Abstract
Background
Characterized as a sudden and temporary loss of consciousness and postural tone, with quick and spontaneous recovery, syncope is caused by an acute reduction of systemic arterial pressure and, therefore, of cerebral blood flow. Unsatisfactory results with the use of drugs allowed the nonpharmacological treatment of neurocardiogenic syncope was contemplated as the first therapeutic option.
Objectives
To compare, in patients with neurocardiogenic syncope, the impact of a moderate intensity aerobic physical training (AFT) and a control intervention on the positivity of head-up tilting test (HUT) and orthostatic tolerance time.
Methods
Were studied 21 patients with a history of recurrent neurocardiogenic syncope and HUT. The patients were randomized into: trained group (TG), n = 11, and control group (CG), n = 10. The TG was submitted to 12 weeks of AFT supervised, in cycle ergometer, and the CG to a control procedure that consisted in 15 minutes of stretching and 15 minutes of light walk.
Results
The TG had a positive effect to physical training, with a significant increase in peak oxygen consumption. The CG did not show any statistically significant change before and after the intervention. After the intervention period, 72.7% of the TG sample had negative results to the HUT, not having syncope in the revaluation.
Conclusion
The program of supervised aerobic physical training for 12 weeks was able to reduce the number of positive HUT, as it was able to increase tolerance time in orthostatic position during the HUT after the intervention period.
Keywords: Aerobic physical training, Neurocardiogenic syncope, Head-up tilting test
Introduction
Syncope is defined as a sudden and temporary loss of consciousness and postural tonus characterized by a quick and spontaneous recovery. It is caused by an acute decrease in systemic arterial blood pressure and cerebral blood flow1-3. Neurocardiogenic syncope (NCS) is the most frequent type of syncope, and, although benign, recurrent symptoms decrease the quality of life of patients in the same manner as chronic diseases4,5.
The passive head-up tilt test (HUT) is an effective and safe method to identify patients with suspected NCS. Its reproducibility, when positive, is approximately 82%6,7.
The physiopathology of NCS remains unclear; however, an impairment of the compensatory reflex mechanisms responsible for maintaining pressure levels in the short term is observed. In NCS, unknown trigger mechanisms activate a specific autonomic efferent response, with a decrease in the sympathetic arteriolar stimulation and/or increase in the parasympathetic tonus at the sinus node, thereby resulting in vasodilatation and/or bradycardia that decreases the systemic arterial pressure3,8. Syncope is usually preceded by prodromes such as pallor, nausea, sweating, dizziness, visual disturbances, and palpitations. It may be triggered by orthostatic stress, particularly in hot environments, pain or emotional stress, low hydration, and prolonged fasting3,9,10.
The various forms of treatment proposed for NCS have not been completely investigated11-13. Well-conducted randomized studies have shown that preventive pharmacological therapy is ineffective14-16, which has led to the possibility of considering nonpharmacological treatments as first-line therapeutic options2-5,17-19. Aerobic physical training (AFT) has been extensively studied as part of the treatment for NCS3. In previous studies, increased orthostasis tolerance time and blood volume21 as well as decreased levels of circulating vasopressin were observed in patients with NCS20-22. Another important effect of physical exercise is the increase in muscle mass, particularly in the lower limbs, which improves venous return via the muscle pump mechanism22. Aerobic training decreases cardiac sympathetic tonus, which may cause a decrease in the chronotropic and inotropic responses that are responsible for triggering the Bezold-Jarisch reflex when venous return is decreased because of orthostasis; in addition, it improves baroreflex sensitvity23-25.
This study aimed to clarify the effectiveness of AFT in the treatment of NCS8.
Objective
This study aimed to compare the effects of moderate-intensity AFT on HUT positivity and orthostasis tolerance time with those of a control intervention in patients with NCS.
Methods
This prospective study included 21 sedentary patients with positive HUT results and a history of recurrent NCS (>2 episodes, with the last episode having occurred during the 6 months before the start of this study). These patients underwent the diagnostic test after being referred to the General Cardiology, Arrhythmia, and Neurology Outpatient Clinics of the Hospital das Clínicas of the School of Medicine of Ribeirão Preto-USP. Following inclusion in the study, the patients were reassessed by a cardiologist to rule out cardiac and neurological causes of syncope. They also underwent complementary tests, including resting electrocardiogram (12 leads), Doppler-echocardiogram, and 24-h electrocardiogram (Holter). The selected individuals were studied in the absence of drugs that could interfere with the response of the analyzed variables. Patients with suspected chronic diseases such as diabetes and coronary artery disease were excluded from the study, along with those who had some orthopedic or functional limitation that prevented them from performing aerobic exercise.
The study was approved by the Research Ethics Committee of our institution. All patients provided written informed consent.
The passive HUT
During the test, the patients were maintained in the supine position with a saddle support for 20 min on a tilt table. The following parameters were assessed at baseline and tilt: surface electrocardiogram, instantaneous heart rate (cardio tachometer), and arterial blood pressure by auscultatory method and beat-by-beat using the plethysmographic method (Finapres - Ohmeda - 2300).
After the resting period in the horizontal supine position, the table was tilted to a vertical position at an angle of 70º (head-up tilt) in less than 10 s and the test was continued for 45 min. The test was immediately interrupted if the patient reached the test positivity criteria6. In this case, the table was quickly returned to the horizontal position or the Trendelenburg position, when necessary, for a period of 5 min or until complete recovery of the patient3,10. Both the initial and re-evaluation tests were performed using the same methodology.
The ergospirometric test (ET)
All patients underwent the maximal cardiopulmonary exercise test. The protocol involved dynamic physical effort to be applied while sitting on a cycle ergometer with an electromagnetic brake (Corival 400, Quinton). The power applied to the cycle ergometer was of the ramp-type, and the intensity was determined individually according to the formula developed by Wasserman et al26, which was based on anthropometric characteristics, age, and gender. The patients were encouraged to apply effort until the point of exhaustion. During the test, the initial ramp was preceded by a period of 4 min at minimum load (3-4 Watts) and a constant speed of 60 rotations per min, with the aim of warming up the physiological systems involved in oxygen delivery and allowing them to reach the steady-state. In this protocol, the ventilatory variables were obtained using an ergospirometry device (CPX/D MedGraphics) that allowed the acquisition, processing, and recording of these variables on a breath-to-breath basis. The peak values of oxygen consumption (VO2) and heart rate (HR) were expressed as the mean of the last 30 s of recording during the effort period.
Intervention protocol
Following confirmation of diagnosis based on the HUT, all patients received general guidance on how to avoid situations and places that could trigger syncope, such as excessive heat, closed crowded environments, dehydration, prolonged fasting, remaining in a vertical position for a long time, and emotional stress24. They received instructions to increase the intake of water, perform isometric contraction maneuvers, and/or lie down on experiencing the first presyncopal symptoms. In addition, they were asked to note, in a diary, the dates of any syncope episodes and/or presyncopal symptoms experienced during the intervention period.
The individuals were randomized into 2 groups: a trained group (TG, n = 11) and a control group (CG, n = 10). The TG was subjected to a 12-week program of supervised AFT using a cycle ergometer. The intensity of training was prescribed such that the HR was maintained between the value corresponding to the ventilatory anaerobic threshold (VAT) and that corresponding to 10% below the respiratory compensation point (RCP). A heart rate monitor (Polar®) was used throughout the session. Training took place twice a week and lasted for 35 min, and the patient was instructed to perform 2 more unsupervised sessions. If the patient was unable to attend the scheduled session, another session was rescheduled for the same week to ensure that all supervised sessions were conducted. To ensure adherence to external training, the unsupervised sessions were subjected to weekly control using a spreadsheet given to the patient that was checked at every session.
The CG was subjected to a control procedure that included 15 min of stretching, 15 min of light walking below the prescribed target HR, and 10 min of relaxation. The HR was monitored throughout the procedure using an HR monitor (Polar®) to ensure that it was maintained below the calculated target HR. Two weekly sessions were performed over a period of 12 weeks.
After 12 weeks, both groups were re-evaluated using HUT and ET.
Statistical analysis
Data are expressed as means ± standard deviations and medians. For intergroup analysis of anthropometric data, analysis of variance (ANOVA) was used27. Fisher's exact test was used to compare the response to the HUT and the intervention28. To compare the number of syncope episodes between groups, the Poisson model with random effects was used29. Spearman's correlation coefficient was used to assess the association between the 2 quantitative variables. The level of statistical significance was 5%.
Results
The anthropometric characteristics and basal hemodynamic parameters are shown in Table 1. There was no statistically significant difference between groups.
Table 1.
Antropometric characteristics and basal hemodynamic parameters in the trained group (TG) and control group (CG)
| Gender | Mean (SD) | |
|---|---|---|
| TG | CG | |
| Male | 2 | 1 |
| Female | 9 | 9 |
| Age (years) | 32 (10) | 26 (8) |
| Height (cm) | 164 (9.4) | 163.5 (5.5) |
| Body mass (kg) | 67 (11.2) | 63 (10.0) |
| Heart rate (bpm) | 64 (8) | 65 (7) |
| Systolic arterial pressure (mmHg) | 110.9 (17.1) | 109.0 (17.1) |
| Diastolic arterial pressure (mmHg) | 71.8 (12.7) | 69.0 (11.0) |
SD: Standard deviation.
The results obtained in the ergospirometric stress tests are presented in Table 2. There was no significant difference between groups during the preintervention period. After 12 weeks of aerobic training, the TG exhibited a tendency for higher peak HR, with VO2 peak and VO2 at AT being significantly higher in the TG than in the CG. The CG did not exhibit any statistically significant changes in ergospirometry variables before and after the training period. After the intervention, the TG exhibited a higher VO2 at AT compared with the CG.
Table 2.
Description of the variables measured in the ergospirometric test before and after intervention in the trained group (TG) and control group (CG)
| Group | Period | Variable | n | Mean | SD | CV | Minimum | Median | Maximum |
|---|---|---|---|---|---|---|---|---|---|
| Control | Before | HR rest | 10 | 65 | 7 | 11 | 56 | 63 | 77 |
| HR peak | 10 | 168 | 14 | 9 | 143 | 173 | 183 | ||
| VO2 peak | 10 | 24.3 | 7.55 | 31.06 | 16 | 22.95 | 40 | ||
| VO2 AT | 10 | 12.4 | 3.27 | 26.39 | 8 | 12 | 20 | ||
| After | HR rest | 10 | 66 | 6 | 9 | 59 | 65 | 82 | |
| HR peak | 10 | 169 | 17 | 10 | 123 | 172 | 181 | ||
| VO2 peak | 10 | 22.84 | 7.25 | 31.72 | 14.3 | 22 | 38 | ||
| VO2 AT | 10 | 11.8§ | 3.12 | 26.44 | 9 | 11 | 19 | ||
| Trained | Before | HR rest | 11 | 64 | 8 | 12 | 51 | 66 | 78 |
| HR peak | 11 | 162 | 16 | 10 | 133 | 166 | 180 | ||
| VO2 peak | 11 | 21,1† | 4.02 | 19.03 | 14 | 21.15 | 26.8 | ||
| VO2 AT | 11 | 12‡ | 1.48 | 12.36 | 9 | 12 | 14 | ||
| After | HR rest | 11 | 66 | 10 | 15 | 52 | 64 | 83 | |
| HR peak | 11 | 169 | 10 | 6 | 150 | 171 | 181 | ||
| VO2 peak | 11 | 25.35† | 3.93 | 15.51 | 21.6 | 25 | 34.8 | ||
| VO2 AT | 11 | 14.27ठ| 2.28 | 16 | 10 | 14 | 19 |
SD: standard deviation; CV: coefficient of variation; HR: heart rate; AT: anaerobic threshold; † p ≤ 0.05; VO2 peak in the trained group before and after the intervention. ‡ p ≤ 0.05; VO2 at AT in the trained group before and after the intervention. § p ≤ 0.05; VO2 at AT between the control and trained groups after the intervention.
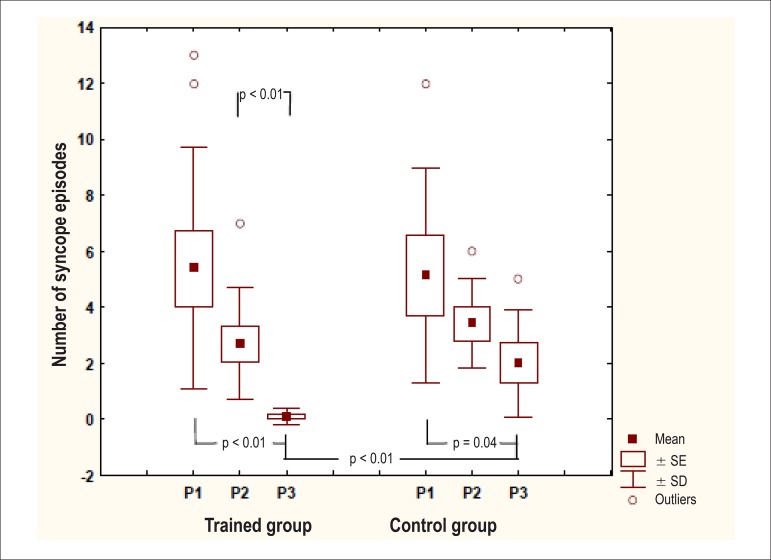
Figure 1 shows an analysis of the number of syncope episodes experienced by the patients during 3 distinct periods: from 1 year to 6 months before the intervention (P1), during the 6 months before the intervention (P2), and 3 months after the start of the intervention (P3). The TG exhibited a statistically significant difference between periods P1 and P3 and between P2 and P3, whereas the CG exhibited a statistically significant difference between P1 and P3. On comparing both groups, a significant difference was observed for P3.
Figure 1.
Number of syncope episodes in the trained group and control group in the 3 periods under study.
In the initial evaluation of both groups, all individuals exhibited a positive HUT result (Table 3). There was no statistically significant difference between groups in orthostasis tolerance time before the intervention (Table 4).
Table 3.
Results of the head-up tilt test before and after the intervention in the trained group (TG) and control group (CG)
| TG (n = 11) | CG (n = 10) | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| Positive | 11 (100%) | 3 (27.3%) | 10 (100%) | 7 (70%) |
| Negative | 0 | 8 (72.7%)* | 0 | 3 (30%)* |
p ≤ 0.05; HUT result between the TG and CG after the intervention.
Table 4.
Orthostasis tolerance time before and after the intervention in the trained group (TG) and control group (CG)
| Group | Time | n | Mean | SD | CV | Minimum | Median | Maximum |
|---|---|---|---|---|---|---|---|---|
| Control | Before | 10 | 31.3 | 12.9 | 41.2 | 9 | 35.5 | 44 |
| After | 10 | 27.6* | 13.6 | 49.3 | 4 | 25.5 | 45 | |
| Trained | Before | 11 | 28.6† | 13.8 | 48.0 | 8 | 32 | 44 |
| After | 11 | 39.5*† | 9.9 | 25.1 | 18 | 45 | 45 |
SD: standard deviation; CV: coefficient of variation; *p ≤ 0.05; orthostasis tolerance time (min) after the intervention between the CG and TG. † p ≤ 0.05; orthostasis tolerance time in the TG before and after the intervention.
After the intervention period, 72.7% patients in the TG exhibited a negative HUT, with no episodes of syncope reported during re-evaluation. In the CG, only 30% patients exhibited a negative HUT; this difference was significant (Table 3). Individuals with a positive HUT result after the intervention, those in the TG exhibited an increased orthostasis tolerance time compared with those in the CG. This difference was statistically significant (Table 4).
There was no correlation between the increase in physical capacity (delta of peak VO2) and increase in orthostasis tolerance time (delta of orthostasis tolerance time) in both groups.
Discussion
NCS is a transient functional impairment of the autonomic nervous system that primarily affects healthy young women who do not exhibit signs of structural heart disease or neurological disease. Patients who experience recurrent episodes experience limitations in their daily and professional activities, which greatly affects their quality of life3,30,31.
The pharmacological treatment of syncope, which is based on medications such as beta blockers, fludrocortisone, and serotonin reuptake inhibitors, is part of the available therapeutic arsenal; however, in the vast majority of patients, pharmacological therapy for the treatment of this dysautonomia does not offer effective results14-16,32.
Therefore, a variety of nonpharmacological approaches have been proposed. These include prevention by avoiding the triggering factors, e.g., increasing water intake throughout the day, avoiding the vertical position at rest, avoiding prolonged fasting, and undergoing passive postural training (tilt training), and the use of maneuvers to avoid syncope when the patient exhibits the so-called prodromes. In these situations, the patients can be instructed to perform maneuvers or adopt postures that prevent the progression of the presyncope to syncope, such as crossing of the legs in the vertical position, isometric effort with the upper limbs, and change to the horizontal position. Among these measures, physical training has been gaining importance because of the positive results observed in terms of a decrease in and/or elimination of NCS episodes3,20,24,33,34.
In our study, AFT resulted in a significant increase in VO2 at stress peak and at VAT in the TG26. Moreover, the CG did not exhibit significant changes in oxygen delivery parameters during stress, either at submaximal levels or at maximal levels. These findings suggest that in the TG, the physiological adaptations induced by aerobic training are responsible for the decrease in the number of individuals with a positive HUT result and increased orthostasis tolerance time after 12 weeks of the intervention.
In 2007, Gardenghi et al24 conducted a 4-month study about the impact of different therapeutic methods (moderate AFT, passive postural training, pharmacological treatment, and control) on the improvement of the baroreceptor reflex in patients with NCS and observed that only AFT was effective in increasing arterial baroreflex sensitivity, which decreased in the studied group. Brum et al25 reported that physical training increased baroreflex sensitivity in normotensive and hypertensive rats. In 1998, Gava et al23 described the decrease in sympathetic cardiac tonus in rats with spontaneous hypertension after physical training, which decreased cardiac chronotropic and inotropic responses that are responsible for triggering the Bezold-Jarisch reflex when venous return is decreased because of gravitational stress.
Mitnangi and Hainsworth33 and Carrol et al21 reported an increase in orthostasis tolerance time in patients with NCS who underwent AFT, a finding consistent with our findings. Increased circulating blood volume and decreased levels of circulating vasopressin were observed in these patients. Convertino et al22 found a significant increase in the circulating plasma volume of individuals subjected to moderate intensity AFT.
In this context, it is worth noting the study conducted by Allen et al35 in 1945, wherein 2 identical twins with positive tilt tests were studied. One of them performed physical training over 3 weeks, whereas the other remained sedentary. After the intervention period, the sedentary twin exhibited a positive HUT result, whereas the active twin exhibited a negative HUT result.
AFT, in addition to promoting beneficial changes in the autonomic modulation of the cardiovascular system in patients with NCS, can also increase blood volume, hemoglobin levels, and muscle tonus in the lower limbs (when exercise involves these muscle groups)30. Moreover, physical training is responsible for the increase in the final diastolic and systolic volumes, which may interfere with the stimulation of ventricular C fibers that trigger NCS3,36.
Although the present study was not designed to explore the physiopathological mechanisms responsible for syncope, which are many and complex, and considering that we assessed the effects of a 12-week physical training program, it is unlikely that the increase in circulating plasma volume is key to the reported findings20,33-35,37. In this study, changes in arterial baroreceptor reflex sensitivity probably played an important role in the effects observed in the trained group, considering that other studies have reported these adaptations to short- and long-duration aerobic physical exercise24,25,38,39.
Conclusions
The 12-week supervised AFT program was effective in decreasing the number of patients with positive HUT results and increasing the orthostasis tolerance time after the intervention period in patients with NCS.
Limitations
The effects of physical training as a nonpharmacological procedure for the treatment of NCS require further investigations. Because of the short follow-up period used in the present study, syncope recurrence could not be evaluated and the clinical impact of the intervention on the natural progression of the disease could not be determined.
Footnotes
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conception and design of the research: Takahagi VCM, Gallo Júnior L; Acquisition of data: Takahagi VCM, Costa DC, Crescêncio JC; Analysis and interpretation of the data and Statistical analysis: Takahagi VCM; Writing of the manuscript: Takahagi VCM, Costa DC; Critical revision of the manuscript for intellectual content: Gallo Júnior L.
Sources of Funding
This study was funded by CAPES.
Study Association
This article is part of the thesis of master submitted by Vanessa Cristina Miranda Takahagi from Faculdade de Medicina de Ribeirão Preto - Universidade de São Paulo.
References
- 1.Chen-Scarabelli C, Scarabelli TM. Neurocardiogenic syncope. BMJ. 2004;329(7461):336–341. doi: 10.1136/bmj.329.7461.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brignole M, Alboni P, Benditt DG, Bergfeldt L, Blanc JJ, Thomsen PE, et al. Task Force on Syncope, European Society of Cardiology Guidelines on management (diagnosis and treatment) of syncope-update 2004. Executive Summary. Eur Heart J. 2004;25(22):2054–2072. doi: 10.1016/j.ehj.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Gardenghi G, Hachul DS, Negrão CE, Sosa E. Síncope neurocardiogênica e exercício. Reblampa. 2004;17(1):3–10. [Google Scholar]
- 4.Kinay O, Yazici M, Nazli C, Acar G, Gedikli O, Altinbas A, et al. Tilt training for recurrent neurocardiogenic syncope: effectiveness, patient compliance, and scheduling the frequency of training sessions. Jpn Heart J. 2004;45(5):833–843. doi: 10.1536/jhj.45.833. [DOI] [PubMed] [Google Scholar]
- 5.Di Girolamo E, Di Iorio C, Leonzio L, Sabatini P, Barsotti A. Usefulness of a tilt training program for the prevention of refractory neurocardiogenic syncope in adolescents: a controlled study. Circulation. 1999;100(17):1798–1801. doi: 10.1161/01.cir.100.17.1798. [DOI] [PubMed] [Google Scholar]
- 6.Wu TC, Hachul D, Scanavacca M, Sosa E. Comparação entre os resultados do teste de inclinação obtidos em diferentes períodos do dia. Arq Bras Cardiol. 2002;79(4):385–394. doi: 10.1590/s0066-782x2002001300006. [DOI] [PubMed] [Google Scholar]
- 7.Hachul D, Sosa E, Consolim F, Magalhães L, Scanavacca M, Martinelli M, et al. Reproducibility of head-up tilt test in patients with neurocardiogenic syncope. Arq Bras Cardiol. 1994;62(5):297–299. [PubMed] [Google Scholar]
- 8.Wieling W, Van Lieshout JJ, Hainsworth R. Extracellular fluid volume expansion in patients with posturally related syncope. Clin Auton Res. 2002;12(4):242–249. doi: 10.1007/s10286-002-0024-z. [DOI] [PubMed] [Google Scholar]
- 9.Krediet CTP, Go-Schön IK, Van Lieshout JJ, Wieling W. Optimizing squatting as a physical maneuver to prevent vasovagal syncope. Clin Auton Res. 2008;18(4):179–186. doi: 10.1007/s10286-008-0481-0. [DOI] [PubMed] [Google Scholar]
- 10.Braunwald E. Tratado de medicina cardiovascular. 5a ed. São Paulo: Editora Roca; 1999. [Google Scholar]
- 11.Parry SW, Kenny RA. The management of vasovagal syncope. QJM. 1999;92(12):697–705. doi: 10.1093/qjmed/92.12.697. [DOI] [PubMed] [Google Scholar]
- 12.Mercader MA, Varghese PJ, Potolicchio SJ, Venkatraman GK, Lee SW. New insights into the mechanism of neurally mediated syncope. Heart. 2002;88(3):217–221. doi: 10.1136/heart.88.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hainsworth R. Syncope: what is the trigger? Heart. 2003;89(2):123–124. doi: 10.1136/heart.89.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madrid AH, Ortega J, Rebollo JG, Manzano JG, Segovia JG, Sánchez A, et al. Lack of efficacy of atenolol for the prevention of neurally mediated syncope in a highly symptomatic population: a prospective, double-blind, randomized and placebo-controlled study. J Am Coll Cardiol. 2001;37(2):544–549. doi: 10.1016/s0735-1097(00)01155-4. [DOI] [PubMed] [Google Scholar]
- 15.Sheldon R, Connolly S, Rose S, Klingenheben T, Krahn A, Morillo C, et al. Prevention of syncope trial (POST): a randomized, placebo-controlled study of metoprolol in the prevention of vasovagal syncope. Circulation. 2006;113(9):1164–1170. doi: 10.1161/CIRCULATIONAHA.105.535161. [DOI] [PubMed] [Google Scholar]
- 16.Raj SR, Rose S, Ritchie D, Sheldon RS. The second prevention of syncope trial (POST II) - a randomized clinical trial of fludrocortisone for the prevention of neurally mediated syncope: rationale and study design. Am Heart J. 2006;151(6):1186. e11-7. doi: 10.1016/j.ahj.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Abe H, Kohshi K, Nakashima Y. Home orthostatic self-training in neurocardiogenic syncope. Pacing Clin Electrophysiol. 2005;28(Suppl 1):S246–S248. doi: 10.1111/j.1540-8159.2005.00023.x. [DOI] [PubMed] [Google Scholar]
- 18.Ector H, Reybrouck T, Heidbuchel H, Gewillig M, Van De Werf F. Tilt training: a new treatment for recurrent neurocardiogenic syncope and severe orthostatic intolerance. Pt 2Pacing Clin Electrophysiol. 1998;21(1):193–196. doi: 10.1111/j.1540-8159.1998.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 19.Foglia-Manzillo G, Giada F, Gagglioli G, Bartoletti A, Lolli G, Dinelli M, et al. Efficacy of tilt training in the treatment of neurally mediated syncope: a randomized study. Europace. 2004;6(3):199–204. doi: 10.1016/j.eupc.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Mtinangi BL, Hainsworth R. Effects of moderate exercise training on plasma volume, baroreceptor sensitivity and orthostatic tolerance in healthy subjects. Exp Physiol. 1999;84(1):121–130. doi: 10.1111/j.1469-445x.1999.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 21.Carrol JF, Wood CE, Pollock MI, Graves JE, Convertino VA, Lowenthal DT. Hormonal responses in elders experiencing pre syncopal symptoms during head-up tilt before and after exercise training. J Gerontol A Biol Sci Med Sci. 1995;50(6):M324–M329. doi: 10.1093/gerona/50a.6.m324. [DOI] [PubMed] [Google Scholar]
- 22.Convertino VA, Montgomery LD, Greenleaf JE. Cardiovascular responses during orthostasis: effect of an increase in VO2max. Aviat Space Environ Med. 1984;55(8):702–708. [PubMed] [Google Scholar]
- 23.Gava NS, Véras-Silva AS, Negrão CE, Krieger EM. Low intensity exercise training attenuates cardiac beta adrenergic tone during exercise in spontaneously hypertensive rats. Pt 2Hypertension. 1995;26(6):1129–1133. doi: 10.1161/01.hyp.26.6.1129. [DOI] [PubMed] [Google Scholar]
- 24.Gardenghi G, Rondon MU, Braga AM, Scanavacca MI, Negrão CE, Sosa E, et al. The effects of exercise training on arterial baroreflex sensitivity in neurally mediated syncope patients. Eur Hear J. 2007;28(22):2749–2755. doi: 10.1093/eurheartj/ehm208. [DOI] [PubMed] [Google Scholar]
- 25.Brum PC, Da Silva GJ, Moreira ED, Ida F, Negrão CE, Krieger EM. Exercise training increases baroreceptor gain activity in normal and hypertensive rats. Hypertension. 2000;36(6):1018–1022. doi: 10.1161/01.hyp.36.6.1018. [DOI] [PubMed] [Google Scholar]
- 26.Wasserman K, Hansen JE, Sue D, Whipp BJ, Casaburi R. Principles of exercise testing and interpretation. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 27.Montgomery DC. Design and analysis of experiments. 5th ed. New York: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 28.Pagano M, Gavreau K. Princípios de bioestatística. 2a ed. São Paulo: Pioneira; 2004. [Google Scholar]
- 29.Coelho-Barros EA, Achcar J, Martinez E, Aragon DC, Pinho EM, Marroni S, et al. Bayesian analysis for Poisson longitudinal data. Rev Mat Estat. 2005;24(3):95–114. [Google Scholar]
- 30.Soteriades ES, Evans JC, Larson MG, Chen MH, Chen L, Benjamin EJ, et al. Incidence and prognosis of syncope. N Engl J Med. 2002;12(12):878–885. doi: 10.1056/NEJMoa012407. [DOI] [PubMed] [Google Scholar]
- 31.Bastos S, Scanavacca M, Darrieux F, Ludovice AC, Sosa E, Hachul DT. Evolução clínica de pacientes com síncope neurocardiogênica após suspensão da terapia específica. Arq Bras Cardiol. 2006;86(4):256–260. doi: 10.1590/s0066-782x2006000400004. [DOI] [PubMed] [Google Scholar]
- 32.Lippman N, Stein KM, Lerman BB. Comparison of methods for the removal of ectopic in the measurements of heart rate variability. Pt 2Am J Physiol. 1994;267(1):H411–H418. doi: 10.1152/ajpheart.1994.267.1.H411. [DOI] [PubMed] [Google Scholar]
- 33.Mitnangi BL, Hainsworth R. Increased orthostatic tolerance following moderate exercise training in patients with unexplained syncope. Heart. 1998;80(6):596–600. doi: 10.1136/hrt.80.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wieling W, Colman N, Krediet CTP, Freeman R. Nonpharmacological treatment of reflex syncope. Clin Auton Res. 2004;14(Suppl 1):62–70. doi: 10.1007/s10286-004-1009-x. [DOI] [PubMed] [Google Scholar]
- 35.Allen SC, Taylor CG, Hall VE. A study of orthostatic insufficiency by the tiltboard method. Am J Physiol. 1945;143:11–20. [Google Scholar]
- 36.Brandão MU, Wajngarten M, Rondon E, Giorgi MC, Hironaka F, Negrão CE. Left ventricular function during dynamic exercise in untrained and moderately trained subjects. J Appl Physiol. 1993;75(5):1989–1995. doi: 10.1152/jappl.1993.75.5.1989. [DOI] [PubMed] [Google Scholar]
- 37.Wieling W, Van Lieshout JJ, Hainsworth R. Extracellular fluid volume expansion in patients with posturally related syncope. Clin Auton Res. 2002;12(4):242–249. doi: 10.1007/s10286-002-0024-z. [DOI] [PubMed] [Google Scholar]
- 38.Negrão CE, Irigoyen MC, Moreira ED, Brum PC, Freire PM, Krieger EM. Effect of exercise training on RSNA, baroreflex control, and blood pressure responsiveness. Pt 2Am J Physiol. 1993;265(2):R365–R370. doi: 10.1152/ajpregu.1993.265.2.R365. [DOI] [PubMed] [Google Scholar]
- 39.Krieger EM, Da Silva GJ, Negrão CE. Effects of exercise training on baroreflex control of the cardiovascular system. Ann N Y Acad Sci. 2001;940:338–347. doi: 10.1111/j.1749-6632.2001.tb03689.x. [DOI] [PubMed] [Google Scholar]