Abstract
Aim:
This study aims to determine the potential impact of positive family history of Metabolic Syndrome (MetS) among two generations, on developing Type 2 Diabetes Mellitus (T2DM) and the potential relation of consanguineous marriage among patients with MetS to the risk of developing T2DM among a sample of Qataris.
Design:
A cross-sectional study.
Setting:
Primary healthcare (PHC) centers.
Materials and Methods:
The survey and measurement were conducted from April 2011 to December 2012 among Qatari nationals above 20 years of age. Of the 2,182 subjects, who were approached to participate in the study, 1,552 (71%) gave their consent. Face-to-face interviews were conducted using a structured questionnaire followed by anthropometric measurements and laboratory tests. Metabolic syndrome was defined using the National Cholesterol Education Program-Third Adult Treatment Panel (ATP III) as well as International Diabetes Federation (IDF).
Results:
Overall, the prevalence of MetS was 26.2% according to ATP III and 36.9% according to IDF (P < 0.0001). The mean age of MetS patients with T2DM was significantly higher than those without T2DM (Mean 48 ± 9.9 vs. 42.5 ± 9.2; P < 0.001). The proportion of females was higher among MetS patients with T2DM as compared to those without T2DM (61% vs. 51%; P = 0.053). In addition, there were significant differences between MetS patients with and without DM in terms of co-morbidities of hypertension, coronary heart disease, and high cholesterol. The proportion of MetS patients with positive family history for MetS was significantly higher in MetS patients with T2DM as compared to those without T2DM (46.7% vs. 33.8%; P = 0.009). The proportion of positive family history of MetS among fathers (35% vs. 21.9%; P = 0.005), mothers (30.5% vs. 18.8%; P = 0.008), maternal aunt (18.3% vs. 11.2%; P = 0.055), and maternal grand father (19.5% vs. 10%; P = 0.010) were significantly higher in MetS patients with T2DM as compared to the counterpart. The proportion of consanguineous marriages was almost two times higher among MetS patients with T2DM as compared to those without T2DM (80.9% vs. 41.9%; P < 0.001). The proportion of MetS patients with T2DM was lower than MetS patients without DM below 45 years, but after 45 years, the proportion of MetS patients with T2DM remained higher than their counterparts.
Conclusion:
Family history of MetS among parents, maternal aunt, maternal grandfather, and consanguineous marriages among patients of MetS are significantly associated with the development of T2DM in Qatar. These results support the necessity of earlier screening for T2DM among MetS patients with positive family history of MetS.
Keywords: Consanguinity, correlates, diabetes mellitus, genetics, metabolic syndrome, prevalence
INTRODUCTION
The metabolic syndrome (MetS) is very common [1,2,3,4] worldwide. In the recent years, MetS has internationally evolved into a recognized clinical entity, assuming an epidemic proportion. [1,2,3] One component of the MetS, diabetes is a multifactorial disease that involves complex interactions between genes, environment, and health behavior. Type 2 diabetes mellitus (T2DM) is a common metabolic disorder, characterized by hyperglycemia caused by impaired glucose homeostasis, and represents a serious public health problem in many developed countries. [1,2,3,4]
Diabetes is a disease that has a strong clustering in families and has a genetic component. It has been widely reported that the occurrence of T2DM is triggered by genetic susceptibility and familial aggregation in several populations. [4,5,6,7] Family history is a well-known risk factor for the developing of T2DM. It was estimated that the risk for diagnosed T2DM increases approximately 2-4 fold when one or both parents are affected. [8] Almost 25-33% of all T2DM patients have family members with diabetes. Having a first degree relative with the disease poses a 40% risk of developing diabetes. [9] T2DM patients are more likely to have diabetic mothers than diabetic fathers. The existence of excess maternal transmission of type 2 diabetes in offspring of affected mothers than affected fathers is currently debated. [10] Family history reflects both inherited genetic susceptibilities and shared environmental exposures that include cultural factors. [11,12] Thus, family history of diabetes may be a useful tool to identify individuals at increased risk of the disease and target behavior modifications that could potentially delay disease onset and improve health outcomes.
Although prevalence of T2DM is generally high in older adults, young T2DM patients are increasingly being reported. [1,2,3,13] Several studies on the etiologic factors of T2DM have been conducted, and, out of many factors, genetic predispositions are found to be important. [4,5,6,7,14,15] Many diabetologists have supposed that a family history of diabetes has influence on the metabolic aspects of patients. However, only few studies have been performed on its effects on metabolic and clinical factors, except studies on the influence of family history of diabetes on T2DM development. [13,14,15,16,17] In addition, metabolic syndrome, an important risk factor of diabetes and a predictor of cardiovascular disease, [6] was observed to be inherited in some studies; [1,2,3,4,13] however, there are few studies on its association with a family history of diabetes and MetS.
Qatar is one of the Arab states, which is known to have high prevalence of T2DM in its population. [11] The most up to date national stepwise survey conducted by the Supreme Council of Health in Qatar stated that the prevalence of T2DM among Qataris is 16.7%. [18] The survey was conducted among 2,496 Qataris using the World Health Organization (WHO) STEPwise approach to Surveillance (STEPS) methodology. In the Arabic culture, there is a high percentage of relative marriages among the population of Qatar estimated to be about 54%. [19] Therefore, the population of Qatar provides a good sample population to conduct this study.
The main aim of the study was to determine the potential impact of positive family history of MetS among two generations on developing T2DM and the relation of consanguineous marriages among patients with MetS to the risk of development of T2DM among a sample of Qataris. Furthermore, comparisons between subjects with MetS who has T2DM and subjects with MetS without T2DM in terms of demographic, biochemical, and clinical characteristics were done.
MATERIALS AND METHODS
This is a cross-sectional study which was conducted among the adult Qatari population above 20 years of age residing in the State of Qatar over a period from April 2011 to March 2013. The study was approved by Hamad Medical Corporation prior to commencing data collection. Only participants who agreed to participate and signed the consent form were included in the study.
Sampling procedure
A multistage stratified cluster sampling design was developed using the administrative divisions of the primary health centers in Qatar that had approximately equal number of inhabitants. In order to secure a representative sample of the study population, sampling was stratified with a view to obtaining proportional representation from urban and semi urban areas. The sample size was determined on a priori presumption that the prevalence rate of metabolic syndrome in Qatar was 26.5%, with 99% confidence level and 2.5% bound on error of estimation, a minimum sample size of 2,064 subjects was required for this study. Of the total of 22 primary healthcare centers available, 12 were selected at random. Of these 10 were located in urban and two in semi-urban areas of Qatar. Finally, subjects were selected systematically 1-in-2 using a sampling procedure. During the study period, 2,064 subjects (men and women) were approached, of whom 1,552 subjects responded to the questionnaire making a response rate of 75.2%.
A questionnaire was designed and tested among 100 subjects as a pilot study to validate the questionnaire. Necessary corrections have been done after considering the minor discrepancies that had been found during the pilot study. The first part included information about socio-demographic and anthropometric characteristics. The second part collected information about central obesity, hypertension, triglyceride, high-density lipoprotein; and family history of diabetes, MetS, and hypertension. The third part collected the information on lifestyle habits of the patients.
Qatar boasts one of the highest rates of consanguinity (54%) in the world with 34.8% of marriages between first cousins and 13.4% between those related more distantly. It was important to identify whether the high prevalence rate of MetS among first and second degree of relatives could be related to the high consanguineous community. [11,12] Qatar is a small country. All the residents of Qatar have a unique Health Card (HC) number which is used for availing the health services provided by different hospitals and primary health centers under Hamad Medical Corporation. Complete medical record of each individual is linked with HC numbers and can be retrieved easily. Information regarding MetS among different family members of the study participants was retrieved from the medical records based on HC numbers provided by the participants.
The most widely accepted criteria to identify the MetS have been proposed by the World Health Organization (WHO), [1] the European group for the study Insulin Resistance (EGIR) [20] and the National Cholesterol Education Program-Third Adult Treatment Panel (NCEP ATPIII). [21] The International Diabetes Federation (IDF) [22] and American Heart Association (AHA)/National Heart, Lung and Blood Institute (NHLB1) [23] recently proposed a new worldwide definition of MetS intended to facilitate its clinical diagnosis and simplify the comparison among data from different countries. In our study sample, we have used ATP III and IDF definitions to
Diagnostic criteria
National cholesterol education program-third adult treatment panel
According to ATP III criteria, [21] a participant has the metabolic syndrome, if she/he has three or more of the following criteria: (1) Fasting Plasma Glucose (FPG) ≥100 mg/dl (5.6 mmol/L), (2) Blood Pressure ≥ 130/85 mmHg, (3) Triglyceride ≥150mg/dl (1.7 mmol/L), (4) High Density Lipoprtoen (HDL) Cholesterol: Men <40 mg/dl (1.03 mmol/L); Women <50 mg/dl (1.29 mmol/L), (5) Men with waist circumference >102 cm and women with waist circumference >88 cm.
International diabetes federation
According to IDF criteria, [22] a participant has the metabolic syndrome, if she/he has a high waist circumference (≥94 cm in men and ≥80 cm in women) plus any two of the following conditions: (1) FPG ≥100 mg/dl (5.6 mmol/L) or previously diagnosed impaired fasting glucose, (2) Blood Pressure ≥130/85 mmHg or treatment for hypertension, (3) Triglyceride ≥150 mg/dl (1.7 mmol/L), (4) HDL Cholesterol: Men < 40 mg/dl (1.03 mmol/L); Women <50 mg/dl (1.29 mmol/L) or treatment for low HDL.
Physical examination and measurements
Physical examination and measurements were performed by a trained nurse. Height was measured in centimeters using a height scale (SECA, Germany) while the subject was standing bare feet and with normal straight posture. Male subjects were requested to remove their head cover (Igaal and Guttra). Weight was measured in kilograms using a weight scale (SECA, Germany). The subjects were asked to remove any objects from their pockets and to stand on the weight scale bare feet with light clothing. Body Mass Index (BMI) was calculated as the ratio of weight (kilogram) to the square of height (meters). Obesity and overweight were classified according to WHO criteria. [16] A person was considered obese if the BMI value was ≥30 kg/m2 , overweight if BMI ≥ 25 kg/m2 and <30 kg/m2 .
Hypertension was taken according to the definition of ATPIII and IDF which is Systolic Blood Pressure (SBP) ≥130 mmHg or Diastolic Blood Pressure (DBP) ≥85 mmHg or using anti-hypertensive medication. Two readings of the SBP and DBP were taken from the subject's left arm while seated and his/her arm at heart level, using a standard zero mercury sphygmomanometer after at least 10-15 minutes of rest. Then the average of the two readings was obtained.
Waist circumference was measured in centimeters with subjects wearing light clothes at midway level between lower rib margin and iliac crest using non-stretchable measuring tape. Waist circumference was measured according to the definition of ATP III and IDF and considered as risk factor for metabolic syndrome.
Laboratory measurements
Fasting blood venous samples were collected from all participants for determination of impaired fasting glucose, low HDL, and triglyceride. The criteria for impaired fasting glucose, low HDL, and triglyceride were according to the definition of ATP III and IDF as classified above.
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS, version # 20) software. Student t-test was used to ascertain the significance of differences between two means of a continuous variable. Chi-square test Fisher's exact test (two-tailed) was performed to test for differences in proportions of categorical variables between two or more groups. A P value of less than 0.05 was considered statistically significant. Venn Diagram was created to map the overlapping between MetS, T2DM, and family history of MetS. Finally, a family pedigree of three generation with MetS and consanguineous was presented.
RESULTS
Overall, the prevalence of MetS among the sample population was 26.2% according to ATP III and 36.9% according to IDF (P < 0.0001).
Table 1 shows the comparison of socio-demographic and clinical characteristics among patients of MetS with and without T2DM. The mean age of MetS patients with T2DM was significantly higher than those without T2DM (Mean 48 ± 9.9 vs. 42.5 ± 9.2; P < 0.001). The proportion of females was higher among MetS patients with T2DM as compared to those without T2DM (61% vs. 51%; P=0.053). Also, the proportion of secondary and above education was significantly higher among MetS with T2DM as compared to those without T2DM (41.5% vs. 28%; P = 0.006). In addition, there were significant differences between MetS patients with and without T2DM in terms of co-morbidities of hypertension (34.6% vs. 16.2%; P < 0.001), coronary heart disease (35.4% vs. 23.1%; P = 0.009), and high cholesterol (45.9% vs. 24.4%; P < 0.001), serum triglycerides level (Mean 1.70 ± 0.75 vs. 1.53 ± 0.91; P = 0.046), waist circumference (109.93 ± 7.13 vs. 103.51 ± 11.03; P < 0.001), and BMI (33.38 ± 6.96 vs. 30.88 ± 7.05; P = 0.010).
Table 1.
Comparison of demographic, biochemical, and clinical characteristics among MetS patients with and without diabetes mellitus in Qatar
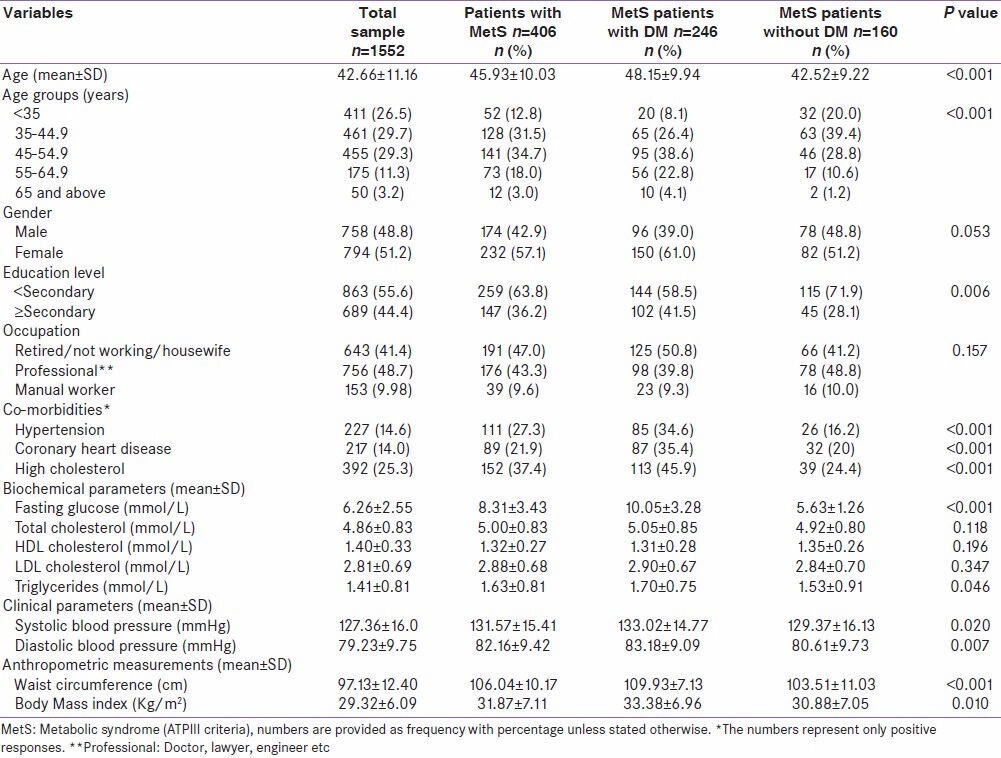
Table 2 shows the distribution of family history of MetS, T2DM, and consanguinity among MetS patients with and without DM. The proportion of MetS patients with positive family history for MetS was significantly higher in MetS patients with DM as compared to those without DM (46.7% vs. 33.8%; P = 0.009). The proportion of positive family history of MetS among fathers (35% vs. 21.9%; 0.005), mothers (30.5% vs. 18.8%; P = 0.008), maternal aunt (18.3% vs. 11.2%; P = 0.055), and maternal grandfather (19.5% vs. 10%; P = 0.010) were significantly higher in MetS patients with T2DM as compared to the counterpart. The proportion of positive family history of MetS among siblings, paternal uncle and aunt, maternal uncle, paternal grandfather and mother and maternal grandmother were not significant between MetS patients with and without T2DM. The proportion of consanguineous marriages was almost two times higher among MetS patients with T2DM as compared to those without T2DM (80.9% vs. 41.9%; P < 0.001).
Table 2.
Family history of MetS, DM, and consanguinity among MetS patients with and without DM in Qatar
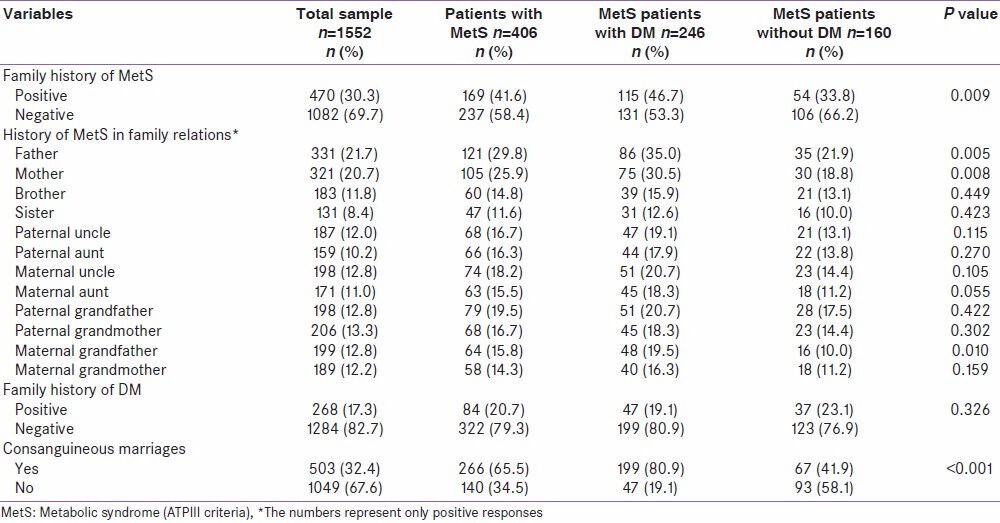
Figure 1 shows the distribution of MetS patients with and without T2DM across different age groups. The proportion of MetS patients with T2DM was lower than MetS patients without T2DM below 45 years but after 45 years the proportion of MetS patients with DM remained higher than their counterparts.
Figure 1.
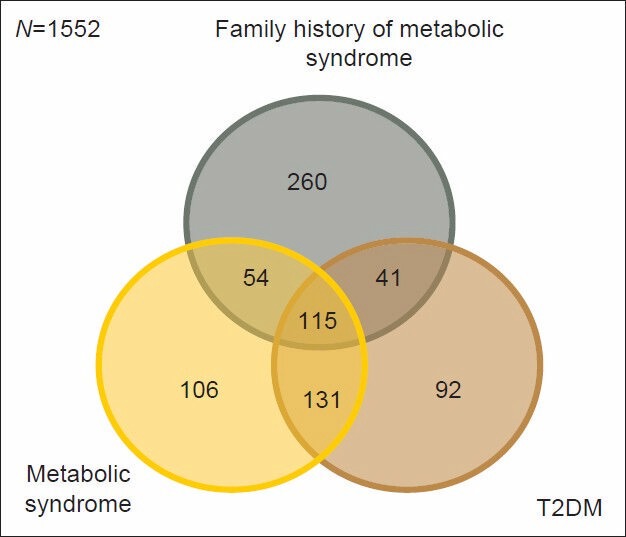
Venn Diagram showing the overlapping of Metabolic syndrome (MetS) with T2DM and family history of MetS (N=1552) Metabolic syndrome: 406 T2DM: 379 Family history of MetS: 470
Figure 2 shows a typical one family with consanguinity and MetS in three generations.
Figure 2.
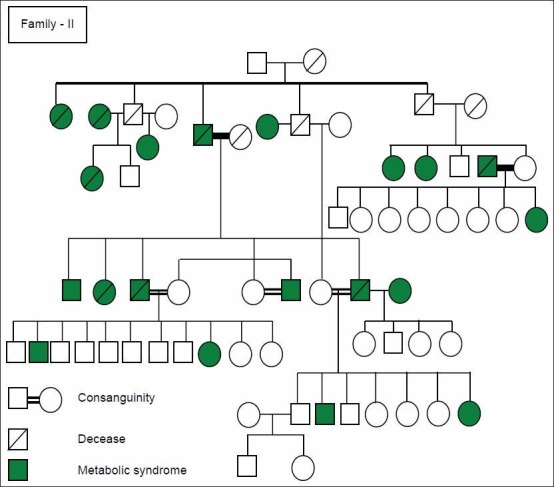
Family pedigree of three generation with Metabolic Syndrome and consanguineous
DISCUSSION
The MetS presents a high prevalence in the world, [1,2,3,4,5,6,7,8,9,10,11] in developed and developing countries. Also, in our study sample, the prevalence of MetS was high among Qataris and varied according to the definition used. The prevalence of MetS was lower with NCEP-ATP III (26.2%) and higher when using IDF definition (36.9%). The MetS prevalence varies depending on the diagnosis criteria; most are higher with IDF than ATP III criteria as seen in our study. As the two definitions are based on much of the same components, the difference in prevalence was mainly related to different waist circumference and to the focus on central obesity as an obligatory component in the IDF definition in contrast to being one out of five equally weighted components in the 2005 ATP III definition.
Many factors may play a role in the development of the metabolic syndrome including age, race, weight, menopause in women, smoking, low income and low socio-economic status, [4] high carbohydrate intake, cigarette smoking, low physical activity, consumption of soft drink, [4,11,17] antipsychotic drugs, T2DM, poor cardiovascular fitness, and genetic factors. [11,12,24,25] Our data support the notion that obesity (classified by BMI) and central obesity in particular is a strong risk factor for type 2 diabetes, a finding that is consistent with those reported previously in various racial/ethnic populations. [4,11,12,16,17,21,23,26,27] Globally, it has been estimated that approximately 58% of T2DM is attributable to overweight and obesity and 90% of T2DM in Western countries is attributed to weight gain. [28] When lower cut-off points for BMI and waist circumference were used, Asians appeared to have a higher prevalence of obesity-especially central obesity-than Caucasians.
Although numerous studies have reported the separate associations between BMI, metabolic syndrome, insulin resistance, and the risk for T2DM, [4,14,15,16,17] we are aware of only one study that has investigated associations between BMI/MetS categories, BMI/insulin resistance categories, and diabetes risk. [10] In the previous study by Meigs et al., [10] all participants with MetS or insulin resistance were at higher risk for diabetes regardless of BMI status, whereas overweight/obese individuals without the MetS were at no increased risk. Moreover, obese participants without insulin resistance were at a threefold higher risk for diabetes relative to normal-weight participants without insulin resistance, whereas overweight individuals without insulin resistance were at no increased risk.
In most studies, increasing age [4,26] was the key factor affecting the prevalence of metabolic syndrome and it also showed in our study. Increasing in BMI was correlated with increasing prevalence of MetS in this study. This is in agreement with other studies. [4,16,17,21,23] There is controversy about the relation between sex and MetS in different studies. In this study, prevalence of MetS was significantly higher in women than men. Other studies were in agreement with our findings. [4] Genetic, cultural, physical activity, and nutritional differences can be the cause of controversies. This association was seen in some other studies. [24,25,26]
Significantly higher proportion of co-morbidities like hypertension, high triglycerides, coronary heart disease, and high cholesterol were found among the MetS with T2DM group. This is expected since T2DM is an established risk factor of cardiovascular diseases [29] and long ago has been found to be associated with the different clusters of the MetS such as high cholesterol, triglycerides, [30] and hypertension. [31]
More recently Meis et al., [32] reported that genetic inheritance predisposes African Americans of components of MetS in obese, glucose-tolerant, first degree relatives of African-American patients with T2DM and study revealed that the prevalence of MetS is higher in a subgroup of African-Americans who were first-degree relatives of patients with type 2 diabetes than that of African-Americans in the National Health and Nutrition Examination Survey (NHANES III); and waist circumference rather than metabolic parameters was the single most important parameter and was more likely to meet the MetS criteria in African-American relatives. This is consistent with the present study.
Furthermore, a parental history of MetS increases the risk of developing MetS among their offspring. This is advocated by the findings that genetic factors may account for as much as 50% of the variable in level of the MetS traits in offspring. [5,6,7,8,14,16] It was clear from our study that the proportions of subjects with maternal family history of MetS who have T2DM were significantly higher than that who did not have T2DM. Further analysis should be made to investigate this interesting finding.
Limitations of study
This study is cross-sectional in nature with no follow up and, therefore, it has inherent difficulty to determine the temporal association between cause and effect. However, the various differences according to family history that were shown in this study might be clinically meaningful for the prevention of T2DM among MetS patients.
CONCLUSION
Family history of MetS among parents, maternal aunt, maternal grandfather, and consanguineous marriages among patients of MetS are significantly associated with the development of T2DM in Qatar. These results support the necessity of earlier screening for T2DM among MetS patients with positive family history of MetS.
ACKNOWLEDGEMENT
The project was supported and funded by the Diabetic Association, Qatar Foundation and Hamad Medical Corporation for generous support and help while this project conducted. We also, would like to thank Hamad Medical Corporation IRB Committee for their approval this study (HMC Research Protocol # 10262/10, April 2011).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications part 1: Diagnosis and classification of diabetes mellitus provisional report of WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: Prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 3.Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: Prevalence in worldwide populations. Endocrinol Metab Clin North Am. 2004;33:351–75. doi: 10.1016/j.ecl.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Bener A, Mohammad A, Ismail AN, Zirie M, Abdullatef WK, Al-Hammaq AO. Gender and age-related differences in patients with the metabolic syndrome in a highly endogamous population. Bosn J Basic Med Sci. 2010;10:210–7. doi: 10.17305/bjbms.2010.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erasmus RT, Blanco Blanco E, Okesina AB, Mesa Arana J, Gqweta Z, Matsha T. Importance of family history in type 2 Black South African diabetic patients. Postgrad Med J. 2001;77:323–5. doi: 10.1136/pmj.77.907.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee Sc, Pu YB, Chow CC, Yeung VT, Ko GT, So WY, et al. Diabetes in Hong Kong Chinese: Evidence for familial clustering and parental effects. Diabetes Care. 2000;23:1365–8. doi: 10.2337/diacare.23.9.1365. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Isomaa B, Taskinen MR, Groop L, Tuomi T. Consequences of a family history of type 1 and type 2 diabetes on the phenotype of patients with type 2 diabetes. Diabetes Care. 2000;23:589–94. doi: 10.2337/diacare.23.5.589. [DOI] [PubMed] [Google Scholar]
- 8.Harrison TA, Hindorff LA, Kim H, Wines RC, Bowen DJ, McGrath BB, et al. Family history of diabetes as a potential public health tool. Am J Prev Med. 2003;24:152–9. doi: 10.1016/s0749-3797(02)00588-3. [DOI] [PubMed] [Google Scholar]
- 9.ADAM. Diabetes: Type 2. 2004. [Last cited on 2013 Mar 18]. Available from: http://adam.about.net/reports/000060_2.htm .
- 10.Meigs JB, Panhuysen CI, Myers RH, Wilson PW, Cupples LA. A genome-wide scan for loci linked to plasma levels of glucose and HbA (1c) in a community-based sample of Caucasian pedigrees: The Framingham offspring study. Diabetes. 2002;51:833–40. doi: 10.2337/diabetes.51.3.833. [DOI] [PubMed] [Google Scholar]
- 11.Bener A, Yousafzai MT, Al-Hamaq AO, Mohammad AG, Defronzo RA. Parental transmission of type 2 diabetes mellitus and its influence in the offspring in a highly endogamous population. World J Diabetes. 2013;4:40–6. doi: 10.4239/wjd.v4.i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bener A, Yousafzai MT, Al-Hamaq AO. Familial Aggregation of T2DM among Arab diabetic population. Int J Diabetes Dev Ctries. 2012;32:90–2. [Google Scholar]
- 13.Jeong SU, Kang DG, Lee DH, Lim DM, Kim BJ, Park KY, et al. Clinical characteristics of type 2 diabetes patients according to family history of diabetes. Korean Diabetes J. 2010;34:222–8. doi: 10.4093/kdj.2010.34.4.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siewert S, Filipuzzi S, Codazzi L, Gonzalez I, Ojeda MS. Impact of metabolic syndrome risk factors in first-degree relatives of type 2 diabetic patients. Rev Diabet Stud. 2007;4:177–84. doi: 10.1900/RDS.2007.4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pankow JS, Jacobs DR, Jr, Steinberger J, Moran A, Sinaiko AR. Insulin resistance and cardiovascular disease risk factors in children of parents with the insulin resistance (metabolic) syndrome. Diabetes Care. 2004;3:775–80. doi: 10.2337/diacare.27.3.775. [DOI] [PubMed] [Google Scholar]
- 16.Groop L, Forsblom C, Lehtovirta M, Tuomi T, Karanko S, Nissen M, et al. Metabolic consequences of a family history of NIDDM (the Botnia study): Evidence for sex-specific parental effects. Diabetes. 1996;45:1585–93. doi: 10.2337/diab.45.11.1585. [DOI] [PubMed] [Google Scholar]
- 17.Bener A, Zirie M, Musallam M, Khader YS, Al-Hamaq AO. Prevalence of metabolic syndrome according to ATP III and IDF criteria: A population-based study. Metab Syndr Relat Disord. 2009;7:221–9. doi: 10.1089/met.2008.0077. [DOI] [PubMed] [Google Scholar]
- 18.Qatar Stepwise Report 2012: Chronic Disease Risk Factors Surveillance. Supreme Council of Health. 2013 Qatar. [Google Scholar]
- 19.Bener A, Alali KA. Consanguineous marriages in a newly developed country: The Qatari population. J Biosoc Sci. 2006;38:239–46. doi: 10.1017/S0021932004007060. [DOI] [PubMed] [Google Scholar]
- 20.Vauhkonen I, Niskanen L, Vanninen E, Kainulainen S, Uusitupa M, Laakso M. Defects in insulin secretion and insulin action in non-insulin-dependent diabetes mellitus are inherited: Metabolic studies on offspring of diabetic probands. J Clin Invest. 1998;101:86–96. doi: 10.1172/JCI716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Third Report of the National Cholesterol Education Program (NCEP). Expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 22.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome: A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 23.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. , Diagnosis and management of the metabolic syndrome, an American Heart Association/National Heart, Lung and Blood Institute Scientific statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 24.Molyneaux L, Constantino M, Yue D. Strong family history predicts a younger age of onset for subjects diagnosed with type 2 diabetes. Diabetes Obes Metab. 2004;6:187–94. doi: 10.1111/j.1462-8902.2004.00330.x. [DOI] [PubMed] [Google Scholar]
- 25.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–12. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 26.Shahbazian H, Latifi SM, Jalali MT, Shahbazian H, Amani R, Nikhoo A, et al. Metabolic syndrome and its correlated factors in an urban population in South West of Iran. J Diabetes Metab Disord. 2013;12:11. doi: 10.1186/2251-6581-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos AC, Ebrahim S, Barros H. Alcohol intake, smoking, sleeping hours, physical activity and the metabolic syndrome. Prev Med. 2007;44:328–34. doi: 10.1016/j.ypmed.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Visscher TL, Seidell JC. The public health impact of obesity. Annu Rev Public Health. 2001;22:355–75. doi: 10.1146/annurev.publhealth.22.1.355. [DOI] [PubMed] [Google Scholar]
- 29.Wei M, Gaskill SP, Haffner SM, Stern MP. Effect of diabetes and level of glycemia on all-cause and cardiovascular mortality: The San Antonio Heart Study. Diabetes Care. 1998;21:1167–72. doi: 10.2337/diacare.21.7.1167. [DOI] [PubMed] [Google Scholar]
- 30.American Heart Association. Cholesterol Abnormalities and Diabetes. 2012. [Last accessed on 2013 Apr 27]. Available from: http://www.heart.org/HEARTORG/Conditions/Diabetes/WhyDiabetesMatters/Cholesterol-Abnormalities-Diabetes_UCM_313868_Article.jsp .
- 31.Barrett-Connor E, Criqui MH, Klauber MR, Holdbrook M. Diabetes and hypertension in a community of older adults. Am J Epidemiol. 1981;113:276–84. doi: 10.1093/oxfordjournals.aje.a113097. [DOI] [PubMed] [Google Scholar]
- 32.Meis SB, Schuster D, Gaillard T, Osei K. Metabolic syndrome in nondiabetic, obese, first-degree relatives of African American patients with type 2 diabetes: African American triglycerides-HDL-C and insulin resistance paradox. Ethn Dis. 2006;16:830–6. [PubMed] [Google Scholar]


