Abstract
Objectives:
To study ovarian morphology by ultrasound in women with or without polycystic ovary syndrome (PCOS) and to establish cut-off values of these parameters in Indian women with PCOS.
Materials and Methods:
A total of 119 consecutive women diagnosed PCOS and 77 apparently healthy women were enrolled. Transabdominal ultrasound examination was carried out to assess ovarian volume, stromal echogenecity, follicle number and size. Cut-off values of the above ovarian parameters with sensitivity, specificity, positive predictive value (PPV) and negative predictive values (NPV) were calculated.
Results:
Sensitivity of 79.49% and specificity of 90.67% was achieved with a cut-off of 8 mL as ovarian volume. A cut-off value of 9 follicles to distinguish between PCOS and control women yielded a sensitivity of 82.35% and specificity of 92.0% while as a follicular size of 5 mm yielded sensitivity and specificity of 74.67% and 78.15% respectively. With all the three parameters sensitivity was 87.39% and specificity 87.84% with 92.04% PPV and 81.25% NPV.
Conclusion:
Using two or three sonographic criteria in combination improves sensitivity and helps diagnose additional patients with PCOS. Our results are at variance with the established cut-off values highlighting the fact that American Society for Reproductive Medicine consensus cut-off values are not reproducible in Indian context.
Keywords: Hyperandrogenism, ovarian volume, polycystic ovaries, thecal hyperechogenicity
INTRODUCTION
Polycystic ovary syndrome (PCOS) first described by Stein and Leventhal [1] in 1935 as a condition characterized by “amenorrhea, hirsutism, obesity and sclerotic ovaries,” is now dominantly characterized by androgen excess and insulin resistance. Hence, PCOS is not merely a gynecological condition, but a systemic endocrinopathy with elevated risk of dyslipidemia, [2] hypertension, cardiovascular disease [3] insulin resistance, [4] glucose intolerance [5] and some cancers. [6] With the advent of clinical USG in 1980's and the ability to directly visualize the ovarian architecture researchers have included USG as an important diagnostic criterion. Popularity of USG is owing to a paradigm shift from purely clinical (i.e., NIH 1990) [7] to the combination of clinical and sonographic criteria as by Rotterdam 2003 [8] or androgen excess-polycystic ovary syndrome (AE-PCOS) 2006. The first widely accepted definition of polycystic ovaries (PCO) on transabdominal ultrasound (TAS) was proposed by Swanson et al. [9] as enlarged and rounded ovaries with a mean volume of 12 mL and containing an increased number of small follicles (2-8 mm). Subsequently, Adams et al. [10] proposed echogenic ovarian stroma apart from increased number (>10) of peripherally arranged follicles, measuring 2-8 mm in diameter. These criteria have been widely used even in the era of transvaginal ultrasound (TVS), which is currently considered the gold standard. Recently, Robert et al. [11] have pointed out that size is not as important as ovarian morphology and even proposed the upper limit of ovarian volume as 5.5 mL. However, TVS and TAS studies in asymptomatic volunteers have demonstrated that PCO can be seen in up to 20% of women of reproductive age group [12] and the clinical significance of PCO in these asymptomatic women is unclear. There is a particular difficulty in USG diagnosis in adolescents where TVS is not feasible and follicle size has less significance and therefore researchers. [13] There is scarce data published from India on the ovarian morphology in women with PCOS. Sikka et al. [14] correlated hormonal and insulin sensitivity parameters with TVS findings of PCO in 100 anovulatory infertile women, but they did not include a control group.
We planned to study the sonographic morphology of ovaries in Indian PCOS women for comparison with Rotterdam 2003 criteria and thereby establish cut-off values for PCO. (Ovarian size and number, and size of follicles, stromal echogenicity).
MATERIALS AND METHODS
Subjects
All women presenting with complaints of menstrual disturbances, male pattern hair growth or infertility to Endocrine and Gynecology clinics of our Institute between July 2011 and December 2012, were informed and asked to participate in the study. A total of 119 consecutive women who had signed an informed consent and qualified NIH 1990 [7] criteria for diagnosis of PCOS (n = 119, cases) were recruited for the study. Oligoamenorrhea was defined as the absence of menstruation for ≥35 days or <8 cycles/year and amenorrhea as no menstruation for >6 months. Clinical hyperandrogenism was identified by modified Ferriman-Gallwey (FG) score ≥8, [15] with or without acne and/or androgenic alopecia. The study was approved from the Institute Ethics Committee.
Controls
Seventy seven age-matched apparently healthy non-pregnant women with normal menstrual cycles acted as controls. These women also consented to participate in the study and were selected from health awareness camps done in University students or from hospital staff. None of them received any medications including steroids androgens, OCP's, etc., especially hormonal treatment and none had hirsutism or acne. Three of these control subjects were found to have PCO and were excluded from the study.
Methods
Anthropometric measurements such as weight, height, waist and hip circumference were measured using standard calibrated digital weighing scale, stadiometer and nonexpendable inch tape. TAS examinations were conducted using a 3 MHz transabdominal transducer (LogiqP6) between 4th and 11th day of the cycle. Random TAS examination was performed in the amenorrheic women. For ultrasound assessment of ovarian morphology, the methodology described by Balen et al. [16] was followed. Follicular size was measured, according to the method described by Pache et al. [17] Echogenicity was scored as normal (=1), moderately increased (=2), on markedly increased (=3). [18] The volume of the ovary was calculated with the formula of ellipse: '1/2 (A × B × C), where A is the longitudinal diameter, B is the anteroposterior diameter; and C is the transverse diameter of the ovary. [19] The intra observer variation checked on cohort was < 6.0%.
Investigations
Baseline fasting serum samples were collected in all subjects for estimation of biochemical (glucose, lipids and liver and kidney functions) and hormonal (PRL, TSH, T4, Cortisol, LH, FSH, 17-OHP, total testosterone and insulin) parameters. The samples for LH, FSH, total testosterone and 17-OHP were collected on days 3-7th (early follicular phase) of spontaneous or medroxy-progesterone induced (in amenorrheic patients) menstrual cycle. Fasting plasma insulin samples were immediately separated in cold centrifuge and stored at −70°C until the assay.
Assays
Hormone estimation was carried out using electrochemiluminescence (Cobas e411, Roche Diagnostics Limited, Charles Avenue, Burgess Hill and West Sussex). The intra and inter-assay coefficients of variation for these were <7.2% and <6.0% respectively. Plasma glucose, lipids, liver and kidney functions were performed on Hitachi 912, Japan using the standard commercial kits.
Statistical analysis
Data was analyzed using (StataCorp LP, 4905 Lakeway Drive, College Station, Texas 77845-4512, USA) and t-test was used to compare the averages of volume, number of follicles and size between the groups. ROC analysis was used to find out the cut-off values of various parameters (ovarian volume, follicular number and follicular size) for diagnosis of PCOS and sensitivity, specificity and positive predictive value (PPV) and negative predictive values (NPV) were calculated with 95% confidence interval for each of these parameters singly and in combination.
RESULTS
The mean age of PCOS subjects was 26.7 ± 4.23 (16-42) versus 27.3 ± 4.91 (18-43) years among controls (P > 0.05). The mean body mass index (BMI) of cases and controls was also comparable (27.76 ± 3.1 vs. 26.22 ± 3.2 kg/m2; P > 0.05). Among the cases, irregular menstrual cycles were seen in 49.5% (59/119) with the menstrual cycle length of 36-99 days (median length of 76 days), hirsutism in 69.7% (83/119) with FG score ranging from 8 to 30, obesity in 48.4% (58/119) with BMI ranging from 25.5 to 33.2 kg/m2 , various grades of acne vulgaris in 37.8% (45/119) and infertility in 66.67% (32 out of 48 patients who were married). For purposes of brevity, biochemical and hormonal parameters are not presented here and have been partly reported elsewhere. [20,21,22]
There was no significant difference in ovarian volume, follicle number, follicular size and stromal echogenicity between the ovaries in the same subject, barring two patients in whom the disease was unilateral. Therefore, averaged values of both ovaries were used for statistical analysis in control as well as PCOS women, except in two patients with unilateral disease. The distribution of mean follicle size, number of follicles and mean ovarian volume in patients with PCOS and control subjects is depicted in Figure 1.
Figure 1.
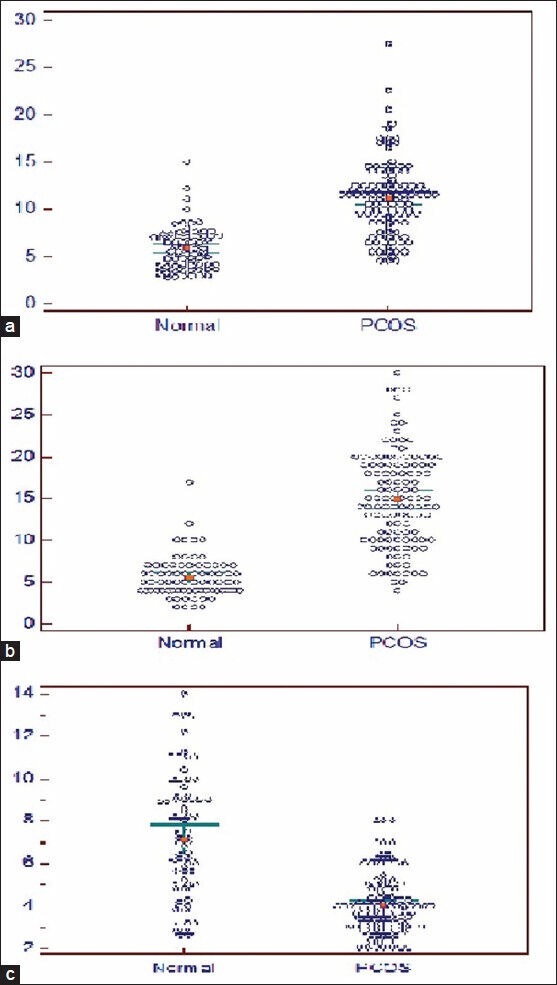
Distribution of ovarian volume (a) mean number of follicles (b) and mean follicular size (c) in healthy women (n = 77) versus women with PCOS (n = 119)*. (a) Ovarian volume (b) Follicular number (c) Follicular size (in mm)
Median ovarian volume was 11 mL in PCOS women and 5.75 mL in controls (P < 0.01). Similarly, median values for follicle size and number were 3.9 mm and 13 in PCOS women versus 7 mm and 5 in controls (P < 0.01). The volume of ovaries in control subjects was never greater than 10.2 mL; the maximum number of follicles in control ovaries was 10. Ovarian stromal echogenicity was markedly increased in 65 (54.6%), moderately increased in 50 (42.01%) and was normal in only 4 PCOS women (0.034%). The difference between stromal echogenicity in PCOS and control women was statistically significant (P < 0.01) as it was normal in all control subjects.
Since there is a lot of overlap between the PCOS and control women in the values of ovarian volume, follicular size and number, a single parameter cannot be used to differentiate polycystic and normal ovaries. Sensitivity of 79.49% and specificity of 90.67% was achieved when an ovarian volume of 8 mL was taken as cut-off to distinguish between PCOS and control women, correctly classifying 83.85% of PCOS cases. Sensitivity of 76.42% and specificity of 91.16% with corresponding PPV of 90.10% and NPV 78.2% was achieved with an ovarian volume cut-off of 10 mL. On raising the cut-off to 12 mL, the sensitivity reached to 74.56% and specificity to 93.31%. Using cut-off value of 9 follicles per ovary to distinguish between PCOS patients and control subjects yielded a sensitivity of 82.35%, specificity of 92.0% with a PPV of 88.4% and NPV of 75.23%, which correctly classified 86.08% of PCOS patients. Accordingly taking a cut-off value of 10 and 12 follicles per ovary to distinguish between PCOS patients and control subjects yielded a sensitivity of 78.41% and 80.34%, specificity of 92.91% and 93.11%, respectively. Follicular size was the least useful indicator when used alone and a cut-off value of 5 mm yielded a sensitivity of 74.67%, specificity of 78.15% and correctly classified 76.8% of PCOS patients.
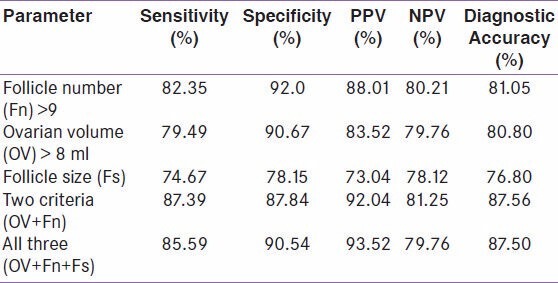
However, it is relatively easy to correctly classify subjects as PCOS or normal, when a combination of parameters is used. When all the three criteria (ovarian volume, follicle size and follicle number) were used, a sensitivity of 85.59% and specificity of 90.54% was achieved with a PPV of 93.52%, NPV of 79.76% and diagnostic accuracy of 87.5%. With the combination of two (ovarian volume and follicular number) or all three components of ovarian morphology, a sensitivity of 87.39% and specificity of 87.84% was achieved with 92.04% PPV, 81.25% NPV and diagnostic accuracy of 87.56%. Using two or three criteria in combination improved sensitivity and additional patients were diagnosed alike since follicular size yielded lower sensitivity and specificity when used for diagnosis of PCOS [Table 1].
Table 1.
ROC of various ovarian morphological parameters showing the discriminatory power between normal and PCOS women
DISCUSSION
Since the paradigm shift from purely clinical (NIH 1990) to inclusion of USG in combination with clinical criteria by Rotterdam 2003 and AE-PCOS 2006, it becomes imperative to distinguish PCO from multi-follicular ovaries. [7,8] The latter condition is known to have larger (up to 10 mm in diameter) and fewer (up to 6 in each ovary) cysts, without hypertrophic echogenic stroma. [10] According to The Rotterdam European Society for Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM)-Sponsored PCOS Consensus Workshop Group of 2004, [8] PCO is one of the three components. PCO on USG is defined as “presence of >12 follicles of 2-9 mm in each ovary and/or increased ovarian volume (>10 mL).” The definition was based on studies conducted by Pache et al. [23] van Santbrink et al. [24] and Jonard et al. [25] In our study, the volume of ovaries in control subjects was never greater than 10.2 mL; the maximum number of follicles in control ovaries was 10. A cut-off value of 8 mL ovarian volume correctly classified 83.85% of PCOS patients with a sensitivity of 79.49% and specificity of 90.67%. Although there was an overlap between PCOS and control ovaries, a cut-off value of 9 follicles to distinguish between PCOS patients and control subjects yielded a sensitivity of 82.35%, specificity of 92.0% and correctly classified 86.08% of PCOS patients. However, in the present study, 6 control women had an ovarian follicular number of 10. Nearly, 100% specificity was achieved when the cut-off for the number of follicles was 19 and for ovarian volume was 16 mL.
In a study on Indian population by Sikka et al., [14] a 100% PPV of the ovarian follicle number of 10 was described for insulin resistance and hyperandrogenism. Also, all patients in that study had an ovarian volume of more than 10 mL, whereas in our study, a significant number of patients with PCOS had volumes less than 10 mL [Figure 1a]. These results may not be applicable in discriminating normal from PCOS women as this study did not include a control group. Our findings highlight that the ESHRE/ASRM cut-off values are not reproducible in Indian women and this fact has been reported by many other investigators for other populations. [26,27,28] All our sonographic findings were recorded by a single observer, removing the confounder of inter observer variability as has been reported earlier by Amer et al. [29] and Lujan et al. [30] Despite limitations of the present study (i.e., the parameters are obtained with TAS and no correlation was carried out with insulin resistance and hormones levels), this is the first Indian study evaluating PCO morphology. As TVS is not practicable in unmarried women, our study may pave the way for new studies with TAS to define the cut-off values for these parameters.
We conclude that the results of sonographic morphology of ovaries raises the concern that large number of PCOS women fall outside the standard definition of PCOS and highlight the need to develop more reliable and accurate ultrasound criteria for diagnosis of PCOS. Besides, it supports the concept that using two or three sonographic criteria in combination improve the sensitivity.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Stein IF, Leventhal ML. Amenorrhoea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–91. [Google Scholar]
- 2.Wild RA, Painter PC, Coulson PB, Carruth KB, Ranney GB. Lipoprotein lipid concentrations and cardiovascular risk in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1985;61:946–51. doi: 10.1210/jcem-61-5-946. [DOI] [PubMed] [Google Scholar]
- 3.Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: A consensus statement by the androgen excess and polycystic ovary syndrome (AE-PCOS) society. J Clin Endocrinol Metab. 2010;95:2038–49. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 4.Carmina E. PCOS: Metabolic impact and long-term management. Minerva Ginecol. 2012;64:501–5. [PubMed] [Google Scholar]
- 5.Ganie MA, Khurana ML, Eunice M, Gupta N, Dwivedi SN, Gulati M, et al. Prevalence of glucose intolerance among adolescent and young women with polycystic ovary syndrome in India. J Clin Endocrinol Metab. 2004;4:9–14. doi: 10.1210/jc.2003-031780. [DOI] [PubMed] [Google Scholar]
- 6.Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: A systematic review. Reprod Biomed Online. 2009;19:398–405. doi: 10.1016/s1472-6483(10)60175-7. [DOI] [PubMed] [Google Scholar]
- 7.Zawadzki JK, Dunaif A. Polycystic Ovary Syndrome: Current Issues in Endocrinology and Metabolism. Vol. 4. Boston: Blackwell Scientific; 1992. Diagnostic criteria for polycystic ovary syndrome: Towards a rational approach; pp. 377–84. [Google Scholar]
- 8.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Swanson M, Sauerbrei EE, Cooperberg PL. Medical implications of ultrasonically detected polycystic ovaries. J Clin Ultrasound. 1981;9:219–22. doi: 10.1002/jcu.1870090504. [DOI] [PubMed] [Google Scholar]
- 10.Adams J, Franks S, Polson DW, Mason HD, Abdulwahid N, Tucker M, et al. Multifollicular ovaries: Clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet. 1985;2:1375–9. doi: 10.1016/s0140-6736(85)92552-8. [DOI] [PubMed] [Google Scholar]
- 11.Robert Y, Dubrulle F, Gaillandre L, Ardaens Y, Thomas-Desrousseaux P, Lemaitre L, et al. Ultrasound assessment of ovarian stroma hypertrophy in hyperandrogenism and ovulation disorders: Visual analysis versus computerized quantification. Fertil Steril. 1995;64:307–12. [PubMed] [Google Scholar]
- 12.Clayton RN, Ogden V, Hodgkinson J, Worswick L, Rodin DA, Dyer S, et al. How common are polycystic ovaries in normal women and what is their significance for the fertility of the population? Clin Endocrinol (Oxf) 1992;37:127–34. doi: 10.1111/j.1365-2265.1992.tb02296.x. [DOI] [PubMed] [Google Scholar]
- 13.Reyss AC, Proust-Richard C, Catteau-Jonard S, Dewailly D. Rotterdam consensus in adolescent girls: Which investigations and how to interpret them to make the diagnosis of PCOS? Gynecol Obstet Fertil. 2006;34:341–6. doi: 10.1016/j.gyobfe.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Sikka P, Gainder S, Dhaliwal LK, Bagga R, Sialy R, Sahdev S. Ultrasonography of the ovaries and its correlation with clinical and endocrine parameters in infertile women with PCOS. Int J Fertil Womens Med. 2007;52:41–7. [PubMed] [Google Scholar]
- 15.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–7. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 16.Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: International consensus definitions. Hum Reprod Update. 2003;9:505–14. doi: 10.1093/humupd/dmg044. [DOI] [PubMed] [Google Scholar]
- 17.Pache TD, Wladimiroff JW, de Jong FH, Hop WC, Fauser BC. Growth patterns of nondominant ovarian follicles during the normal menstrual cycle. Fertil Steril. 1990;54:638–42. doi: 10.1016/s0015-0282(16)53821-7. [DOI] [PubMed] [Google Scholar]
- 18.Pache TD, Hop WC, Wladimiroff JW, Schipper J, Fauser BC. Transvaginal sonography and abnormal ovarian appearance in menstrual cycle disturbances. Ultrasound Med Biol. 1991;17:589–93. doi: 10.1016/0301-5629(91)90029-v. [DOI] [PubMed] [Google Scholar]
- 19.Sample WF, Lippe BM, Gyepes MT. Gray-scale ultrasonography of the normal female pelvis. Radiology. 1977;125:477–83. doi: 10.1148/125.2.477. [DOI] [PubMed] [Google Scholar]
- 20.Ganie MA, Laway BA, Wani TA, Zargar MA, Nisar S, Ahamed F, et al. Association of subclinical hypothyroidism and phenotype, insulin resistance, and lipid parameters in young women with polycystic ovary syndrome. Fertil Steril. 2011;95:2039–43. doi: 10.1016/j.fertnstert.2011.01.149. [DOI] [PubMed] [Google Scholar]
- 21.Ganie MA, Farooqui KJ, Bhat MA, Mir MM, Shah ZA, Douhath S, et al. Pattern of urinary albumin excretion in normotensive young and adolescent Indian women with polycystic ovary syndrome. Indian J Endocrinol Metab. 2012;16:277–82. doi: 10.4103/2230-8210.93752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganie MA, Khurana ML, Eunice M, Gupta N, Gulati M, Dwivedi SN, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: An open-labeled study. J Clin Endocrinol Metab. 2004;89:2756–62. doi: 10.1210/jc.2003-031780. [DOI] [PubMed] [Google Scholar]
- 23.Pache TD, Hop WC, Wladimiroff JW, Schipper J, Fauser BC. How to discriminate between normal and polycystic ovaries. Radiol. 1992;17:589–93. doi: 10.1148/radiology.183.2.1561343. [DOI] [PubMed] [Google Scholar]
- 24.van Santbrink EJ, Hop WC, Fauser BC. Classification of normogonadotropic infertility: Polycystic ovaries diagnosed by ultrasound versus endocrine characteristics of polycystic ovary syndrome. Fertil Steril. 1997;67:452–8. doi: 10.1016/s0015-0282(97)80068-4. [DOI] [PubMed] [Google Scholar]
- 25.Jonard S, Robert Y, Cortet-Rudelli C, Pigny P, Decanter C, Dewailly D. Ultrasound examination of polycystic ovaries: Is it worth counting the follicles? Hum Reprod. 2003;18:598–603. doi: 10.1093/humrep/deg115. [DOI] [PubMed] [Google Scholar]
- 26.Allemand MC, Tummon IS, Phy JL, Foong SC, Dumesic DA, Session DR. Diagnosis of polycystic ovaries by three-dimensional transvaginal ultrasound. Fertil Steril. 2006;85:214–9. doi: 10.1016/j.fertnstert.2005.07.1279. [DOI] [PubMed] [Google Scholar]
- 27.Baerwald AR, Adams GP, Pierson RA. Characterization of ovarian follicular wave dynamics in women. Biol Reprod. 2003;69:1023–31. doi: 10.1095/biolreprod.103.017772. [DOI] [PubMed] [Google Scholar]
- 28.Diamanti-Kandarakis E, Panidis D. Unravelling the phenotypic map of polycystic ovary syndrome (PCOS): A prospective study of 634 women with PCOS. Clin Endocrinol (Oxf) 2007;67:735–42. doi: 10.1111/j.1365-2265.2007.02954.x. [DOI] [PubMed] [Google Scholar]
- 29.Amer SA, Li TC, Bygrave C, Sprigg A, Saravelos H, Cooke ID. An evaluation of the inter-observer and intra-observer variability of the ultrasound diagnosis of polycystic ovaries. Hum Reprod. 2002;17:1616–22. doi: 10.1093/humrep/17.6.1616. [DOI] [PubMed] [Google Scholar]
- 30.Lujan ME, Chizen DR, Peppin AK, Dhir A, Pierson RA. Assessment of ultrasonographic features of polycystic ovaries is associated with modest levels of inter-observer agreement. J Ovarian Res. 2009;2:6. doi: 10.1186/1757-2215-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


