to say which diagnostic modality is best for diagnosing osteoporosis and predicting fracture risk in adolescent and adult patients with T1DM. Moreover, there is a need for more randomized trial in the therapeutic area to know as to which drug is the best for treatment of osteoporosis in patients with TIDM. We hope that in future, there will be evidence-based answers to all these questions.
CONCLUSION
Patients with T1DM are at high risk for osteopenia and osteoporosis in later life. There is clear epidemiological evidence that prevalence of osteoporosis and fracture is higher in patients with T1DM than general population. There are number of factors which influence BMD in such individuals. Physicians involved in care of patients with T1DM should carefully evaluate the risk factors for osteoporosis. Promotion of physical activities since the diagnosis of disease, adequate calcium and vitamin D supplementation are corner stone for prevention of osteoporosis in T1DM. The treatment of osteoporosis in T1DM is similar to osteoporosis in general. However, more studies are needed to say which treatment modality is better in patients with T1DM.
ACKNOWLEDGEMENT
We acknowledge the help of Lisa Meyers, Barbara Davis Center for Editing and reviewing this manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kalra S, Kalra B, Sharma A. Prevalence of type 1 diabetes mellitus in Karnal district, Haryana state, India. Diabetol Metab Syndr. 2010;2:14. doi: 10.1186/1758-5996-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prasanna Kumar KM, Krishna P, Reddy SC, Gurrappa M, Aravind SR, Munichoodappa C. Incidence of type 1 diabetes mellitus and associated complications among children and young adults: results from Karnataka Diabetes Registry 1995-2008. J Indian Med Assoc. 2008;106:708–11. [PubMed] [Google Scholar]
- 3.Ramachandran A, Snehalatha C, Krishnaswamy CV. The Incidence of IDDM in children in urban population in southern India. Madras South India. Diabetes Res Clin Pract. 1996;34:79–82. doi: 10.1016/s0168-8227(96)01338-1. [DOI] [PubMed] [Google Scholar]
- 4.Ramachandran A, Snehalatha C, Khader O, Joseph TA, Visvanathan M. Prevalence of childhood diabetes in urban population in South India. Diabetes Res Clin Pract. 1992;17:227–31. doi: 10.1016/0168-8227(92)90098-c. [DOI] [PubMed] [Google Scholar]
- 5.Shah VN, Moser EG, Blau A, Dhingra M, Garg SK. The future of Basal insulin. Diabetes Technol Ther. 2013;15:727–32. doi: 10.1089/dia.2013.0228. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2013 doi: 10.1016/S0140-6736(13)60591-7. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ. Improvements in the life expectancy of type 1 diabetes: The Pittsburgh Epidemiology of Diabetes Complications study cohort. Diabetes. 2012;61:2987–92. doi: 10.2337/db11-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sue Kirkman M, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, et al. Consensus Development Conference on Diabetes and Older Adults. Diabetes in older adults: A consensus report. J Am Geriatr Soc. 2012;60:2342–56. doi: 10.1111/jgs.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albright F, Reifenstein EC. Bone development in diabetic children: A roentgen study. Am J Med Sci. 1948;174:313–9. [Google Scholar]
- 10.Levin ME, Boisseau VC, Avioli LV. Effects of diabetes mellitus on bone mass in juvenile and adult-onset diabetes. N Engl J Med. 1976;294:241–5. doi: 10.1056/NEJM197601292940502. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbloom AL, Lezotte DC, Weber FT, Gudat J, Heller DR, Weber ML, et al. Diminution of bone mass in childhood diabetes. Diabetes. 1977;26:1052–5. doi: 10.2337/diab.26.11.1052. [DOI] [PubMed] [Google Scholar]
- 12.Auwerx J, Dequeker J, Bouillon R, Geusens P, Nijs J. Mineral metabolism and bone mass at peripheral and axial skeleton in diabetes mellitus. Diabetes. 1988;37:8–12. doi: 10.2337/diab.37.1.8. [DOI] [PubMed] [Google Scholar]
- 13.Ponder SW, McCormick DP, Fawcett HD, Tran AD, Ogelsby GW, Brouhard BH, et al. Bone mineral density of the lumbar vertebrae in children and adolescents with insulin-dependent diabetes mellitus. J Pediatr. 1992;120:541–5. doi: 10.1016/s0022-3476(05)82479-5. [DOI] [PubMed] [Google Scholar]
- 14.Olmos JM, Pérez-Castrillón JL, García MT, Garrido JC, Amado JA, González-Macías J. Bone densitometry and biochemical bone remodeling markers in type 1 diabetes mellitus. Bone Miner. 1994;26:1–8. doi: 10.1016/s0169-6009(08)80157-2. [DOI] [PubMed] [Google Scholar]
- 15.Gunczler P, Lanes R, Paz-Martinez V, Martinis R, Esaa S, Colmenares V. Decreased lumbar spine bone mass and low bone turnover in children and adolescents with insulin dependent diabetes mellitus followed longitudinally. J Pediatr Endocrinol Metab. 1998;11:413–9. doi: 10.1515/jpem.1998.11.3.413. [DOI] [PubMed] [Google Scholar]
- 16.Valerio G, del Puente A, Esposito-del Puente A, Buono P, Mozzillo E, Franzese A. The lumbar bone mineral density is affected by long-term poor metabolic control in adolescents with type 1 diabetes mellitus. Horm Res. 2002;58:266–72. doi: 10.1159/000066441. [DOI] [PubMed] [Google Scholar]
- 17.Saha MT, Sievanen H, Salo MK, Tulokas S, Saha HH. Bone mass and structure in adolescents with type 1 diabetes compared to healthy peers. Osteoporos Int. 2009;20:1401–6. doi: 10.1007/s00198-008-0810-0. [DOI] [PubMed] [Google Scholar]
- 18.Hadjidakis DJ, Raptis AE, Sfakianakis M, Mylonakis A, Raptis SA. Bone mineral density of both genders in type 1 diabetes according to bone composition. J Diabetes Complications. 2006;20:302–7. doi: 10.1016/j.jdiacomp.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton EJ, Rakic V, Davis WA, Paul Chubb SA, Kamber N, Prince RL, et al. A five-year prospective study of bone mineral density in men and women with diabetes: The Fremantle Diabetes Study. Acta Diabetol. 2012;49:153–8. doi: 10.1007/s00592-011-0324-7. [DOI] [PubMed] [Google Scholar]
- 20.Liu EY, Wactawski-Wende J, Donahue RP, Dmochowski J, Hovey KM, Ouattrin T. Does Low bone mineral density start in post-teenage years in women with type 1 diabetes? Diabetes Care. 2003;26:2365–9. doi: 10.2337/diacare.26.8.2365. [DOI] [PubMed] [Google Scholar]
- 21.Hofbauer LC, Brueck CC, Singh SK, Dobnig H. Osteoporosis in patients with diabetes mellitus. J Bone Miner Res. 2007;22:1317–28. doi: 10.1359/jbmr.070510. [DOI] [PubMed] [Google Scholar]
- 22.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes: A meta-analysis. Osteoporos Int. 2007;18:427–44. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 23.Joshi A, Varthakavi P, Chadha M, Bhagwat N. A study of bone mineral density and its determinants in type 1 diabetes. J Osteoporos. 2013;2013:397814. doi: 10.1155/2013/397814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roggen I, Gies I, Vanbesien J, Louis O, De Schepper J. Trabecular bone mineral density and bone geometry of the distal radius at completion of pubertal growth in childhood type 1 diabetes. Horm Res Paediatr. 2013;79:68–74. doi: 10.1159/000346686. [DOI] [PubMed] [Google Scholar]
- 25.Hadjidakis DJ, Raptis AE, Sfakianakis M, Mylonakis A, Raptis SA. Bone mineral density of both genders in Type 1 diabetes according to bone composition. J Diabetes Complications. 2006;20:302–7. doi: 10.1016/j.jdiacomp.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Kemink SA, Hermus AR, Swinkels LM, Lutterman JA, Smals AG. Osteopenia in insulin-dependent diabetes mellitus; prevalence and aspects of pathophysiology. J Endocrinol Invest. 2000;23:295–303. doi: 10.1007/BF03343726. [DOI] [PubMed] [Google Scholar]
- 27.Lunt H, Florkowski CM, Cundy T, Kendall D, Brown LJ, Elliot JR, et al. A population-based study of bone mineral density in women with longstanding type 1 (insulin dependent) diabetes. Diabetes Res Clin Pract. 1998;40:31–8. doi: 10.1016/s0168-8227(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 28.López-Alvarenga JC, Zariñán T, Olivares A, González-Barranco J, Veldhuis JD, Ulloa-Aguirre A. Poorly controlled type I diabetes mellitus in young men selectively suppresses luteinizing hormone secretory burst mass. J Clin Endocrinol Metab. 2002;87:5507–15. doi: 10.1210/jc.2002-020803. [DOI] [PubMed] [Google Scholar]
- 29.Orwoll ES, Klein RF. Osteoporosis in men. Endocr Rev. 1995;16:87–116. doi: 10.1210/edrv-16-1-87. [DOI] [PubMed] [Google Scholar]
- 30.Thalassinos NC, Hadjiyanni P, Tzanela M, Alevizaki C, Philokiprou D. Calcium metabolism in diabetes mellitus: Effect of improved blood glucose control. Diabet Med. 1993;10:341–4. doi: 10.1111/j.1464-5491.1993.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 31.Daga RA, Laway BA, Shah ZA, Mir SA, Kotwal SK, Zargar AH. High prevalence of vitamin D deficiency among newly diagnosed youth-onset diabetes mellitus in north India. Arq Bras Endocrinol Metabol. 2012;56:423–8. doi: 10.1590/s0004-27302012000700003. [DOI] [PubMed] [Google Scholar]
- 32.Borkar VV, Devidayal, Verma S, Bhalla AK. Low levels of vitamin D in North Indian children with newly diagnosed type 1 diabetes. Pediatr Diabetes. 2010;11:345–50. doi: 10.1111/j.1399-5448.2009.00589.x. [DOI] [PubMed] [Google Scholar]
- 33.Munoz-Torres M, Jodar E, Escobar-Jimenez F, Lopez-Ibarra PJ, Luna JD. Bone mineral density measured by dual X-ray absorptiometry in Spanish patients with insulin dependent diabetes mellitus. Calcif Tissue Int. 1996;58:31–9. doi: 10.1007/BF02509378. [DOI] [PubMed] [Google Scholar]
- 34.Rix M, Andreassen H, Eskildsen P. Impact of peripheral neuropathy on bone density in patients with type 1 diabetes. Diabetes Care. 1999;22:827–31. doi: 10.2337/diacare.22.5.827. [DOI] [PubMed] [Google Scholar]
- 35.Bouillon R. Diabetic bone disease. Calcif Tissue Int. 1991;49:155–60. doi: 10.1007/BF02556109. [DOI] [PubMed] [Google Scholar]
- 36.Rejnmark L, Vestergaard P, Heickendorff L, Andreasen F, Mosekilala L. Loop diuretics increase bone turnover and decrease BMD in osteopenic postmenopausal women: Results from a randomized controlled study with bumetanide. J Bone Miner Res. 2006;21:163–70. doi: 10.1359/JBMR.051003. [DOI] [PubMed] [Google Scholar]
- 37.Szypowska A, B³azik M, Groele L, Pankowska E. The prevalence of autoimmune thyroid disease and celiac disease in children and adolescents with type 1 diabetes mellitus. Pediatr Endocrinol Diabetes Metab. 2008;14:221–4. [PubMed] [Google Scholar]
- 38.Lombardi F, Franzese A, Iafusco D, del Puente A, Esposito A, Prisco F, et al. Bone involvement in clusters of autoimmune diseases: Just a complication. Bone. 2010;46:551–5. doi: 10.1016/j.bone.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 39.Kota SK, Meher LK, Jammula S, Kota SK, Modi KD. Clinical profile of coexisting conditions in type 1 diabetes mellitus patients. Diabetes Metab Syndr. 2012;6:70–6. doi: 10.1016/j.dsx.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Gravholt CH, Vestergaard P, Hermann AP, Mosekilde L, Brixen K, Christiansen JS. Increased fracture rates in Turner's syndrome: A nationwide questionnaire survey. Clin Endocrinol (Oxf) 2003;59:89–96. doi: 10.1046/j.1365-2265.2003.01807.x. [DOI] [PubMed] [Google Scholar]
- 41.Horowitz M, Wishart JM, O'Loughlin PD, Morris HA, Need AG, Nordin BE. Osteoporosis and Klinefelter's syndrome. Clin Endocrinol (Oxf) 1992;36:113–8. doi: 10.1111/j.1365-2265.1992.tb02910.x. [DOI] [PubMed] [Google Scholar]
- 42.Saggese G, Baroncelli GI, Bertelloni S. Puberty and bone development. Best Pract Res Clin Endocrinol Metab. 2002;16:53–64. doi: 10.1053/beem.2001.0180. [DOI] [PubMed] [Google Scholar]
- 43.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-gamma2transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–89. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. J Cell Biochem. 1999;74:357–71. [PubMed] [Google Scholar]
- 45.Botolin S, McCabe LR. Inhibition of PPAR-Gamma prevents type I diabetic bone marrow adiposity but not bone loss. J Cell Physiol. 2006;209:967–76. doi: 10.1002/jcp.20804. [DOI] [PubMed] [Google Scholar]
- 46.Bazelier MT, de Vries F, Vestergaard P, Herings RM, Gallagher AM, Leufkens HG, et al. Risk of fracture with thiazolidinediones: An individual patient data meta-analysis. Front Endocrinol (Lausanne) 2013;4:11. doi: 10.3389/fendo.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Habib ZA, Havstad SL, Wells K, Divine G, Pladevall M, Williams LK. Thiazolidinedione use and the longitudinal risk of fractures in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95:592–600. doi: 10.1210/jc.2009-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gopalakrishnan V, Vignesh RC, Arunakaran J, Aruldhas MM, Srinivasan N. Effects of glucose and its modulation by insulin and estradiol on BMSC differentiation into osteoblastic lineages. Biochem Cell Biol. 2006;84:93–101. doi: 10.1139/o05-163. [DOI] [PubMed] [Google Scholar]
- 49.Lu H, Kraut D, Gerstenfeld LC, Graves DT. Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology. 2003;144:346–52. doi: 10.1210/en.2002-220072. [DOI] [PubMed] [Google Scholar]
- 50.Lechleitner M, Koch T, Herold M, Dzien A, Hoppichler F. Tumour necrosis factor-alpha plasma level in patients with type 1 diabetes mellitus and its association with glycaemic control and cardiovascular risk. J Intern Med. 2000;248:67–76. doi: 10.1046/j.1365-2796.2000.00705.x. [DOI] [PubMed] [Google Scholar]
- 51.Coe LM, Irwin R, Lippner D, McCabe LR. The bone marrow microenvironment contributes to type I diabetes induced osteoblast death. J Cell Physiol. 2011;226:477–83. doi: 10.1002/jcp.22357. [DOI] [PubMed] [Google Scholar]
- 52.Fraser JH, Helfrich MH, Wallace HM, Ralston SH. Hydrogen peroxide, but not superoxide, stimulates bone resorption in mouse calvariae. Bone. 1996;19:223–6. doi: 10.1016/8756-3282(96)00177-9. [DOI] [PubMed] [Google Scholar]
- 53.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 54.Viégas M, Costa C, Lopes A, Griz L, Medeiro MA, Bandeira F. Prevalence of osteoporosis and vertebral fractures in postmenopausal women with type 2 diabetes mellitus and their relationship with duration of the disease and chronic complications. J Diabetes Complications. 2011;25:216–21. doi: 10.1016/j.jdiacomp.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Kim JH, Jung MH, Lee JM, Son HS, Cha BY, Chang SA. Diabetic peripheral neuropathy is highly associated with nontraumatic fractures in Korean patients with type 2 diabetes mellitus. Clin Endocrinol (Oxf) 2012;77:51–5. doi: 10.1111/j.1365-2265.2011.04222.x. [DOI] [PubMed] [Google Scholar]
- 56.Campos Pastor MM, López-Ibarra PJ, Escobar-Jiménez F, Serrano Pardo MD, García-Cervigón AG. Intensive insulin therapy and bone mineral density in type 1 diabetes mellitus: A prospective study. Osteoporos Int. 2000;11:455–9. doi: 10.1007/s001980070114. [DOI] [PubMed] [Google Scholar]
- 57.Sealand R, Razavi C, Adler RA. Diabetes mellitus and osteoporosis. Curr Diab Rep. 2013;13:411–8. doi: 10.1007/s11892-013-0376-x. [DOI] [PubMed] [Google Scholar]
- 58.Hamann C, Kirschner S, Günther KP, Hofbauer LC. Bone, sweet bone--osteoporotic fractures in diabetes mellitus. Nat Rev Endocrinol. 2012;8:297–305. doi: 10.1038/nrendo.2011.233. [DOI] [PubMed] [Google Scholar]
- 59.Nixon AJ, Lillich JT, Burton-Wurster N, Lust G, Mohammad HO. Differentiated cellular function in fetal chondrocytes cultured with insulin-like growth factor-I and transforming growth factor-beta. J Orthop Res. 1998;16:531–41. doi: 10.1002/jor.1100160503. [DOI] [PubMed] [Google Scholar]
- 60.Moyer-Mileur LJ, Slater H, Jordan KC, Murray MA. IGF-1 and IGF-binding proteins and bone mass, geometry, and strength: Relation to metabolic control in adolescent girls with type 1 diabetes. J Bone Miner Res. 2008;23:188–91. doi: 10.1359/jbmr.080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horcajada-Molten MN, Chanteranne B, Lebecque P, Davicco MJ, Coxam V, Young A, et al. Amylin and bone metabolism in streptozotocin-induced diabetic rats. J Bone Miner Res. 2001;16:958–65. doi: 10.1359/jbmr.2001.16.5.958. [DOI] [PubMed] [Google Scholar]
- 62.Bhadada SK, Kochhar R, Bhansali A, Dutta U, Kumar PR, Poornachandra KS, et al. Prevalence and clinical profile of celiac disease in type 1 diabetes mellitus in north India. J Gastroenterol Hepatol. 2011;26:378–81. doi: 10.1111/j.1440-1746.2010.06508.x. [DOI] [PubMed] [Google Scholar]
- 63.Lucendo AJ, García-Manzanares A. Bone mineral density in adult coeliac disease: An updated review. Rev Esp Enferm Dig. 2013;105:154–62. doi: 10.4321/s1130-01082013000300006. [DOI] [PubMed] [Google Scholar]
- 64.Raisz LG. Clinical practice. Screening for osteoporosis. N Engl J Med. 2005;353:164–71. doi: 10.1056/NEJMcp042092. [DOI] [PubMed] [Google Scholar]
- 65.Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S, et al. Dual energy x-ray absorptiometry interpretation and reporting in children and adolescents: The 2007 ISCD pediatric official positions. J Clin Densitom. 2008;11:43–58. doi: 10.1016/j.jocd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 66.Leib ES, Lewiecki EM, Binkley N, Hamdy RC. Official positions of the International Society for Clinical Densitometry. J Clin Densitom. 2004;7:1–6. doi: 10.1385/jcd:7:1:1. [DOI] [PubMed] [Google Scholar]
- 67.Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, et al. The Bone Mineral Density in Childhood Study (BMDCS): Bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92:2087–99. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 68.Bachrach LK, Sills IN. Clinical report-Bone densitometry in children and adolescents. Pediatrics. 2011;127:189–94. doi: 10.1542/peds.2010-2961. [DOI] [PubMed] [Google Scholar]
- 69.Seeman E, Delmas PD. Bone quality-The material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–61. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 70.Kanis JA, McClokkey EU, Johansson H, Oden A, Strom O, Borgstrom F. Development and use of FRAX in osteoporosis. Osteoporos Int. 2010;21(Suppl 2):S407–13. doi: 10.1007/s00198-010-1253-y. [DOI] [PubMed] [Google Scholar]
- 71.Kalra S, Kalra B, Baruah MP. Diagnosing osteoporosis made easier. FRAX tool gets better. J Med Nutr Nutr. 2012;1:61–2. [Google Scholar]
- 72.Gogate Y, Bhadada SK. FRAX: Facts and Fantasy. Indian J Endocrinol Metab. 2012;16:S224–6. doi: 10.4103/2230-8210.104044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gunter KB, Almstedt HC, Janz KF. Physical activity in childhood may be the key to optimizing lifespan skeletal health. Exerc Sport Sci Rev. 2012;40:13–21. doi: 10.1097/JES.0b013e318236e5ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maggio AB, Rizzoli RR, Marchand LM, Ferrari S, Beghetti M, Farpour-Lambert NJ. Physical activity increases bone mineral density in children with type 1 diabetes. Med Sci Sports Exerc. 2012;44:1206–11. doi: 10.1249/MSS.0b013e3182496a25. [DOI] [PubMed] [Google Scholar]
- 75.The Diabetes Control and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 76.Blazina S, Bratanic N, Campa AS, Blagus R, Orel R. Bone mineral density and importance of strict gluten-free diet in children and adolescents with celiac disease. Bone. 2010;47:598–603. doi: 10.1016/j.bone.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 77.Tau C, Mautalen C, De Rosa S, Roca A, Valenzuela X. Bone mineral density in children with celiac disease. Effect of a Gluten-free diet. Eur J Clin Nutr. 2006;60:358–63. doi: 10.1038/sj.ejcn.1602323. [DOI] [PubMed] [Google Scholar]
- 78.Vestergaard P, Rejnmark L, Mosekilde L. Are antiresorptive drugs effective against fractures in patients with diabetes? Calcif Tissue Int. 2011;88:209–14. doi: 10.1007/s00223-010-9450-4. [DOI] [PubMed] [Google Scholar]
- 79.Motyl KJ, McCauley LK, McCabe LR. Amelioration of type I diabetes-induced osteoporosis by parathyroid hormone is associated with improved osteoblast survival. J Cell Physiol. 2012;227:1326–34. doi: 10.1002/jcp.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nikkel LE, Iyer SP, Mohan S, Zhang A, McMahon DJ, Tanriover B, et al. Pancreas-kidney transplantation is associated with reduced fracture risk compared with kidney-alone transplantation in men with type 1 diabetes. Kidney Int. 2013;83:471–8. doi: 10.1038/ki.2012.430. [DOI] [PMC free article] [PubMed] [Google Scholar]


