Abstract
Treat-to-target is a therapeutic concept that considers well defined and specific physiologic targets as aims in controlling the pathophysiology of the disease. It has been widely used in diseases that pathophysiology includes, chronic metabolic and physiological disturbances, namely rheumatic conditions, vascular medicine and diabetes. In diabetes, the availability of “gold-standard” quantitative measures like fasting plasma glucose and glycated hemoglobin make the application of treat-to-target trials especially pertinent. Treatment modalities which have used single therapeutic agents or combinations or in combination with a variety of titration algorithms and implementation protocols have broadened our understanding of diabetes management with specific reference to insulin initiation and maintenance. Treat-to-target trials have been used to investigate a wide variety of questions including efficacy, safety, effect of treatment on comorbidities and patient satisfaction, ideal mechanisms to implement insulin initiation etc. A more generalized acceptance and implementation of treat-to-target trials may finally revolutionize diabetes management by combining aspects of individual care with standard treatment protocols.
Keywords: Clinical trials, diabetes, efficacy, safety
INTRODUCTION
“Treat-to-target” is a concept used in designing therapeutic strategies, with treatment modalities oriented towards achieving a well-defined, clinically relevant end-target. A dynamic and responsive treatment plan that guides adjustments in the administration of an intervention and facilitates target achievement lies at the base of a treat-to target approach.[1] The chosen targets are based on specific quantitative measures and the rationale for a specific target is based on comprehensive, evidence based, generally accepted target values. Usually treat-to-target strategy consists of measures proposed by medical national and or international bodies.[2,3,4] The principle of ‘treat-to-target’ is founded on an important management approach used against some of the most prevalent diseases like arterial hypertension and coronary heart disease.[2] The implementation of treat-to-target in other therapeutic areas was facilitated by a better understanding of risk factors (as in cardiovascular medicine) or the treatment paradigm as in rheumatology (treatment of rheumatoid arthritis).[1,5] In cardiovascular medicine, “gold-standard” measures like blood pressure, low density lipoprotein-cholesterol (LDL-C) etc., have long been popular for setting treatment targets in clinical practice.[5,6] Even in the case of rheumatoid arthritis, which has a relative lack of standard measures reflective of overall clinical status, pooled indices of several measures, based on a Core Data Set of seven measures have been used for defining targets.[7]
Cardiovascular pathologies, as well as rheumatoid arthritis are diseases involving normal physiologic functions dysregulation, which results in long-term organ damage if not treated-a pattern of pathophysiology shared with diabetes.[5,8] Thus, the principle of treatment for achieving a certain target is also apposite to diabetes management. Treatment to achieve glucose level targets has been the cardinal principle in diabetes management, due to the availability of different standardized glucose measurements and their utility in accurately reflecting the clinical status of patients.[9,10,11] Nonetheless, while the treat-to-target approach has been discussed in clinical settings for a long time, its systematic use has been generalized only in the past decade, a fact that finally unveiled its full clinical potential.[12,13] One of the first studies to employ this approach was, the United Kingdom Prospective Diabetes Study (UKPDS) which prospectively investigated outcomes in patients treated with insulin plus oral antidiabetic drugs (OADs). While a specific glycemic target and subsequent titration algorithm were not a part of its design, it investigated the risk of complications at glycated hemoglobin (HbA1c) levels representing “near-normal” glycemic control; this attempt thus, represents the evaluation of complications in a glycemic target-bound context.[14] The treat-to-target approach in diabetes is facilitated by several disease specific factors. The availability of “gold standard” measures like HbA1c, fasting plasma glucose (FPG) and post prandial plasma glucose (PPPG) which are informative in all individual patients greatly helps in setting quantitative targets which provide a common context for interpretation of individual results when accurate results are available.[15] Based on a large body of evidence, the national and international organizations have established targets for glucose control, making treat-to-target trials interpretable in a standard clinical context. A majority of stakeholders recommend a target of HbA1c <7% and corresponding self-measured plasma glucose measurements to obtain a glycemic control that reduces the risk of late diabetes complications.[3,4,16]
Furthermore, these targets and the algorithms designed to achieve them have been under continued debate thus, making available targets and algorithms, open to continual scrutiny and updates; for example in comparison to other guidelines, a joint task force of the European Society of Cardiology and the European Association for the Study of Diabetes recommended a target for HbA1c <6.5% for patients with diabetes and cardiovascular disease (CVD) in its 2007 clinical guidelines.[2,3,16,17,18] Figure 1 summarizes the ADA-EASD recommended algorithm for the management of hyperglycemia in type 2 diabetes mellitus (T2D).[16] The suitability of employing these targets and treatment algorithms, with available therapeutic options in clinical practice becomes demonstrable by the use of treat-to-target trials and can inform the academic discourse on this topic.[19] The failure of a large number of patients with diabetes in reaching glycemic targets despite the availability of a large pool of different therapeutic options for diabetes is noteworthy.[1] Thus, models of individualized care with a variety of agents, individually or in combination, need to be evaluated within the ambit of guiding treatment principles. The treat-to-target approach in trials, enables examining such simultaneous targeting of individual needs within the scope of objective principles of care.[20]
Figure 1.
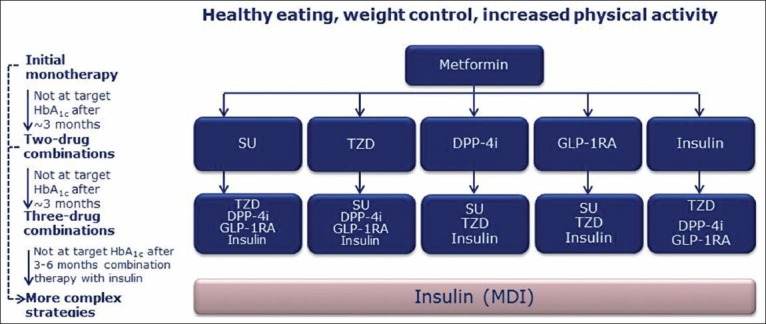
ADA-EASD algorithm for antihyperglycemic therapy in type 2 diabetes[16]. ADA: American Diabetes Association, EASD: European Association for Study of Diabetes, HbA1c: Glycosylated haemoglobin, GI: Gastrointestinal, TZD: Thiazolidinedione, DPP-4 I: Dipeptidyl peptidase-4 inhibitor, GLP-1 RA: Glucagon like peptide-1 receptor agonist, MDI: Multiple daily injections, SU: Sulphonylurea. (a) Consider beginning at this stage in patients with very high HbA1c (e.g., ≥9%), (b) Consider rapid-acting, nonsulfonylurea secretagogues (meglitinides) in patients with irregular meal schedules or who develop late postprandial hypoglycemia on sulfonylureas, (c) additional potential adverse effects and risks may be seen, (d) Usually a basal insulin (NPH, glargine, detemir) in combination with noninsulin agents, (e) Certain noninsulin agents may be continued with insulin
Clinical trials employing the treat-to-target approach have been used to examine a diverse array of outcomes. Efficacy and safety outcomes have been the main focus but other outcomes relating to treatment satisfaction, health-related quality of life etc., have also been tested.[21,22,23] Pertinent questions such as the initiation of different insulins either singly or in combination with oral anti-hyperglycemic drugs, or their relative safety at a given target, or efficacy in the face of fixed safety targets etc., have been examined.[13] A narrow focus on one target (e.g. blood glucose) may result in the neglect of other consequences (e.g. risk of hypoglycemia) with untoward effects, thus treat-to-target trials have also studied the effect of treatment modalities on diabetes associated comorbidities like cardiovascular diseases (CVD) etc.[24] Similarly, non-pharmaceutical interventions (e.g. effect of group vs. individual education) have also been evaluated for their effect in complementing the efficacy of pharmaceutical interventions.[25] Given the importance and range of evidence emerging from treat-to-target trials, the current review will endeavor to present an overview of the application, advantages and limitations of treat-to-target trials in diabetes, prominent trials and their results and the major considerations operative in them.
APPLYING TREAT-TO-TARGET IN DIABETES: CONSIDERATIONS, ADVANTAGES AND DISADVANTAGES
The treat-to-target concept has been used in several clinical trials in the past decade with the application of uniform titration algorithms, primarily, those initiating insulin. The availability of a wide range of new long and intermediate acting, basal and mixed insulin and the idea of treat-to-target, are paradigms that allow investigators to develop new management protocols for the treatment of diabetes.[13] A variety of factors including the stringency with which the algorithm is applied in the study, whether titrations are physician- or patient-directed, frequency of dose adjustments, characteristics of the patients, including their disease status and what oral drugs are concomitant and/or discontinued at the start of insulin therapy, are important modulators of the outcomes in treat-to-target trials.[26]
Titration in treat-to-target trials: Plasma glucose targets
The question of an appropriate and regularly monitored glycemic measure is central to treat-to-target trials. As evident from the 24-hour patterns of plasma glucose observed pre- and post-treatment with the usual methods of pharmacologic intervention, treatment of basal hyperglycemia has been more effective than control of postprandial hyperglycemia. FPG can often be reduced to 120 mg/dL or less, but PPG increments usually remain unaffected.[13] Riddle (2006) opines that a decline in HbA1c levels from 8.5%, to levels ranging from 6.5 to 7.5% is possible with such improvement of basal hyperglycemia alone. Glycemic targets in treat-to-target trials include both FPG and HbA1c, and rarely PPG and this is consistent with the fact that many treat-to-target trials include basal/pre-mixed insulin intervention either singly or in combination with OADs. Use of PPG for interventions targeting post-meal glucose levels is possible.
Given the target bound nature of treat-to-target trials, a well-defined target level of measures is required. The Glycaemia Optimization Trial (GOT), which randomized patients to five different plasma glucose targets ranging from 80 to 120 mg/dL (while other factors were similar) provides us with clear evidence supporting 100 mg/dL as an ideal target.[27] While, the rates of hypoglycemia were similar with targets from 100 to 120 mg/dL, for a target between 100 to 90 mg/dL the rate of hypoglycemia doubled and further aggravated at a target of 80 mg/dL.[27] Other trials have confirmed the increased incidence of severe hypoglycemia at targets lower than 100 mg/dL, with incidences of severe hypoglycemia between 5 to 9.3% for a target of 95 mg/dL.[28,29] For a FBG within the range of 80 to 120, HbA1cdiffered in a range of only 0.25%. A small reduction was obtained despite a high insulin dose (more than 20 U) that was used to achieve the lowest FPG target.[27] Thus, differences in the absolute morning FPG target within the 80 to 120 mg/dL range have a very marginal effect on achieved HbA1c values and 100 mg/dL represents a safe target while representing no significant loss of glycemic control compared to lower FPG values.[27] The insulin dose at the start of insulin dose titration for achieving FPG-100 mg/dL is usually 10 U to 20 U, or based on the measured morning FPG using the formula of Holman and Turner [(FPG in mg/dL-50)/10], which typically yields doses just under 20 U.[30]
Implementation of titration mechanism
The implementation of the titration mechanism is of key importance in treat-to-target trials. The implementation methodology has a major impact on the adherence to the protocol algorithm. In a review on treat-to-target trial, Strange (2007) mentioned that “specific algorithms in the protocol offer a glycemic control advantage over a guideline goal range which leaves titration to the physician's discretion”. While some trials simply state a guideline target and leave titration up to the physician's discretion, others have implemented titration through various mechanisms.[26] The mechanisms broadly are a result of two primary questions; firstly, whether centralized control of titration is more applicable in a given context and secondly whether patient-directed titration protocols perform as well as physician-directed ones. The final titration algorithm has elements of all or some of these. These mechanisms include titration through physician discretion with no specific enforcement measures put in place centrally, physician directed but centrally monitored and enforced measures, patient-directed titration with close clinic oversight, or patient-directed titration.[26]
Centralized oversight of algorithm adherence through a rigorous application of titration can help realize the best possible HbA1c with a therapy. This is seen in the results of two clinical trials [4001 and Treat-To-Target (TTT)] which used the same titration algorithm and study insulins, with the only major difference between the trials being physician discretion in 4001 trial versus the centralized oversight of algorithm adherence in TTT trial. The average end HbA1c values for the two insulins under investigation were 8.3 and 8.1% in 4001 trial[31] and 7.0 and 7.0%, in TTT trial.[12] The centralized enforcement methodology proves its utility in answering questions about efficacy target best achievable with specific drugs, but in clinical practice it is very resource intensive and its plausibility is questionable.[26]
Another important question which needs attention is the extent of patient involvement in titration. Patient-directed algorithms can yield significant benefits as seen from the AT.LANTUS trial which compared two treatment algorithms for insulin glargine initiation and titration: Algorithm 1 (physician-directed) versus algorithm 2 (patient-directed). The patient-directed titration group showed significantly better improvements in HbA1c and lower incidence of hypoglycemia. While the specific effect of a patient-directed titration in this study is not completely clear due to the differences in the algorithms in the two comparison groups, nonetheless the overall impact of patient-directed titration can be seen.[32] Blonde et al., while not comparing patient-directed with physician-directed titration, reported that patient-directed titration in their study had led to a majority of subjects achieving the ADA recommended guideline of HbA1c <7% at the end of the study with low rates of hypoglycemia.[33] Patient-directed algorithms can save on crucial clinical resources and patients' involvement in administration of therapy is known to be associated with better glycemic outcomes.[34] There have also been attempts to implement computerized titration algorithms[35] but these attempts have drawn criticism on the grounds that the “electronic system may have limited the ability of both patient and physician to make appropriately individualized decisions when needed, leading to mediocre results from a one size fits all scheme”.[20] Patient direction is considerably easier to implement in clinical practice but reflects the relative effectiveness of a therapy with patients rather than its efficacy in them.
Treat-to-target trials: Advantages and limitations
The treat-to-target trials allow combining individualized treatment decisions with standardized treatment algorithms into a therapeutic option that better reflects the contemporary clinical practice. Looking into crucial questions of comparison which reflect on the efficacy and safety of therapeutic options is thus possible due to a closely related titration protocol; it is possible to evaluate efficacy at a fixed rate of hypoglycemia or evaluating hypoglycemia at an equal HbA1c target value.[12] The inclusion of insulin as a part of therapy much earlier than was thought possible is a result of the understanding gained from treat-to-target trials.[12]
While, questions of efficacy and safety are central within the purview of clinical trials, the possibility of patient-directed protocols or trials which incorporate elements of patient-directed titration protocols make results interpretable in terms of the actual effectiveness of the drug with the end-user.[26] Thus the impact of diabetes treatment on patient quality of life (QoL), and the appropriate curative course of action against it, is more clear.[36] Concerns about efficacy also often cloud the impact that treatment can have on diabetes comorbidities due to focus on glycemic control.[37] As the final glycemic target is the same for the patients in treat-to-target trials, insulin is often titrated to variable doses in different individuals as in clinical practice; while the extra-glycemic impact of treatment at a fixed dose might not be entirely apparent, it is clearer when the target rather than the dose is fixed. The measurement of treatment impact on diabetes comorbidities in a glycemic target-bound clinical environment is thus easier in treat-to-target trials.[19]
The variety and applicability of results from treat-to-target results have been reported to be sometimes confounded due to the effect of sample size. The true effect of treat-to-target approach, in a specific algorithm, can be overshadowed by a large sample size in another algorithm.[24] Furthermore, outcomes in treat-to-target trials are dependent on the rigor with which the algorithm is enforced in the study.[26] Thus, in the absence of centralized monitoring of titration protocol, higher than normal subjectivity in the data can be expected.
TREAT-TO-TARGET IN DIABETES CLINICAL TRIALS
Efficacy and safety
A wide array of measures has been used in treat-to-target trials yielding widely applicable data which has enabled us to extend and deepen our understanding of diabetes. In an early trial, TTT, the question of evaluating efficacy at a fixed rate of hypoglycemia or evaluating hypoglycemia at an equal HbA1c target value was addressed. The study was a randomized, open-label, parallel and 24-week multicenter trial whose aim was to achieve a target FPG of 5.5 mmol/L. The trial involved overweight subjects with inadequate glycemic control (HbA1c >7.5%), who received bedtime glargine or NPH once daily, titrated using an algorithm along with one or two pre-study oral agents. Outcome measures were FPG, HbA1c, hypoglycemia, and percentage of patients reaching HbA1c ≤7% without documented nocturnal hypoglycemia. The study showed that systematic titration of bed-time basal insulin added to oral therapy can safely achieve an HbA1c target of 7% in a majority of overweight patients with T2D and HbA1c between 7.5-10.0% on oral agents alone; glargine caused significantly less nocturnal hypoglycemia than NPH.[12]
Other recent studies have since used different insulin formulations. Zinman et al., compared ultra-long-acting insulin degludec with glargine for efficacy and safety in insulin-naive patients with T2D inadequately controlled with OADs. Insulin was titrated to achieve a pre-breakfast plasma glucose of 3.9 to 4.9 mmol/L and consistent with the treat-to-target design, reduction of HbA1c from baseline to end of trial was similar between treatments. Degludec and glargine in combination with OADs showed similar long-term glycemic control while lower rates of nocturnal hypoglycemia was seen with degludec (0.25 vs. 0.39 episodes/patient-year of exposure; P = 0.038).[38] The study also demonstrated a significant reduction in the level of FPG in the degludec group compared to the glargine group. Though the difference in confirmed hypoglycemia was not statistically significant, the rate of confirmed hypoglycemia in the degludec group was 18% lower.
The treat-to-target concept has also been exploited in studies evaluating the utility of therapeutic option specifically either in type 1 or type 2 diabetes In the BEGIN Basal-Bolus Type 1 study, insulin degludec was tested versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (T1D). The basal insulin dose was titrated with the aim of achieving before-breakfast self-measured plasma glucose (SMPG) concentration of 3.9 to 5.0 mmol/L. The bolus insulin doses were titrated with the aim of achieving SMPG concentrations of 3.9 to 5.0mmol/L before the next meal at bedtime. For a decrease of HbA1c by 0.4% in both the treatment groups, the rate of nocturnal confirmed hypoglycemia was 25% lower with degludec than with glargine (4.41 vs. 5.86 episodes per patient-year of exposure; P = 0.021).The authors suggest that insulin degludec might be useful as basal insulin for patients with T1D because it provides effective glycemic control while lowering the risk of nocturnal hypoglycemia.[39]
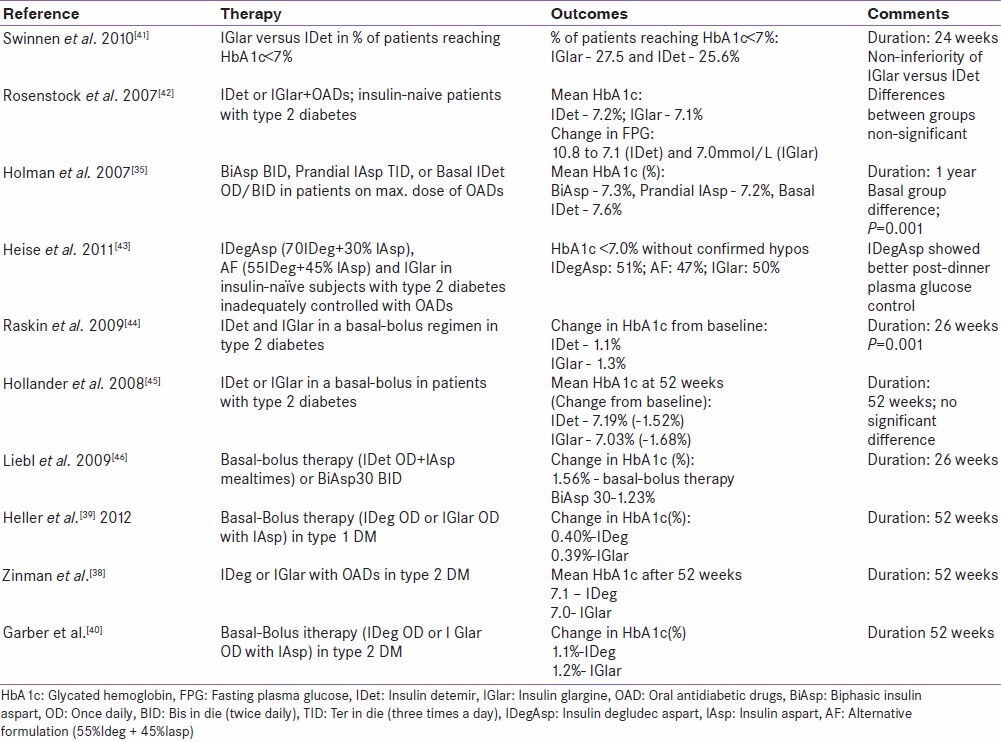
Similarly, the BEGIN Basal-Bolus Type 2 trial tested the same basal bolus combinations in patients with T2D. The study found a significant difference in the rates of nocturnal confirmed hypoglycemia in the two treatment groups with degludec showing a lower rate of hypoglycemia compared to glargine.[40] These studies while reflecting upon the relative efficacy and safety of different therapeutic options, specifically address a long-held notion favoring suboptimal glycemic control to hypoglycemia. The common thread that runs through the results of these studies is that new basal insulins such as degludec, that are associated with lower risk of hypoglycemia, might enable an early initiation of insulin in patients with diabetes. An overview of treat-to-target trials and their prominent results has been presented in [Table 1].[35,41,42,43,44,45,46]
Table 1.
Overview of results from treat-to-target trials
Apart from testing different therapeutic options for their efficacy and safety, treat-to-target studies have also investigated optimal methods to initiate and maintain insulin therapy in different treatment algorithms. The AT.LANTUS study investigated initiation of once-daily glargine therapy in patients sub optimally controlled on multiple OADs using two treatment algorithms.[22] Algorithm 1 was a clinic-driven titration and algorithm 2 was a patient-driven titration with titration being based on target FPG ≤5.5mmol/L. HbA1c decreased significantly between baseline and endpoint for patients receiving glargine plus 1 OAD (algorithm 1= −1.3% vs. algorithm 2= −1.5%; P = 0.03) and glargine plus > 1 OAD (algorithm 1= −1.5% vs. algorithm 2= −1.8%; P = 0.001).[22] Greater reduction in HbA1c was seen in patients randomized to the patient-driven algorithm (algorithm 2) on glargine plus >1 OAD.
Patient education about insulin therapy and empowering patients to initiate and maintain insulin therapy has been previously suggested to be associated with better outcomes.[34] The treat to target approach in achieving LDL-C targets was explored in the MIND.IT study.[24] This approach resulted in nearly twice the number of patients receiving statins with an aim of achieving the target range compared to those on usual care (43% versus 28%; P < 0.001).[24] However; these impressive figures were dependent on adherence to the prescribed therapy which can be effectively improved with a structured patient education program, for example, DAFNE in T1D and DESMOND or X-PERT in T2D.[47] The MEMO trial demonstrated that we can achieve stringent metabolic targets in those patients in whom caution is advised about intensification of therapy (based on ACCORD, ADVANCE and VADT), with a treat-to-target approach fortified with a structured education program.[48] Such studies, examining optimal methods of administering therapy have the potential to inform and enrich the current discourse on insulin therapy and its optimal use.
Diabetes: Comorbidities, quality of life and patient satisfaction
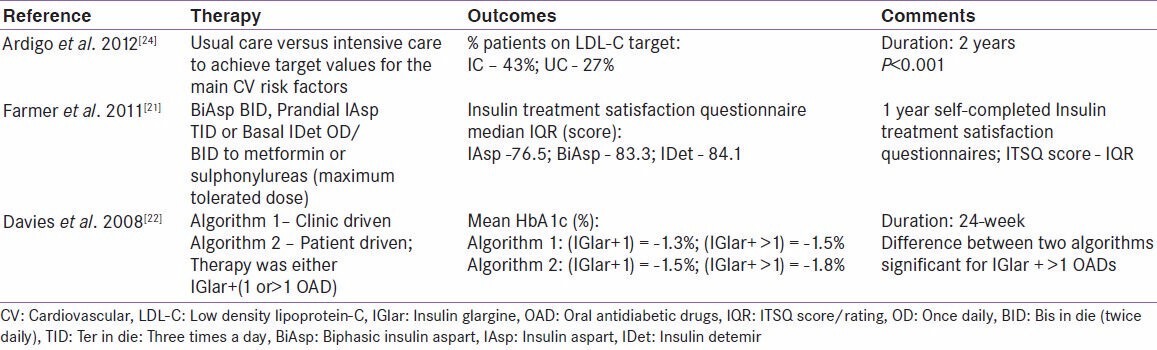
Diabetes is a multidimensional disease showing a range of physiological and psychological effects and is often associated with multiple comorbidities. Often, desired management of diabetes (e.g., by insulin initiation) is complicated by the incidence of these comorbidities or due to the effect of therapy on QoL.[34] Treat-to-target trials have also been used to investigate measures associated with comorbidities like CVD, and measures of QoL, patient satisfaction etc., Ardigo et al., examined the effectiveness of treat-to-target strategy for LDL-C control in T2D. The study compared usual care of CVD prevention with a multifactorial intensive care approach aiming to achieve target values for the main CV risk factors. A significant improvement in LDL-C beyond usual practice was seen to be associated with the application of a multifactorial treat-to-target intervention. While the results in this study might be significantly impacted by the larger sample size, a variety of variables including the rate of statin prescription, withdrawal from treatment and LDL-C levels offer a comprehensive overview of CV risk factors in diabetes patients.[24] In another planned study currently underway, the effect of an 18 m treatment with one of three insulin analogue regimens (1. insulin detemir before bedtime; 2. biphasic insulin aspart 30 before dinner with the possibility to increase to 2/3 injections daily; 3. insulin aspart before the main meals thrice daily and insulin detemir before) and metformin vs. placebo is proposed to be evaluated, the primary outcome measure being carotid intima-medial thickness in T2D patients aiming for an HbA1c ≤7.0%.[23] While, reduction in HbA1c levels is associated with reduced CV risk, the risk of CV aggravation is a persistent factor in the treatment of diabetes patients.[37,49] While the central concern of glycemic control and CV risk in diabetes are being addressed separately in clinical trials, treat-to-target trials have the potential to help us understand diabetes comorbidities in a clinical scenario where glycemic targets need primary attention.[23] Such understanding might go a long way in deciphering new benefits of diabetes treatment or point out hidden dangers, and thus contribute to the use of right options in the right context. Table 2 presents an overview of studies which measured CV and patient satisfaction outcomes.
Table 2.
Treat-to-target trials: cardiovascular and patient satisfaction outcomes
Fremantle et al., 2012 compared the effect of insulin degludec and insulin glargine on health-related QoL, in patients with T2D starting on insulin therapy, using the 36-item Short Form (SF-36) version 2 questionnaire. At endpoint, the overall physical health component score was significantly better with degludec versus glargine (+0.66), primarily due to a difference (+1.10) in the bodily pain domain score. In the mental domains, vitality was significantly higher with insulin degludec versus insulin glargine (+0.81). The study concluded that compared with insulin glargine, insulin degludec leads to improvements in both mental and physical health status for patients with T2D initiating insulin therapy.[36]
Farmer et al., 2011 examined differences in treatment satisfaction following randomized addition of biphasic (biphasic insulin aspart), prandial (insulin aspart) or basal insulin (insulin detemir) to oral therapy in T2D using self-completed Insulin Treatment Satisfaction Questionnaires (ITSQ). Prandial insulin compared with the basal or biphasic groups generally showed lower scores for 1-year adjusted ITSQ scores and significantly different scores were observed between groups for each of the ITSQ domains. Median ITSQ scores were lower in patients with a gain in body mass index (BMI) >1.23 kg/m² over 1 year compared to those with a lesser or no gain in BMI and in those with occurrence of hypoglycemia compared to those with no hypoglycemia.[21] This suggests a relationship between increased weight and lower insulin treatment satisfaction. Different treat-to-target studies have reported weight gain between 0.7 to 3.9kg during their respective study periods.[12,25,31,50] Concerns of weight gain and fear of hypos are well-known factors that may delay insulin initiation.[34] Clear identification of therapy-specific concerns are possible using treat-to-target trials when different therapeutic options are being employed to achieve similar targets.
CONCLUSIONS
Treat-to-target trials represent, the combination of individualized care within standard diabetes management protocols seen within the context of a target driven clinical practice. In contemporary clinical practice, a large number of patients do not reach required glycemic targets despite the availability of a wide variety of therapeutic options.[34] Understanding the utility of currently available therapeutic options, especially, long- and intermediate-acting insulins in terms of the best efficacy and safety outcomes achievable using them in individuals being treated in a target-bound manner is important. This affords us the opportunity to design appropriate treatment protocols. Further vigorous application of treatment modalities keeping in view single targets may have multifarious effects on the patients. Understanding these effects within the context of overall diabetes management protocols is another major contribution of treat-to-target trials. Most importantly, though, the evaluation of actual treatment algorithms and titration protocols summates the available data and yields information about the mechanistic aspects diabetes management. Treat-to-target trials thus have the potential to redefine our understanding of diabetes management practices in a context which reflects the target-oriented nature of diabetes clinical practice and their effectiveness with individual patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Castrejón I, Pincus T. Differences in treat-to-target in patients with rheumatoid arthritis versus hypertension and diabetes-consequences for clinical care. Bull NYU Hosp Jt Dis. 2011;69:104–10. [PubMed] [Google Scholar]
- 2.Atar D, Birkeland KI, Uhlig T. ‘Treat to target’: moving targets from hypertension, hyperlipidaemia and diabetes to rheumatoid arthritis. Ann Rheum Dis. 2010;69:629–30. doi: 10.1136/ard.2010.128462. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Standards of medical care in diabetes-2012. Diabetes Care. 2012;35:S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Handelsman Y, Mechanick JI, Blonde L, Grunberger G, Bloomgarden ZT, Bray GA, et al. AACE Task Force for Developing Diabetes Comprehensive Care Plan. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract. 2011;17:1–53. doi: 10.4158/ep.17.s2.1. [DOI] [PubMed] [Google Scholar]
- 5.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European Association for Cardiovascular Prevention and Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33:1635–701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 6.Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, et al. European Society of Cardiology (ESC); European Association for Cardiovascular Prevention and Rehabilitation (EACPR); Council on Cardiovascular Nursing; European Association for Study of Diabetes (EASD); International Diabetes Federation Europe (IDF-Europe); European Stroke Initiative (EUSI); Society of Behavioural Medicine (ISBM); European Society of Hypertension (ESH); WONCA Europe (European Society of General Practice/Family Medicine); European Heart Network (EHN); European Atherosclerosis Society (EAS). European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) Eur J Cardiovasc Prev Rehabil. 2007;14:S1–13. [Google Scholar]
- 7.Felson DT. Choosing a core set of disease activity measures for rheumatoid arthritis clinical trials. J Rheumatol. 1993;20:531–4. [PubMed] [Google Scholar]
- 8.Serra-Bonett N, Rodríguez MA. The swollen joint, the thickened artery, and the smoking gun: Tobacco exposure, citrullination and rheumatoid arthritis. Rheumatol Int. 2011;31:567–72. doi: 10.1007/s00296-010-1644-6. [DOI] [PubMed] [Google Scholar]
- 9.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 10.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–57. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 11.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 12.Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–6. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 13.Riddle MC. The Treat-to-Target Trial and related studies. Endocr Pract. 2006;12:71–9. doi: 10.4158/EP.12.S1.71. [DOI] [PubMed] [Google Scholar]
- 14.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 15.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27:1761–73. doi: 10.2337/diacare.27.7.1761. [DOI] [PubMed] [Google Scholar]
- 16.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD).Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–79. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodbard HW, Jellinger PS. Comment on: Inzucchi et al. Management of hyperglycemia in type 2 diabetes: A patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–79. doi: 10.2337/dc12-0413. Diabetes Care 2012;35:e70; author replye72-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rydén L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, et al. Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC); European Association for the Study of Diabetes (EASD). Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28:88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 19.Hermansen K, Mortensen LS, Hermansen ML. Combining insulins with oral antidiabetic agents: Effect on hyperglycemic control, markers of cardiovascular risk and disease. Vasc Health Risk Manag. 2008;4:561–74. doi: 10.2147/vhrm.s1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riddle MC. Starting and advancing insulin for type 2 diabetes: Algorithms and individualized methods are both necessary. J Clin Endocrinol Metab. 2008;93:372–4. doi: 10.1210/jc.2007-2576. [DOI] [PubMed] [Google Scholar]
- 21.Farmer AJ, Oke J, Stevens R, Holman RR. Differences in insulin treatment satisfaction following randomized addition of biphasic, prandial or basal insulin to oral therapy in type 2 diabetes. Diabetes Obes Metab. 2011;13:1136–41. doi: 10.1111/j.1463-1326.2011.01475.x. [DOI] [PubMed] [Google Scholar]
- 22.Davies M, Lavalle-González F, Storms F, Gomis R; AT. AT.LANTUS Study Group. Initiation of insulin glargine therapy in type 2 diabetes subjects suboptimally controlled on oral antidiabetic agents: Results from the AT.LANTUS trial. Diabetes Obes Metab. 2008;10:387–99. doi: 10.1111/j.1463-1326.2008.00873.x. [DOI] [PubMed] [Google Scholar]
- 23.Lundby Christensen L, Almdal T, Boesgaard T, Breum L, Dunn E, Gade-Rasmussen B, et al. CIMT Trial Group. Study rationale and design of the CIMT trial: The Copenhagen Insulin and Metformin Therapy trial. Diabetes Obes Metab. 2009;11:315–22. doi: 10.1111/j.1463-1326.2008.00959.x. [DOI] [PubMed] [Google Scholar]
- 24.Ardigò D, Vaccaro O, Cavalot F, Rivellese AA, Franzini L, Miccoli R, et al. Effectiveness of treat-to-target strategy for LDL-cholesterol control in type 2 diabetes: Post-hoc analysis of data from the MIND. IT study. Eur J Prev Cardiol. 2012 doi: 10.1177/2047487312467746. [In press] [DOI] [PubMed] [Google Scholar]
- 25.Yki-Järvinen H, Dressler A, Ziemen M. HOE 901/300s Study Group. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130–6. doi: 10.2337/diacare.23.8.1130. [DOI] [PubMed] [Google Scholar]
- 26.Strange P. Treat-to-target insulin titration algorithms when initiating long or intermediate acting insulin in type 2 diabetes. J Diabetes Sci Technol. 2007;1:540–8. doi: 10.1177/193229680700100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanenberg R, Zisman A, Stewart J. Glycemia optimization treatment (GOT): Glycemic control and rate of severe hypoglycemia for five different dosing algorithms of insulin glargine (GLAR) in patients with type 2 diabetes mellitus (T2DM). 66th Sci Sess Am Diabetes Assoc (ADA), Washington, DC (Jun 2006), Abstr. 567-P. Diabetes. 2006;55:A135. [Google Scholar]
- 28.Meneghini L, Schwartz S, Soltes Rak E, Harris A, Strange P. Improved glycemic control with insulin glargine vs pioglitazone as add-on therapy in patients with type 2 diabetes uncontrolled on sulfonylurea or metformin monotherapy. Late breaking abstract poster at the ADA annual meeting.2005. [Google Scholar]
- 29.Hollander P, Sugimoto D, Kilo C, Harris A, Vlajnic A. Combination therapy with insulin glargine plus metformin but not glargine plus sulfonylurea provides similar glycemic control to triple oral combination in patients with type 2 diabetes failing dual oral agents. Late breaking abstract poster at the ADA annual meeting; 2005. [DOI] [PubMed] [Google Scholar]
- 30.Holman RR, Turner RC. A practical guide to basal and prandial insulin therapy. Diabet Med. 1985;2:45–53. doi: 10.1111/j.1464-5491.1985.tb00592.x. [DOI] [PubMed] [Google Scholar]
- 31.Fritsche A, Schweitzer MA, Haring HU. Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med. 2003;138:952–9. doi: 10.7326/0003-4819-138-12-200306170-00006. [DOI] [PubMed] [Google Scholar]
- 32.Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R. AT.LANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: Comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28:1282–8. doi: 10.2337/diacare.28.6.1282. [DOI] [PubMed] [Google Scholar]
- 33.Blonde L, Merilainen M, Karwe V, Raskin P. TITRATE Study Group. Patient-directed titration for achieving glycaemic goals using a once-daily basal insulin analogue: an assessment of two different fasting plasma glucose targets-The TITRATE study. Diabetes Obes Metab. 2009;11:623–31. doi: 10.1111/j.1463-1326.2009.01060.x. [DOI] [PubMed] [Google Scholar]
- 34.Brunton S, Gough S, Hicks D, Weng J, Moghissi E, Peyrot M, et al. A look into the future: Improving diabetes care by 2015. Curr Med Res Opin. 2011;27:65–72. doi: 10.1185/03007995.2011.603300. [DOI] [PubMed] [Google Scholar]
- 35.Holman RR, Thorne KI, Farmer AJ, Davies MJ, Keenan JF, Paul S, et al. 4-T Study Group. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357:1716–30. doi: 10.1056/NEJMoa075392. [DOI] [PubMed] [Google Scholar]
- 36.Freemantle N, Meneghini L, Christensen T, Wolden ML, Jendle J, Ratner R, et al. Insulin degludec improves health-related quality of life (SF-36(®) compared with insulin glargine in people with Type 2 diabetes starting on basal insulin: A meta-analysis of phase 3a trials. Diabet Med. 2013;30:226–32. doi: 10.1111/dme.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fonseca VA. Ongoing clinical trials evaluating the cardiovascular safety and efficacy of therapeutic approaches to diabetes mellitus. Am J Cardiol. 2011;108:52B–8. doi: 10.1016/j.amjcard.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Zinman B, Philis-Tsimikas A, Cariou B, Handelsman Y, Rodbard HW, Johansen T, et al. NN1250-3579 (BEGIN Once Long) Trial Investigators. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: A 1-year, randomized, treat-to-target trial (BEGIN Once Long) Diabetes Care. 2012;35:2464–71. doi: 10.2337/dc12-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heller S, Buse J, Fisher M, Garg S, Marre M, Merker L, et al. BEGIN Basal-Bolus Type 1 Trial Investigators. Insulin degludec, an ultra-long acting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1489–97. doi: 10.1016/S0140-6736(12)60204-9. [DOI] [PubMed] [Google Scholar]
- 40.Garber AJ, King AB, Del Prato S, Sreenan S, Balci MK, Muñoz-Torres M, et al. NN1250-3582 (BEGIN BB T2D) Trial Investigators. Insulin degludec, an ultra-long acting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): A phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1498–507. doi: 10.1016/S0140-6736(12)60205-0. [DOI] [PubMed] [Google Scholar]
- 41.Swinnen SG, Dain MP, Aronson R, Davies M, Gerstein HC, Pfeiffer AF, et al. A 24-week, randomized, treat-to-target trial comparing initiation of insulin glargine once-daily with insulin detemir twice-daily in patients with type 2 diabetes inadequately controlled on oral glucose-lowering drugs. Diabetes Care. 2010;33:1176–8. doi: 10.2337/dc09-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naïve people with type 2 diabetes. Diabetologia. 2008;51:408–16. doi: 10.1007/s00125-007-0911-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heise T, Tack CJ, Cuddihy R, Davidson J, Gouet D, Liebl A, et al. A new-generation ultra-long-acting basal insulin with a bolus boost compared with insulin glargine in insulin-naive people with type 2 diabetes: A randomized, controlled trial. Diabetes Care. 2011;34:669–74. doi: 10.2337/dc10-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raskin P, Gylvin T, Weng W, Chaykin L. Comparison of insulin detemir and insulin glargine using a basal-bolus regimen in a randomized, controlled clinical study in patients with type 2 diabetes. Diabetes Metab Res Rev. 2009;25:542–8. doi: 10.1002/dmrr.989. [DOI] [PubMed] [Google Scholar]
- 45.Hollander P, Cooper J, Bregnhøj J, Pedersen CB. A 52-week, multinational, open-label, parallel-group, noninferiority, treat-to-target trial comparing insulin detemir with insulin glargine in a basal-bolus regimen with mealtime insulin aspart in patients with type 2 diabetes. Clin Ther. 2008;30:1976–87. doi: 10.1016/j.clinthera.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Liebl A, Prager R, Binz K, Kaiser M, Bergenstal R, Gallwitz B. PREFER Study Group. Comparison of insulin analogue regimens in people with type 2 diabetes mellitus in the PREFER Study: A randomized controlled trial. Diabetes Obes Metab. 2009;11:45–52. doi: 10.1111/j.1463-1326.2008.00915.x. [DOI] [PubMed] [Google Scholar]
- 47.Carey M, Khunti K, Davies M. Structured education in diabetes: A review of the evidence. Diabetes Prim Care. 2012;14:154–62. [Google Scholar]
- 48.Crasto W, Jarvis J, Khunti K, Skinner TC, Gray LJ, Brela J, et al. Multifactorial intervention in individuals with type 2 diabetes and microalbuminuria: The Microalbuminuria Education and Medication Optimisation (MEMO) study. Diabetes Res Clin Pract. 2011;93:328–36. doi: 10.1016/j.diabres.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 49.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ. 2000;321:405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther. 2006;28:1569–81. doi: 10.1016/j.clinthera.2006.10.020. [DOI] [PubMed] [Google Scholar]


