Abstract
Pheochromocytoma, a tumor characterized by catecholamine excess, is usually associated with impaired glucose tolerance. Hypoglycemia may occur after the abrupt withdrawal of catecholamines in the postoperative period. Rarely, insulin secretion by stimulation of β-2 adrenoreceptors may overwhelm the glucagon production, thereby causing hypoglycemia. Here, we describe a female with pheochromocytoma, who presented with postprandial hypoglycemia.
Keywords: Hypoglycemia, pheochromocytoma, postprandial/reactive hypoglycemia
INTRODUCTION
Pheochromocytomas are adreno-medullary tumors characterized by catecholamine excess. Impaired glucose tolerance and diabetes mellitus have been reported in 26-50% cases of pheochromocytoma in different series.[1] This has been attributed to multiple mechanisms including insulin resistance at the level of skeletal muscle and liver, enhanced gluconeogenesis but the major effect is inhibition of insulin release from the pancreas.[2]
Hypoglycemia in patients with pheochromocytoma has usually been reported in the postoperative phase.[3,4,5] There have been anecdotal case reports of hypoglycemia in patients with pheochromocytoma prior to surgical removal of the tumor. In these six cases, hypoglycemia was attributed to the predominant β-adrenoreceptor stimulatory effect for the release of insulin. Here, we describe an unusual case of pheochromocytoma who presented as reactive hypoglycemia.
CASE REPORT
A 51-year-old female initially presented to the Department of Surgery with complaint of recurrent nonbilious vomiting. USG abdomen revealed cholelithiasisand a right adrenal mass. Patient denied complaints of episodic palpitations, headache, sweating or weight loss. There was no history of early satiety or constipation. There was a history of frequent intake of meals at an interval of 3-4 hrs. She denied any history suggestive of postprandial hypoglycemia. She was diagnosed to be hypertensive around 3 years back, but she was not taking regular medication. She had been taking tablet Amlodipine 10 mg and Olmesartan 40 mg and referred to the Endocrine Unit. There was no family history suggestive of Hypertension, DM, CAD or MEN.
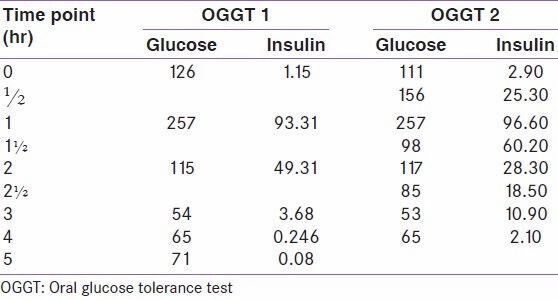
Examination revealed a thin built female of height 5′3″, weight 43 kg, BMI: 16.79 Kg/m2. Blood pressure was 115/80 mmHg in supine position and 104/80 mmHg in standing position. There was no postural drop in blood pressure. Systemic examination was unremarkable. Biochemical investigations revealed normal hemogram, liver function test and kidney function test. The 24-hour urine catecholamines were elevated [VMA: 36.63 mg/g creatinine (1.6-4.2 mg/g), Epinephrine: 81.42 pg/ml (<67 pg/ml), Norepinephrine: 876.47 ng/ml (95-446 ng/ml), Dopamine: 51.61 pg/ml]. Postprandial glucose levels were reported as 30 mg/dL and 54 mg/dL on two different occasions. An extended oral glucose tolerance test was done which showed reactive hypoglycemia and hyperinsulinemia at 3 hrs [Table 1]. HbA1C was 6.20%. Thyroid function test and serum cortisol levels were normal. CT abdomen and pelvis showed partially necrotic, otherwise well defined mass in the right adrenal measuring 42 × 39 mm in size. An incidental finding of mural thickening of antrum of stomach was found. Echocardiography revealed severe concentric Left ventricular hypertrophy, mild MR, AR and LV diastolic dysfunction with left ventricular ejection fraction of 60%.
Table 1.
Extended oral GTT with 75 g of glucose
Patient underwent surgery after appropriate hypertensive management with α and β-blockers. Histopathology revealed tumor cells with abundant amphophilic granular cytoplasm, vesicular nuclei and few cells showed nucleoli with occasional mitotic figures suggestive of pheochromocytoma [Figure 1].
Figure 1.
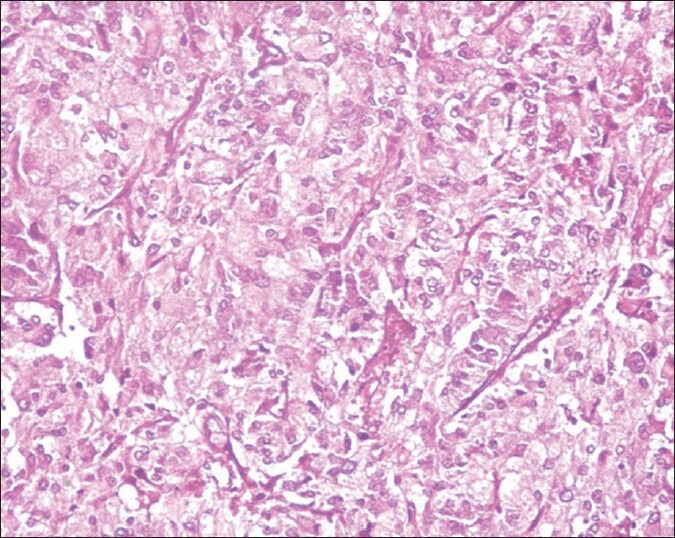
Histopathology showing tumor cells of pheochromocytoma
DISCUSSION
Abnormalities in glucose homeostasis are frequently reported in patients of pheochromocytoma. Impaired glucose tolerance and diabetes mellitus has been reported to be in as many as 26-50% patients in a semilogical study of 2585 patients, including 11 with pheochromocytoma.[1] Anecdotal reports of diabetic ketoacidosis have also been described in patients with pheochromocytoma. Isotani et al. reported a young patient with NA predominant pheochromocytoma presenting with DKA.[6] Another case of DKA in a 29-year-old patient was reported by Edelman in the year 1992 which was attributed to catecholamine excess.[7]
Catecholamines exert their action on glucose homeostasis through α and β-adrenoreceptors. Stimulation of β-adrenoreceptors by catecholamines causes enhanced glycogenolysis and gluconeogenesis by the liver resulting in transient increase in glucose production.[8] Direct stimulation by noradrenaline on α-adrenoreceptors of pancreas, causes inhibition of insulin release whereas stimulation of β-adrenoreceptors by adrenaline, results in insulin release, specially in conditions where glycogen stores are depleted.[9] In most situations the α-receptor-mediated insulin inhibition predominates over β-insulin-releasing actions causing impairment of glucose homeostasis.[10,11]
Some cases of hypoglycemia after surgical removal of pheochromocytoma have been described. Hypoglycemia in these cases is believed to be due to sudden loss of catecholamine effect on glucose homeostasis. There are only anecdotal case reports of hypoglycemia in patients of pheochromocytoma in the preoperative phase. Till date only six such cases have been reported [Table 2]. Reactive or postprandial hypoglycemia, occurs exclusively after meals, typically within 4 hours after food ingestion. The biochemical criteria for defining postprandial hypoglycemia are a plasma glucose concentration less than 3.0 mmol/L (54 mg/dL) and an insulin concentration greater than 18 pmol/L (3.0 pmol/L).[12,13]
Table 2.
Previous cases of hypoglycemia reported in patients with pheochromocytoma
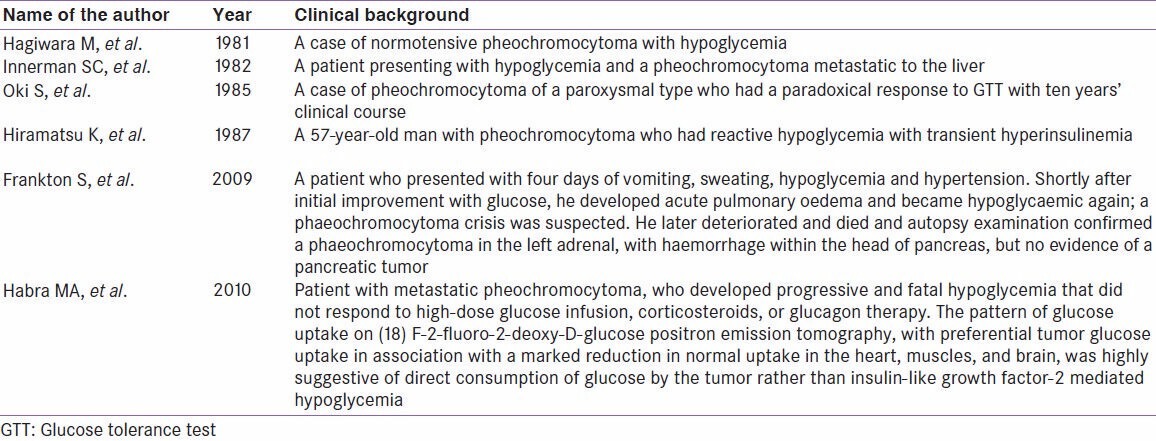
The first case of hypoglycemia in a normotensive pheochromocytoma was reported by Hagiwara in the year 1981.[14] Innerman and his colleagues in 1982 reported a patient of pheochromocytoma that had metastasized to the liver. The author proposed that hypoglycemia was due to secretion of insulin or a substance with insulin-like activity by the tumor, increased utilization of glucose by the malignant cells and a local effect of the tumor on the hepatic parenchyma.[15] Oki et al. reported a case of pheochromocytoma of paroxysmal type with paradoxical response to glucose tolerance test.[16] In 1987, Kazuko Hiramatsu reported a case of pheochromocytoma with dilated cardiomyopathy, in whom transient hyperinsulinemia and reactive hypoglycemia were reported on one occasion during extended GTT, which could not be reproduced on subsequent glucose load. The author pointed out that the glucose tolerance abnormalities were transient.[17] Frankton et al. (2008) reported a case of pheochromocytoma crisis presenting with profound hypoglycemia and subsequent hypertension. The author postulated that in conditions of depleted stores of glycogen in liver and skeletal muscles, stimulation of β-adrenoreceptors by adrenaline, causes increase release of insulin thereby causing hypoglycemia.[18] Recently fatal hypoglycemia in malignant pheochromocytoma, was reported by Hambra in 2010. He postulated that hypoglycemia in this patient was because of increased consumption of glucose by the rapidly dividing tumor cells.[19]
Our patient had impaired fasting glucose with glucose levels rising to more than 250 mg/dl at 1 hr. Postprandial glucose fell down to 54 mg/dL at 3 hrs. There was an earlier report of postprandial glucose level of 30 mg/dL. Hyperinsulinemia was observed at 1 and 2 hrs. While the patient had frequent food intake and occasional headaches, classical symptoms of hypoglycemia were not elicited. An incidental finding of mural thickness at gastric outlet cannot explain postprandial hypoglycemia in our patient, rather delayed absorption and delayed increase in plasma glucose levels would be expected. Since the patient has presented with vomiting, it is possible that the glycogen stores in muscle and liver were depleted further contributing to hypoglycemia. Another interesting thing in our patient is that she is never been symptomatic for hypoglycemic symptoms even when the plasma glucose levels were 30 mg/dL.
CONCLUSION
The present case was rare case of pheochromocytoma presenting as reactive hypoglycemia.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Plouin PF, Degoulet P, Tugayé A, Ducrocq MB, Ménard J. Screening for phaeochromocytoma: In which hypertensive patients. A semiological study of 2585 patients, including 11 with phaeochromocytoma (author's transl)? Nouv Presse Med. 1981;10:869–72. [PubMed] [Google Scholar]
- 2.Pacak K, Timmers ML, Eisenhofer G. In: Pheochromocytoma: Endocrinology adult and pediatric. 6th ed. Jameson LJ, Groot DJ, editors. vol. 2. Saunders, an affiliate of Elsevier Inc Publications; 2010. pp. 1990–2018. [Google Scholar]
- 3.Wilkins GE, Schmidt N, Doll WA. Hypoglycemia following excision of pheochromocytoma. Can Med Assoc J. 1977;116:367–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Costello GT, Moorthy SS, Vane DW, Dierdorf SF. Hypoglycemia following bilateral adrenalectomy for pheochromocytoma. Crit Care Med. 1988;16:562–3. doi: 10.1097/00003246-198805000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Akiba M, Kodama T, Ito Y, Obara T, Fujimoto Y. Hypoglycemia induced by excessive rebound secretion of insulin after removal of pheochromocytoma. World J Surg. 1990;14:317–24. doi: 10.1007/BF01658514. [DOI] [PubMed] [Google Scholar]
- 6.Isotani H, Fujimura Y, Furukawa K, Morita K. Diabetic ketoacidosis associated with the pheochromocytoma of youth. Diabetes Res Clin Pract. 1996;34:57–60. doi: 10.1016/s0168-8227(96)01330-7. [DOI] [PubMed] [Google Scholar]
- 7.Edelman ER, Stuenkel CA, Rutherford JD, Williams GH. Diabetic ketoacidosis associated with pheochromocytoma. Cleve Clin J Med. 1992;59:423–7. doi: 10.3949/ccjm.59.4.423. [DOI] [PubMed] [Google Scholar]
- 8.Porte D., Jr A receptor mechanism for the inhibition of insulin release by epinephrine in man. J Clin Invest. 1967;46:86–94. doi: 10.1172/JCI105514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerasi E, Effendic S, Luft R. Role of adrenergic receptors in glucose-induced insulin secretion in man. Lancet. 1969;9:301–2. doi: 10.1016/s0140-6736(69)90059-2. [DOI] [PubMed] [Google Scholar]
- 10.Ullrich S, Wollheim CB. Expression of both alpha 1- and alpha 2-adrenoceptors in an insulin-secreting cell line. Parallel studies of cytosolic free Ca 2+ and insulin release. Mol Pharm. 1985;28:100–6. [PubMed] [Google Scholar]
- 11.Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000;43:533–49. doi: 10.1007/s001250051341. [DOI] [PubMed] [Google Scholar]
- 12.Thompson GB, Service FJ, Andrews JC, Lloyd RV, Natt N, van Heerden JA, et al. Noninsulinomapancreatogenous hypoglycemia syndrome: An update in 10 surgically treated patients. Surgery. 2000;128:937–45. doi: 10.1067/msy.2000.110243. [DOI] [PubMed] [Google Scholar]
- 13.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric bypass surgery. N Engl J Med. 2005;353:249–54. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 14.Hagiwara M, Koyama Y, Deguchi O. A case of normotensive pheochromocytoma with hypoglycemia. Horumon to Rinsho (Toya) 1981;29:287. [Google Scholar]
- 15.Immerman SC, Sener SF, Khandekar JD. Causes and evaluation of tumour-induced hypoglycaemia. Arch Surg. 1982;117:905–8. doi: 10.1001/archsurg.1982.01380310025006. [DOI] [PubMed] [Google Scholar]
- 16.Oki S, Kuno S, Nakao K, Imura H, Endo K, Torizuka K. A case of pheochromocytoma of paroxysmal type detected from a paradoxical response in GTT with ten years' clinical course. Nihon Naika Gakkai Zasshi. 1985;74:1720–5. doi: 10.2169/naika.74.1720. [DOI] [PubMed] [Google Scholar]
- 17.Hiramatsu K, Takahashi K, Kanemoto N, Arimori S. A case of pheochromocytomawith transient hyperinsulinemia and reactive hypoglycemia. Jpn J Med. 1987;26:88–90. doi: 10.2169/internalmedicine1962.26.88. [DOI] [PubMed] [Google Scholar]
- 18.Frankton S, Baithun S, Husain E, Davis K, Grossman AB. Phaeochromocytoma crisis presenting with profound hypoglycemia and subsequent hypertension. Hormones (Athens) 2009;8:65–70. doi: 10.14310/horm.2002.1224. [DOI] [PubMed] [Google Scholar]
- 19.Habra MA, Núñez R, Chuang H, Ayala-Ramirez M, Rich T, Kyle K, et al. Fatal hypoglycemia in malignant pheochromocytoma: Direct glucose consumption as suggested by (18) F-2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography imaging. Endocrine. 2010;37:209–12. doi: 10.1007/s12020-009-9300-1. [DOI] [PubMed] [Google Scholar]


