Abstract
Background:
Polycystic ovary syndrome (PCOS) is a common disorder. PCOS women are at a high risk for insulin resistance and metabolic syndrome (MS). Adiponectin is positively related to insulin sensitivity. It has a preventive role in atherogenesis and MS. The present work was conducted to study the correlation between serum adiponectin levels and clinical characteristics and biochemical parameters in PCOS patients.
Materials and Methods:
A prospective study in 49 newly diagnosed (as per Rotterdam criteria) Indian PCOS women was conducted. PCOS women were clinically examined and investigated for biochemical parameters.
Results:
The mean serum adiponectin was 12 ± 9.4 μg/mL (range 0.47-45). Hypoadiponectinemia (serum adiponectin <4 μg/mL) was present in 22% patients. Age and adiponectin correlated significantly and inversely (r = −0.42, P = 0.027). Overweight/obese patients had lower mean adiponectin levels than normal weight (11.62 ± 9.5 vs 13.58 ± 9.5, P = 0.56). It was significantly lower in patients with acanthosis nigricans (AN) as compared with those without AN (8.4 ± 5.9 vs 15 ± 11, P = 0.038). Hirsute patients showed lower mean adiponectin levels than nonhirsute (10 ± 7.3 vs 13 ± 10, P = 0.57). A positive, insignificant correlation was observed between serum adiponectin and cholesterol, low-density lipoprotein, follicle stimulating hormone (FSH), thyroid stimulating hormone, levels. A negative insignificant correlation existed between serum adiponectin and luteinizing hormone (LH), LH: FSH ratio, prolactin, dehydroepiandrosterone, testosterone, triglyceride, high-density lipoprotein, fasting blood glucose, fasting insulin, and Homeostasis Model Assessment.
Conclusion:
Hypoadiponectinemia is present in one-fifth of women with PCOS. Adiponectin levels decrease as age advances. Low levels of adiponectin possibly contributes to the development of dermal manifestation (AN) of insulin resistance.
Keywords: Acanthosis nigricans, adiponectin, insulin resistance, PCOS
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a common endocrinological disorder affecting about 10% of female population [1] and is responsible for 50%-70% of cases with anovulatory infertility. [2,3] PCOS may present with clinical features, such as irregular menses, infertility, signs and symptoms of hyperandrogenemia, acanthosis nigricans (AN), and biochemical profile showing increased luteinizing hormone (LH): Follicle stimulating hormone (FSH) ratio, increased androgen levels, hyperinsulinemia, dyslipidemia. Insulin resistance is common in PCOS women. Adiponectin, also referred to as Adipocyte Complement-Related Protein (ACRP30) was first identified in 1995. [4] High adiponectin levels are independently associated with increased insulin sensitivity [5,6] and reduced risk of type 2 diabetes. [7]
PCOS patients are not homogenous in their clinical presentation and biochemical profiles. PCOS shows ethnic variation. [8,9] Due to limited studies in Indian women, the present research work was undertaken to study the role of adiponectin and its correlation with clinical characteristics and biochemical parameters in women with PCOS.
MATERIALS AND METHODS
This prospective study was carried out in 49 newly diagnosed postpubertal PCOS patients. PCOS was diagnosed as per Rotterdam criteria. [1] Patients having major systemic illness, congenital adrenal hyperplasia, hyperprolactinemia, acromegaly, functional hypothalamic amenorrhea, and patients receiving drugs for any other systemic illness were excluded. The study was approved by Institutional Ethics committee. All the guidelines of Helsinki were followed.
A detailed menstrual history, marital status, and parity were recorded. Patients were clinically examined. They were investigated for the biochemical parameters, namely, serum adiponectin, LH, FSH, prolactin (PRL), thyroid stimulating hormone (TSH), dehydroepiandrosterone (DHEA), testosterone (T), fasting blood glucose (FBG), and fasting insulin. Since patients had irregular menses, investigations were done independent of day of menstrual cycle. LH: FSH ratio and Homeostasis Model Assessment (HOMA) were calculated. Patients willing to give fasting samples (18 patients) were investigated for lipid profile, FBG, and fasting insulin. The cutoff body mass index as Standard Consensus Statement for Indian population was considered. [10]
Assay methods
In vitro biochemical investigations were carried out using kits Quantitative determination of hormones was carried out by electrochemiluminescence immunoassay (Roche- Hitachi Cobas e 411). Blood glucose was measured on autoanalyzer (Vital Scientific Microlab 300) using oxidase method. Lipid profile was determined by enzymatic colorimetric method. Serum adiponectin levels were measured by Enzyme- Linked Immunosorbent Assay (ELISA) technique (adiponetin kit manufacturer Techo- Medical AG Quidel product USA) (normal laboratory range was 4-12 μg/mL).
Statistical tests
Data were analyzed using software (Graph pad prism version 5). Intergroup comparison was done by nonparametric: Mann-Whitney U test. Correlation was analyzed by Spearman test. Fischer's exact test was used for difference between proportions. P value less than 0.05 is considered significant. (18) precludes this statistics.
RESULTS
DHEA, dehydroepiandrosterone; FBG, fasting blood glucose; FSH, follicle stimulating hormone; HOMA, Homeostasis Model Assessment; LDL, low-density lipoprotein; LH, luteinizing hormone; SD, standard deviation; TSH, thyroid stimulating hormone; HDL, high-density lipoprotein; n, number of patients; PCOS, polycystic ovary syndrome.
The mean age of PCOS patients was 22 ± 5.0 years (range, 14-35). Hypoadiponectinemia (serum adiponectin < 4 μg/mL) was observed in 22% of the patients (normal weight 1/11, overweight/obese 10/38) and was independent of obesity Figure 1 shows distribution of adiponectin values and inverse correlation between adiponectin and age. The relation is highly significant with correlation coefficient of −0.42** (P value, −0.027).
Figure 1.
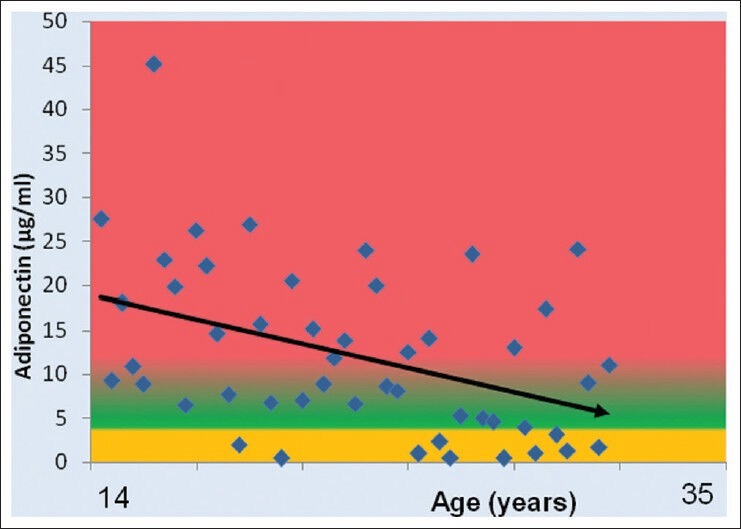
Correlation between adiponectin and age in women with polycystic ovary syndrome (r = −0.42, P = 0.0027**)
DISCUSSION
Adiponectin and clinical characters
In the present study, adiponectin levels in the PCOS patients showed a wide range. Although the mean adiponectin was within normal range, hypoadiponectinemia was present in one-fifth of the PCOS patients. As presence or absence of different clinical features in PCOS guides to determine the underlying pathology in a particular individual and also helps to decide the mode of therapeutic intervention, we tried to explore correlation of adiponectin levels with clinical features [Figure 2] and different biochemical parameters [Figures 3 and 4] as discussed further.
Figure 2.
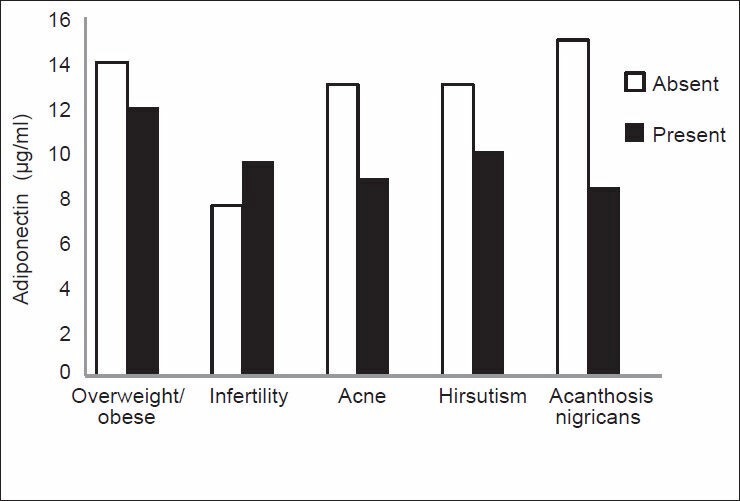
Comparison between mean adiponectin levels in polycystic ovary syndrome patients showing different clinical characteristics (**highly significant, no. of patients = 49, infertility in 8 of 18 married women)
Figure 3.
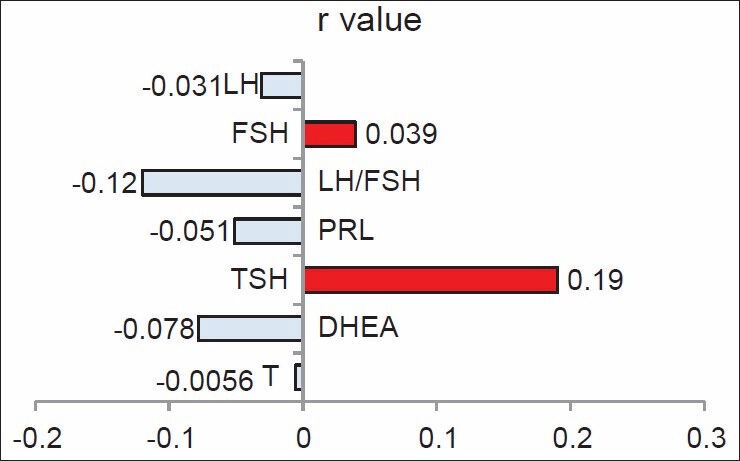
Correlation between serum adiponectin and hormonal profile in women with polycystic ovary syndrome. DHEA, dehydroepiandrosterone; LH, luteinizing hormone; PRL, prolactin; TSH, thyroid stimulating hormone. r, Spearman correlation coefficient
Figure 4.
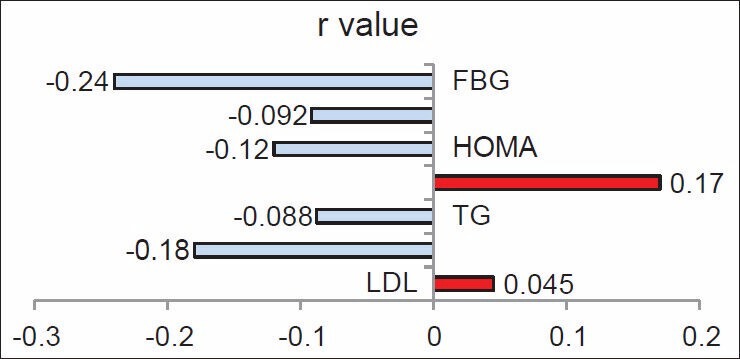
Correlation between serum adiponectin and lipid profile and glycemic status. FBG, fasting blood glucose; HOMA, Homeostasis Model Assessment; TG, triglycerides; LDL, low-density lipoprotein
Age
PCOS patients in the present study showed a highly significant inverse correlation between adiponectin and age [Figure 1]. The result indicates a change in adipocyte functioning as age advances, resulting in reduced adiponectin levels and is suggestive of dominant role of age in the adiponectin-related pathophysiology of women with PCOS. This finding endorses the importance of early detection and intervention in women with PCOS, especially with the knowledge of protective role of adiponectin in the MS and atherogenesis.
Obesity
Adiponectin levels are decreased in obese, compared with normal weight individuals. [11] The PCOS patients in the present study are not diverse. The current result shows lower mean adiponectin level in overweight/obese women with PCOS as compared with normal weight patients [Figure 2]. This finding is similar to that reported by Panidis et al. The authors found reduced serum adiponectin levels in obese PCOS patients when compared with lean PCOS patients and lean nonhyperandrogenic controls. [12] Weight reduction in obese PCOS patients often is advocated. The present findings support this mode of intervention. Weight reduction in addition to other beneficial effects may rectify hypoadiponectinemia and its consequences with a better therapeutic outcome.
Infertility
The mean adiponectin levels in married fertile and infertile women were within normal range. There was insignificant difference between the two groups, infertile group had higher mean adiponectin levels [Figure 2]. Establishment of relationship between infertility in PCOS and adiponectin levels if any needs further studies.
Acanthosis nigricans
Significant low levels of serum adiponectin in PCOS patients with the presence of AN as compared with those without AN were observed in the present study, indicating the inverse relationship between adiponectin and insulin resistance [Figure 2]. We have reported a significant positive correlation between AN and obesity in Indian PCOS patients in a previous study in which all the obese PCOS patients did not show the presence of AN, we had postulated that, either insulin resistance is not present in all obese PCOS patients or development of AN in insulin-resistant obese PCOS patients requires presence of some cofactor. [13] From the current data, we suggest that hypoadiponectinemia may be a contributor to AN and that adiponectin prevents the development of AN. We are not aware of previous studies reporting a relationship between AN and adiponectin levels in PCOS patients. We also suggest that the presence of AN should espouse estimation of adiponectin levels in PCOS.
Acne and hirsutism
Lower levels of adiponectin in patients showing clinical signs of hyperandrogenism, namely, acne and hirsutism as compared with their counterparts were observed in the present study. As some of these also had AN, we excluded patients with AN and further compared the mean adiponectin levels. Hyperandrogenic women showed lower values. The difference was insignificant (data not shown).
Although previous studies report decreased levels of adiponectin as well as insulin resistance in hyperandrogenic PCOS patients, we are not aware of comparison made between PCOS patients showing presence or absence of clinical features, namely, AN, acne, and hirsutism.
Adiponectin and biochemical parameters
Luteinizing hormone and follicle stimulating hormone
Reversed LH: FSH ratio is a common pathognomic feature of PCOS. In the present study, the mean LH: FSH ratio in the patients was more than two and showed insignificant inverse correlation with adiponectin. Stratifying the data into patients showing hypoadiponectinemia group (n = 10) and otherwise, an inverse correlation was observed between LH and adiponectin levels [Figure 3]. It was significant in the former group (data not shown, r = −0.64, P = 0.04). This observation is interesting more so if the lesser number of hypoadiponectenimic patients is considered. The result supports the study conducted by Lu et al. The authors have demonstrated a reduction in basal and Gonadotropin releasing hormone (GnRH)-stimulated LH secretion, acutely in animal model. [14]
Prolactin
A weak insignificant inverse correlation was seen between adiponectin and PRL in the present study. Asai-Sato et al. have reported that prolactin inhibits the production and secretion of adiponectin by primary adipocytes in lean lactating women. [15]
Thyroid stimulating hormone
The present study in euthyroid shows insignificant positive correlation between adiponectin and TSH. Iacobellis et al., have reported significant inverse relationship between adiponectin and TSH in euthyroid obese women. [16]
Dehydroepiandrosterone and testosterone
Previous studies are discordant in reporting any relationship between adiponectin and testosterone and DHEA. Lack of significant correlation between adiponectin and testosterone, DHEA levels in the present study is in accordance with the results reported by Orio et al., [17] and different from those reported by Escobar-Morreale et al. The authors have suggested that hyperandrogenism and abdominal adiposity, by reducing serum levels of insulin sensitizer adiponectin might contribute to insulin resistance in PCOS. [18] The strong relationship between adiponectin and AN and the insignificant correlation with clinical hyperandrogenism or androgen levels does not favor this concept. Their study differs from the current study with respect to the selection of PCOS patients, as the inclusion was based on clinical or biochemical hyperandrogenism and ultrasonography (USG) inclusion criteria was not considered.
Glycemic status
The mean HOMA was increased in the PCOS patients [Table 1] and serum adiponectin levels showed a negative correlation with FBG, insulin, and HOMA, although it was statistically not significant. Similar inverse correlation between adiponectin and FBG, insulin and HOMA in PCOS patients have been reported by Shin et al. [19] Significant correlation with AN and lack of the same with HOMA is observed in the present study. This discrepancy can be explained based on the less number of patients in whom HOMA was assessed. The other possibility may be that the association of adiponectin in peripheral and hepatic glucose homeostasis is significant on different scales as assessment of HOMA is a measure of hepatic insulin sensitivity and glucose tolerance test is a measure of peripheral insulin sensitivity. [20] Luo et al. have reported association of adiponectin levels with impaired glucose tolerance (IGT) and 2-h postprandial glucose in Asian Indian women. [21] Summing up our result and association of adiponectin levels with IGT in Asian Indian females we suggest that in PCOS women reduced adiponectin contributes to peripheral insulin resistance and the occurrence of AN.
Table 1.
Mean hormonal, FBG, and lipid levels in women with PCOS
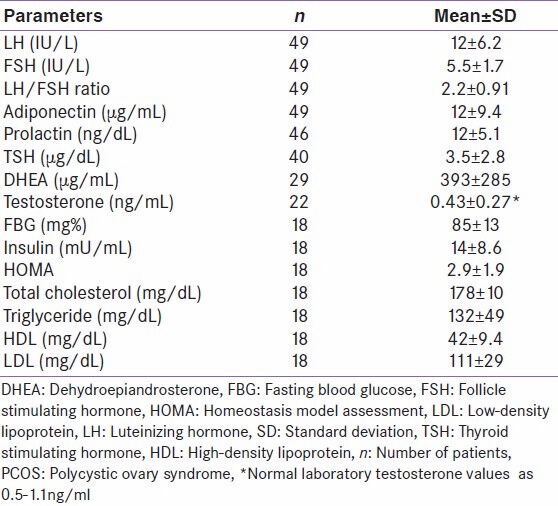
Orio Jr et al. did not find significant correlation between adiponectin levels and LH, FSH, PRL, glucose, and insulin in women with PCOS. [17] We have observed similar findings in the present study.
Adiponectin and lipid profile
In the present study, neither the positive correlation with cholesterol and LDL nor the inverse correlation with HDL or adiponectin was significant. These results are different from the previous studies. Yadav et al., in their study on Indian population, found a significant positive correlation between adiponectin and HDL. [22] Although insignificant, the inverse relationship observed between adiponectin and triglycerides is in accordance with the study reported by Shin et al. [19]
Groth has recommended that adiponectin potentially could be a marker for disease risk in PCOS and had suggested need of further studies. [23] Our present study serves this purpose. However, the limitations of the present study are, PCOS patients were not compared with age- and weight-matched healthy women. All investigations were not carried out in all the patients, although there was no investigator bias. Nonetheless, our study is suggestive of the important role of adiponectin in the pathophysiology of PCOS, its inverse relationship with clinical characteristics, particularly AN focuses its association with insulin resistance and inverse correlation with age substantiates the need for earliest possible therapeutic intervention for better outcome.
CONCLUSION
Hypoadiponectinemia is present in a subset of PCOS patients. In Indian women with PCOS, serum adiponectin is inversely correlated with age, indicating a trend of change in adipocyte homeostasis as the age advances. An early detection and therapeutic intervention of PCOS is a welcome considering the antiatherogenic and preventive role of adiponectin in the metabolic syndrome. Low levels of adiponectin possibly facilitates development of dermal manifestation of insulin resistance (AN) in PCOS patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Adams J, Polson DW, Franks S. Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Br Med J (Clin Res Ed) 1986;293:355–9. doi: 10.1136/bmj.293.6543.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hull MG. Epidemiology of infertility and polycystic ovarian disease: Endocrinological and demographic studies. Gynaecol Endocrinol. 1987;1:235–45. doi: 10.3109/09513598709023610. [DOI] [PubMed] [Google Scholar]
- 4.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 5.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–5. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 6.Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: More than just another fat cell hormone? Diabetes Care. 2003;26:2442–50. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- 7.Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–8. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 8.Williamson K, Gunn AJ, Johnson N, Milsom SR. The impact of ethnicity on the presentation of polycystic ovarian syndrome. Aust N Z J Obstet Gynaecol. 2001;41:202–6. doi: 10.1111/j.1479-828x.2001.tb01210.x. [DOI] [PubMed] [Google Scholar]
- 9.Norman RJ, Mahabeer S, Masters S. Ethnic differences in insulin and glucose response to glucose between white and Indian women with polycystic ovary syndrome. Fertil Steril. 1995;63:58–62. doi: 10.1016/s0015-0282(16)57297-5. [DOI] [PubMed] [Google Scholar]
- 10.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–70. [PubMed] [Google Scholar]
- 11.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 12.Panidis D, Kourtis A, Farmakiotis D, Mouslech T, Rousso D, Koliakos G. Serum adiponectin levels in women with polycystic ovary syndrome. Hum Reprod. 2003;18:1790–6. doi: 10.1093/humrep/deg353. [DOI] [PubMed] [Google Scholar]
- 13.Ramanand SJ, Ghonghane BB, Ramanand JB, Patwardhan MH, Ghanghas RR, Jain SS. Clinical characteristics of polycystic ovary syndrome in Indian women. Indian J Endocrinol Metab. 2013;17:138–45. doi: 10.4103/2230-8210.107858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu M, Tang Q, Olefsky JM, Mellon PL, Webster NJ. Adiponectin activates adenosine monophosphate-activated protein kinase and decreases luteinizing hormone secretion in LbetaT2 gonadotropes. Mol Endocrinol. 2008;22:760–71. doi: 10.1210/me.2007-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asai-Sato M, Okamoto M, Endo M, Yoshida H, Murase M, Ikeda M, et al. Hypoadiponectinemia in lean lactating women: Prolactin inhibits adiponectin secretion from human adipocytes. Endocr J. 2006;53:555–62. doi: 10.1507/endocrj.k06-026. [DOI] [PubMed] [Google Scholar]
- 16.Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relationship of thyroid function with body mass index, leptin, insulin sensitivity and adiponectin in euthyroid obese women. Clin Endocrinol (Oxf) 2005;62:487–91. doi: 10.1111/j.1365-2265.2005.02247.x. [DOI] [PubMed] [Google Scholar]
- 17.Orio F, Jr, Palomba S, Cascella T, Milan G, Mioni R, Pagano C, et al. Adiponectin levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2619–23. doi: 10.1210/jc.2002-022033. [DOI] [PubMed] [Google Scholar]
- 18.Escobar-Morreale HF, Villuendas G, Botella-Carretero JI, Alvarez-Blasco F, Sanchon R, Luque-Ramirez M, et al. Adiponectin and resistin in PCOS: A clinical, biochemical and molecular genetic study. Hum Reprod. 2006;21:2257–65. doi: 10.1093/humrep/del146. [DOI] [PubMed] [Google Scholar]
- 19.Shin HY, Lee DC, Lee JW. Adiponectin in women with polycystic ovary syndrome. Korean J Fam Med. 2011;32:243–8. doi: 10.4082/kjfm.2011.32.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radziuk J. Insulin sensitivity and its measurement: Structural commonalities among the methods. J Clin Endocrinol Metab. 2000;85:4426–33. doi: 10.1210/jcem.85.12.7025. [DOI] [PubMed] [Google Scholar]
- 21.Luo M, Oza-Frank R, Venkat Narayan KM, Gokulakrishnan K, Mohan V. Serum total adiponectin is associated with impaired glucose tolerance in Asian Indian females but not in males. J Diabetes Sci Technol. 2010;4:645–51. doi: 10.1177/193229681000400319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yadav A, Jyoti P, Jain SK, Bhattacharjee J. Correlation of adiponectin and leptin with insulin resistance: A pilot study in healthy north Indian population. Indian J Clin Biochem. 2011;26:193–6. doi: 10.1007/s12291-011-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groth SW. Adiponectin and polycystic ovary syndrome. Biol Res Nurs. 2010;12:62–72. doi: 10.1177/1099800410371824. [DOI] [PMC free article] [PubMed] [Google Scholar]


