Abstract
Background
Stress and ethanol are both, independently, important cardiovascular risk factors.
Objective
To evaluate the cardiovascular risk of ethanol consumption and stress exposure, isolated and in association, in male adult rats.
Methods
Rats were separated into 4 groups: Control, ethanol (20% in drinking water for 6 weeks), stress (immobilization 1h day/5 days a week for 6 weeks) and stress/ethanol. Concentration-responses curves to noradrenaline - in the absence and presence of yohimbine, L-NAME or indomethacin - or to phenylephrine were determined in thoracic aortas with and without endothelium. EC50 and maximum response (n=8-12) were compared using two-way ANOVA/Bonferroni method.
Results
Either stress or stress in association with ethanol consumption increased the noradrenaline maximum responses in intact aortas. This hyper-reactivity was eliminated by endothelium removal or by the presence of either indomethacin or yohimbine, but was not altered by the presence of L-NAME. Meanwhile, ethanol consumption did not alter the reactivity to noradrenaline. The phenylephrine responses in aortas both with and without endothelium also remained unaffected regardless of protocol.
Conclusion
Chronic stress increased rat aortic responses to noradrenaline. This effect is dependent upon the vascular endothelium and involves the release of vasoconstrictor prostanoids via stimulation of endothelial alpha-2 adrenoceptors. Moreover, chronic ethanol consumption appeared to neither influence noradrenaline responses in rat thoracic aorta, nor did it modify the increase of such responses observed as a consequence of stress exposure.
Keywords: Stress, Alcohol Drinking, Norepinephrine, Adrenergic alpha-2 Receptor Agonists, Rats
Introduction
Chronic stress is an important risk factor for the development of cardiovascular pathologies1 such as atherosclerosis2 endothelial dysfunction3 and hypertension4. However, the severity of the cardiovascular damage caused by stress exposure depends on the nature of the stressor, as well as its intensity and duration5.
The literature reports that chronic stress impairs endothelial function, evidenced by a decrease of acetylcholine-induced vasorelaxation2, as well as a reduction in aortic nitric oxide synthase (NOS) activity6. In turn, acute stress exposure reduces noradrenaline-induced contraction and increases acetylcholine-induced relaxation of rat aorta1,7.
Chronic ethanol consumption may also cause cardiocirculatory modifications that contribute to the development of cardiovascular diseases8,9. In line with this possible association, the consumption of 20% ethanol over the course of 6 weeks increased the mesenteric vascular bed response to phenylephrine in Wistar rats8. Ethanol consumption also increased responses to sympathetic agonists within vessels of conductance - vessels of which endothelial mechanisms of modulation work differently from those of resistance vessels. In fact, consumption of 20% ethanol for 6 weeks increased the aorta responses to phenylephrine in Wistar rats9. The consumption of 36% ethanol over 18 weeks also elevated the noradrenaline responses in rat mesenteric artery10. Moreover, the chronic consumption of 10% ethanol increased noradrenaline response in the aorta of male Sprague-Dawley11 rats. On the other hand, the chronic consumption of 20% ethanol either decreased12 or did not influence13 phenylephrine responses in rat aortas. These discrepancies may be attributed to differences between experimental protocols or characteristics of the animal model14.
Although both ethanol and stress play important roles in the development of cardiovascular disease, doubts still persist about their effects upon vascular responsiveness, specifically. These doubts are even more numerous when considering the effects of both of these factors in association. Thus, the goal of this study was to address these persisting questions. Specifically, the aim of this study was to investigate, in adult rats, the vascular adaptive response to sympathetic agonists induced by ethanol consumption and exposure to stress (both alone and in association) and to examine its mechanism.
Methods
Experimental design
Adult male Wistar rats (100 to 120 days old) were housed in plastic cages under a 12 hour light-dark cycle at (23±2ºC) and fed regular lab chow. For the entirety of the 6 weeks, these animals were separated into four groups: control, which received water "ad libitum"; stress, which were immobilized in a metallic containment tube (1 hour/day, 5 days/week) to completely restrict their movements, preserving only breathing; ethanol, which received a 20% ethanol solution instead of drinking water15; stress/ethanol, which was submitted to immobilization stress and received a 20% ethanol solution. Animals from the ethanol and stress/ethanol groups were adapted to ethanol consumption by gradually increasing the concentration of ethanol in their solution (5% in the first week, 10% in the second week and 20% from the third week onward).
All experiments and procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals, published by the U.S. National Institutes of Health16 and were approved by the Research Ethics Committee of the Biosciences Institute of Botucatu, Universidade Estadual Paulista.
Blood ethanol
Blood (5 mL per rat) was collected from the aorta of anesthetized rats using heparinized syringes. Samples (1 mL) were placed in 10 mL headspace vials, to which were added - 1.0 g sodium chloride, 1.0 mL water, and 100 mL internal standard (acetonitrile, 1 mL L−1). Ethanol analysis was carried out using a CG-17A gas chromatographer (Shimadzu, Kyoto, Japan) equipped with a flame-ionization detector and an HSS-4A headspace sampler (Shimadzu). Calibration standards (0.10-3.16 mg mL−1) were prepared in headspace vials. Ethanol concentrations are expressed in mg mL-1 blood.
Aorta rings
Animals were killed using a guillotine device. After thoracotomy, the descending thoracic aortas were removed and dissected in 3-4 mm segments (two rings). The rings were mounted into a 2-mL organ chamber containing Krebs-Henseleit solution (NaCl 130; KCl 4.7; CaCl2 1.6; KH2PO4 1.2; MgSO4 1.2; NaHCO3 15; glucose 11.1; in mmol/L). In the organ chamber, rings were fixed to a stainless-steel hook that was attached to a stationary support, as well as to a second hook, which was connected to an isometric force transducer. The Krebs-Henseleit solution was kept at pH 7.4 and 37 °C and bubbled continuously with a mixture of 95% O2 and 5% CO2. Tension was monitored continuously and recorded using a Powerlab 8/30 data-acquisition system (ADInstruments, Castle Hill, NSW, Australia). Prior to the concentration-response curve to agonists, the rings were equilibrated for 60 min under a resting tension of 1.5 g, which is optimal for inducing maximum contraction.
The functional state of the endothelium was tested at the beginning of the concentration-response curve by the ability of 10-4M acetylcholine to elicit vasodilator responses in preparations pre-contracted by 10-5M phenylephrine. Preparations that showed more than 80% of relaxation were considered as having an intact endothelium, whereas those that showed no relaxation were considered completely devoid of endothelium. Some rings were submitted to mechanical endothelium denudation. Preparations with and without endothelium were studied in parallel.
Organ bath assay
Noradrenaline (10-10 M to 10-4 M; Sigma-Aldrich) or phenylephrine (10-10 M to 10-4 M; Sigma-Aldrich) were cumulatively added into the organ bath, and the evoked responses (g) were plotted to obtain a concentration-response curve. When appropriate, the noradrenaline responses were also determined in preparations pretreated for 30 min with 10-4M L-NAME (Sigma-Aldrich) or 10-5M indomethacin (Sigma-Aldrich) - non-selective inhibitors of NOS and cyclooxygenase (COX), respectively. In another series of experiments, noradrenaline responses were determined in rings pretreated for 30 min with 10-6 M yohimbine (Sigma-Aldrich), and α2-adrenoceptor antagonist. The inhibitors and the antagonist were added during the last 30 min stabilization period and remained in contact with preparations until the end of the experiment.
Non-linear regression (variable slope) of the obtained concentration-effect curves revealed the Rmax (maximal response; highest point of each concentration-response curve) and the EC50 (negative logarithm of the concentration that evoked 50% of the maximal response). The EC50 is indicative of sensitivity to the drug studied.
Determination of nitrite/nitrate (NO2/NO3)
100 µL aliquots of plasma samples from different experimental groups were deproteinated with 200 µL absolute ethanol, in the freezer (-20˚ C) for 30 minutes. They were then submitted to centrifugation (10000 rpm for 5 minutes) and the supernatant was collected. In order to determine total (NO2/NO3) in plasma, the following mixture was used as a reaction medium: sodium phosphate buffer 20 mM pH 7.4, cofactors (final concentration 100μM NADPH and FAD 5 mM) and nitrate reductase of Aspergillus (Sigma) at a concentration of 0.1 U/mL. The samples were then incubated for 3 hours in a water bath at 37° C before being added to Griess Reagent I (1% sulfanilamide in 5% phosphoric acid) and Griess Reagent II (naftil-ethylenediamine 0.1%). Next, the compound's chromophore was read using a spectrophotometer at 540 nm. The concentration of the samples were calculated using a standard curve with known concentrations of NaNO2 (0.40 - 200 μl) and expressed in mM/L.
Statistical analysis
The concentration of vasoactive agents producing a response that was 50% of the maximum (EC50) was calculated in each experiment. Data are presented as mean ± SEM. The maximal responses (Rmax) and EC50 values were compared by two-way ANOVA followed by Bonferroni's post-test, as one variable was ethanol consumption and the other, exposure to immobilization stress. P < 0.05 was considered statistically significant.
Results
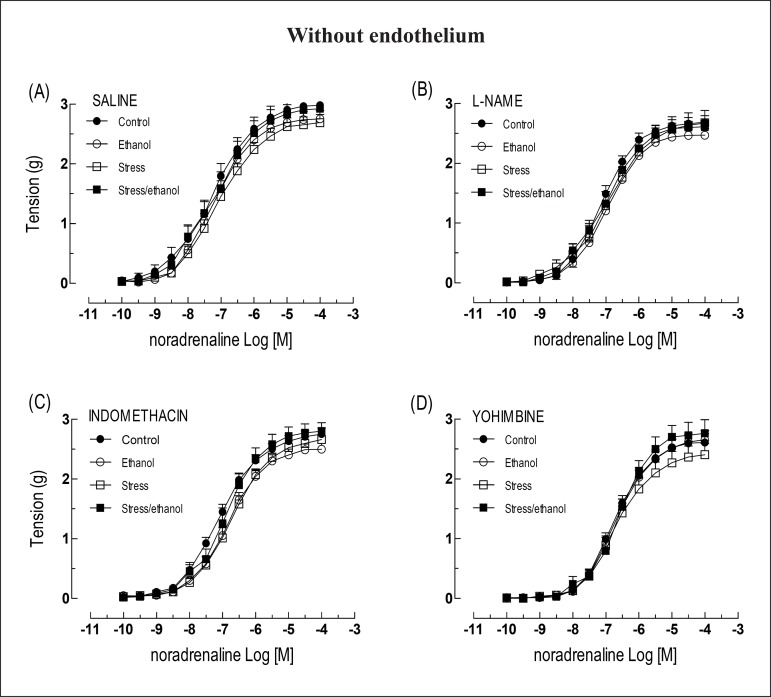
The noradrenaline responses observed in intact aortas taken from animals exposed to both stress and stress/ethanol in combination, were higher than those observed in intact aortas taken from control animals. These outcomes resulted in increased values of Rmax. Animals exposed to ethanol alone, however, did not demonstrate higher responses than controls (Figure 1A). The presence of L-NAME increased the noradrenaline Rmax in aortic rings taken from control and ethanol groups, permitting responses of similar magnitude with preparations taken from stress and stress/ethanol groups (Figures 1B). Inversely, indomethacin abolished the elevation of noradrenaline responses in aortas from stress and stress/ethanol groups, thereby resulting in values of Rmax at the level of the control group (Figure 1C). Similarly, yohimbine also abolished the hyper-reactivity to noradrenaline in thoracic aorta taken from stress and stress/ethanol animals (Figure 1D). Finally, regarding EC50, no differences were detected among groups regardless of absence or presence of L-NAME, indomethacin or yohimbine (Table 1).
Figure 1.
Concentration-response curves to noradrenaline obtained from intact thoracic aorta rings taken from animals exposed to ethanol consumption and/or stress, in the absence or presence of L-NAME (10-4 M), indomethacin (10-5 M) or yohimbine (10-6 M). Values are expressed as means ± SEM. The number of independent determinations was 8-10. *Indicates a significant difference (p < 0.05) in relation to the control animals.
Table 1.
Values of EC50 to noradrenaline obtained from aortic rings with and without endothelium, in presence of absence of L-NAME, indomethacin or yohimbine from adult male rats exposed or not to chronic stress and ethanol, alone or in combination
| Control EC50 (x10-7 M) | Stress EC50 (x10-7 M) | Ethanol EC50 (x10-7 M) | Stress/ethanol EC50 (x10-7 M) | |
|---|---|---|---|---|
| With endothelium | ||||
| Saline | 3.97± 0.06 (10) | 3.85 ± 0.04 (10) | 4.88 ± 0.06 (10) | 4.29 ± 0.08 (10) |
| L-NAME (10-4 M) | 2.48 ± 0.05 (09) | 2.36 ± 0.05 (09) | 2.60 ± 0.04 (09) | 2.64 ± 0.08 (09) |
| Indomethacin (10-5 M) | 3.85 ± 0.05 (08) | 3.56 ± 0.06 (08) | 5.39 ± 0.05 (08) | 3.58 ± 0.06 (08) |
| Yohimbine (10-6 M) | 10.76 ± 0.06 (09) | 10.19 ± 0.06 (09) | 10.23 ± 0.04 (09) | 9.00 ± 0.06 (09) |
| Without endothelium | ||||
| Saline | 0.61 ± 0.10* (10) | 0.83 ± 0.07* (10) | 0.64 ± 0.06 (10) | 0.67 ± 0.09* (10) |
| L-NAME (10-4 M) | 0.77 ± 0.06* (09) | 1.18 ± 0.11* (09) | 1.10 ± 0.07* (09) | 0.90 ± 0.06* (09) |
| Indomethacin (10-5 M) | 0.83 ± 0.05* (08) | 1.88 ± 0.06* (08) | 1.47 ± 0.05* (08) | 1.30 ± 0.08* (08) |
| Yohimbine (10-6 M) | 1.82 ± 0.05* (09) | 1.94 ± 0.08* (09) | 2.10 ± 0.05* (09) | 2.75 ± 0.07* (09) |
Control: received water “ad libitum”; ethanol: received ethanol solution 20% instead of drinking water; stress: immobilization in a metallic containment tube (1 hour/day, 5 days/week for 6 weeks); chronic stress/ethanol: submitted to immobilization stress and exposed to 20% ethanol. Data expressed as mean ± SEM and, between parentheses, the number of independent determinati ons.
p < 0.05 in relation to the respective aorta with endothe lium.
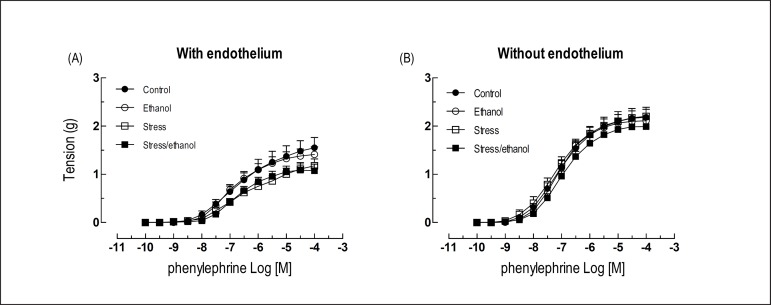
The removal of the endothelium increased the sensitivity to noradrenaline in relation to the respective aorta with intact endothelium (Table 1). Moreover, the removal of the endothelium eliminated the aortic hyper-reactivity to noradrenaline observed in stress and stress/ethanol groups (Figure 2A). None of the protocols altered the reactivity to noradrenaline in denuded aortas (Figures 2B, 2C and 2D), nor did they affect the reactivity to phenylephrine (Figures 3A and 3B) in intact or denuded aortas. Similar phenylephrine EC50 were also observed among the groups (Table 2).
Figure 2.
Concentration-response curves to noradrenaline obtained from denuded thoracic aorta rings taken from animals exposed to ethanol consumption and/or stress, in the absence or presence of L-NAME (10-4 M), indomethacin (10-5 M) or yohimbine (10-6 M). Values are expressed as means ± SEM. The number of independent determinations was 8-10.
Figure 3.
Concentration-response curves to phenylephrine obtained from rings of thoracic aorta with and without endothelium taken from animals exposed to ethanol consumption and/or stress. Values are expressed as means ± SEM. The number of independent determinations was 8-10.
Table 2.
Values of EC50 to phenylephrine obtained from aortic rings with and without endothelium from adult male rats exposed or not to chronic stress or ethanol, alone or in combination
| Control EC50 (x10-7 M) | Stress EC50 (x10-7 M) | Ethanol EC50 (x10-7 M) | Stress/Ehanol EC50 (x10-7 M) | |
|---|---|---|---|---|
| With endothelium | 1.58 ± 0.15 (09) | 2.25 ± 0.12 (09) | 1.14 ± 0.10 (09) | 1.79 ± 0.10 (09) |
| Without endothelium | 0.88 ± 0.09* (09) | 0.67 ± 0.08* (09) | 0.80 ± 0.04* (09) | 1.10 ± 0.07* (09) |
Control: received water “ad libitum”; ethanol: received ethanol solution 20% instead of drinking water; stress: immobilization in a metallic containment tube (1 hour/day, 5 days/week for 6 weeks); chronic stress/ethanol: submitted to immobilization stress and exposed to 20% ethanol. Data expressed as mean ± SEM and, between parentheses, the number of independent determinations.
P < 0.05 in relation to the respective aorta with endothelium.
Blood ethanol concentration reached 0.42 ± 0.09 mg/mL in rats exposed to ethanol for 6 weeks, n=8, while concentrations reached 0.57 ± 0.13 mg/mL in the stress/ethanol group, n = 10.
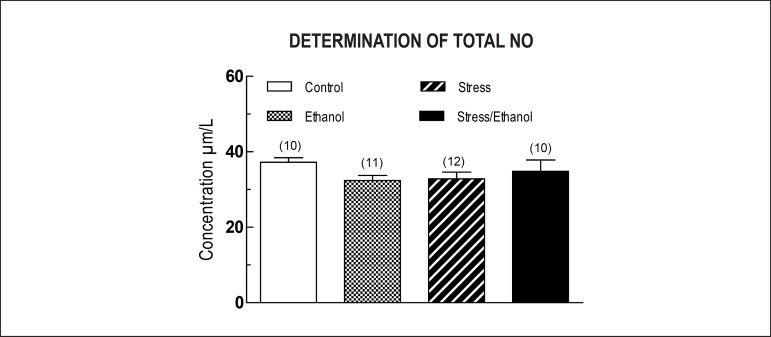
The concentration in plasma of nitrite/nitrate, determined by the Griess Reaction, was not significantly different among groups (Figure 4).
Figure 4.
Plasma concentration of nitrite/nitrate determined by the Griess Reaction taken from animals exposed to ethanol consumption and/or stress. Values are expressed as means ± SEM. The number of independent determinations was 10-12.
Discussion
Experimental and epidemiological evidence suggests that both ethanol and stress play an important role in the development of cardiovascular diseases2,17,18. However, there are discrepancies in the literature about the effects of chronic ethanol consumption, as well as exposure to stress, on the vascular responses to vasoactive agents. Furthermore, the literature is scarce regarding the cardiovascular risks of the association of these factors.
The data reported herein have shown that, regardless of ethanol consumption, stress induced aortic hyper-reactivity to noradrenaline. This response appears to have been dependent upon endothelial cell integrity, as it was abolished by the removal of the endothelium. On the other hand, chronic ethanol consumption did not influence the noradrenaline responses in rat thoracic aorta. Ethanol consumption also showed no signs of influence in the stress-induced noradrenaline hyper-reactivity. Considering that the absence of endothelium potentiates the contractile response to noradrenaline in rats not exposed to stress, the heightened response to noradrenaline observed in intact aorta from stressed rats could indicate an endothelial dysfunction in this condition. These findings corroborate previous reports showing that stress promotes endothelial dysfunction2,3,19,20. However, other studies reported that exposure to stress may actually enhance, rather than impair, endothelial function1,21,22.
Additionally, the presented data corroborate studies showing that the consumption of 20% ethanol for 12 weeks did not alter the phenylephrine responses in aorta of Fisher rats13. On the other hand, it contradicts studies showing increased response to noradrenaline in aorta taken from Sprague-Dawley rats after being exposed to 10% ethanol for 5 months10. Moreover, previous studies have reported increased phenylephrine responses in aorta taken from Wistar rats also exposed to 20% ethanol for 6 weeks8,9,15. Interestingly, although using exactly the same experimental protocol, lower blood ethanol concentrations were determined in the present study. It is possible that these lower blood ethanol concentrations were not sufficient to elicit the aortic responses to norepinephrine or phenylephrine that have been seen in previous research. Indeed, the potential for ethanol-induced modifications to aortic responses to norepinephrine or phenylephrine in rats was not completely discarded by the present findings. Rather, these discrepancies demonstrate the need for complementary studies, in which differences of blood ethanol concentrations should also be assessed.
Many studies have shown that the modulation of vascular tonus may involve endothelium-derived vasoconstrictor and vasodilator prostanoids23. Although the rate of production of COX metabolites in normal cells appears to facilitate vasodilators, it is possible that this rate may vary in response to certain disease states, such as hypertension24.
In order to investigate the involvement of prostanoids in the stress-induced modifications of noradrenaline responses, experiments were performed in aortas pretreated with indomethacin. The presence of this COX inhibitor abolished the increase of the maximum response to noradrenaline observed in intact aorta of stressed rats. After being restored, this response reached a value similar to that observed in rat aorta with endothelium taken from control rats in the absence of indomethacin. These data suggest that the aortic hyper-reactivity to noradrenaline induced by chronic immobilization stress occurs due to the release of vasoconstrictor prostanoids. The release of vasoconstrictor prostanoids has been previously observed in micro and macrovessels of spontaneously hypertensive rats25 and in the aorta of DOCA-salt hypertensive rats23,24.
Once the influence of vasoconstrictor prostanoids in the stress-induced increase of noradrenaline responses in rat aorta is established, it is pertinent to examine "how" this sympathetic agonist is able to activate this endothelial mechanism. Previous studies report that α1 and α2-adrenoceptors are expressed either in smooth muscle or in endothelial cells26-28. Furthermore, the stimulation of α2-adrenoceptors located in the endothelium releases nitric oxide (NO)29,30 and/or prostanoids29 ,) thus counterbalancing the vasoconstrictive effects of noradrenaline mediated by α1-adrenoceptors present in the smooth muscle layer. It has also been shown that thoracic aortic responses to clonidine, a selective α2 adrenoceptor agonist, may be modulated negatively by NO and positively by vasoconstrictive prostanoids31.
In the present study, the presence of yohimbine abolished the stress-induced increase of noradrenaline responses in rat thoracic aorta. Moreover, neither exposure to stress nor ethanol consumption altered the thoracic aorta responses to phenylephrine, a selective α1-adrenoceptor agonist. These data suggest that endothelial α2-adrenoceptor stimulation may release vasoconstrictor prostanoids, thus increasing the noradrenaline responses in aortas from stress-exposed rats.
The literature reports the release of mediators that induce not only contraction, but also relaxation in aorta of DOCA salt hypertensive rats23,24,32. Therefore, it is not yet clear in the present study whether only vasoconstrictor prostanoids are responsible for the increased response to noradrenaline in aorta of stressed animals, or if they act in conjunction with L-arginine/NO mechanisms.
Given this uncertainty, we investigated the role of the L-arginine/NO pathway in the aortic response to noradrenaline in chronically stressed rats (with and without association with ethanol consumption). The results show that the inhibition of NOS by L-NAME increased the noradrenaline Rmax in thoracic aortas taken from animals in both the control and ethanol groups, permitting responses of similar magnitude regarding preparations taken from stress and stress/ethanol groups, in the absence or presence of L-NAME. These data suggest that there is no change in the NO-pathway induced by exposure to stress and chronic ethanol consumption, either alone, or in combination. This hypothesis is also supported by the observation of similar plasma concentrations of nitrite/nitrate among the different experimental groups.
In summary, the present study found that chronic stress increased rat aortic responses to noradrenaline. This effect is dependent upon the vascular endothelium and involves vasoconstrictor prostanoids released by stimulation of endothelial alpha-2 adrenoceptors. Moreover, chronic ethanol consumption appeared to neither influence noradrenaline responses in rat thoracic aorta, nor did it modify the increase of such responses observed in consequence of stress exposure.
Agradecimentos
Esse estudo foi financiado pela CAPES. Agradecemos ao Sr. Alisson Douglas Ventura Neves (Laboratório de Farmacologia da Faculdade de Medicina de Marília, São Paulo, Brasil) pela assistência técnica.
Footnotes
Fontes de Financiamento
O presente estudo foi financiado pelas CAPES
Vinculação Acadêmica
Este artigo é parte de tese de Doutorado de Rafaela de Fátima Ferreira Baptista pela Universidade Estadual Paulista "Júlio de Mesquita Filho" UNESP/Botucatu.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conception and design of the research, Statistical analysis, Obtaining funding, Writing of the manuscript: Baptista RFF, Chies AB, Cordellini S; Acquisition of data: Baptista RFF, Taipeiro EF, Queiroz RHC; Analysis and interpretation of the data: Baptista RFF, Taipeiro EF, Queiroz RHC, Chies AB, Cordellini S; Critical revision of the manuscript for intellectual content: Baptista RFF, Taipeiro EF, Chies AB, Cordellini S.
References
- 1.Cordellini S, Vassilief VS. Decreased endothelium-dependent vasoconstriction to noradrenaline in acute-stressed rats is potentiated by previous chronic stress: nitric oxide involvement. Gen Pharmacol. 1998;30(1):79–83. doi: 10.1016/s0306-3623(97)00074-8. [DOI] [PubMed] [Google Scholar]
- 2.Chung IM, Kim YM, Yoo MH, Shin MK, Kim CK, Suh SH. Immobilization stress induces endothelial dysfunction by oxidative stress via the activation of the angiotensin II/its type I receptor pathway. Atherosclerosis. 2010;213(1):109–114. doi: 10.1016/j.atherosclerosis.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 3.Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, et al. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102(20):2473–2478. doi: 10.1161/01.cir.102.20.2473. [DOI] [PubMed] [Google Scholar]
- 4.Diaconu C, Tartau L, Lupusoru CE. Experimental research on the influence of stress factors in an animal model of hypertension. Rev Med Chir Soc Med Nat Iasi. 2011;115(2):349–353. [PubMed] [Google Scholar]
- 5.Hjemdahl P. Stress and the metabolic syndrome: an interesting but enigmatic association. Circulation. 2002;106(21):2634–2636. doi: 10.1161/01.cir.0000041502.43564.79. [DOI] [PubMed] [Google Scholar]
- 6.Bernatova I, Csizmadiova Z. Effect of chronic social stress on nitric oxide synthesis and vascular function in rats with family history of hypertension. Life Sci. 2006;78(15):1726–1732. doi: 10.1016/j.lfs.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Navarro-Oliveira CM, Vassilieff VS, Cordellini S. The sympathetic adrenomedullary system, but not the hypothalamic-pituitary-adrenal axis, participates in aorta adaptive response to stress: nitric oxide involvement. Auton Nerv System. 2000;83(3):140–147. doi: 10.1016/S1566-0702(00)00157-0. [DOI] [PubMed] [Google Scholar]
- 8.Tirapelli CR, Fukada SY, Yogi A, Chignalia AZ, Tostes RC, Bonaventura D, et al. Gender-specific vascular effects elicited by chronic ethanol consumption in rats: a role for inducible nitric oxide synthase. Br J Pharmacol. 2008;153(3):468–479. doi: 10.1038/sj.bjp.0707589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tirapelli CR, Leone AF, Coelho EB, Resstel LB, Lanchote VL, Uyemura SA, et al. Effect of ethanol consumption on blood pressure and rat mesenteric arterial bed, aorta and carotid responsiveness. J Pharmacol. 2007;59(7):985–993. doi: 10.1211/jpp.59.7.0011. [DOI] [PubMed] [Google Scholar]
- 10.Hatton DC, Bukoski RD, Edgar S, Mccarron DA. Chronic alcohol consumption lowers blood pressure but enhances vascular contractility in Wistar rats. J Hypertens. 1992;(6):529–537. doi: 10.1097/00004872-199206000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Lapido CO, Adigun SA, Nwaigwe CI, Adegunloye BJ. Chronic ethanol consumption alters vascular smooth muscle responses in rats. Clin Exp Pharmacol Physiol. 2002;29(8):707–709. doi: 10.1046/j.1440-1681.2002.03721.x. [DOI] [PubMed] [Google Scholar]
- 12.Strickland JA, Wooles WR. Effect of acute and chronic ethanol on the agonist responses of vascular smooth muscle. Eur J Pharmacol. 1988;152(1-2):83–91. doi: 10.1016/0014-2999(88)90838-2. [DOI] [PubMed] [Google Scholar]
- 13.Husain K, Vazquez M, Ansari RA, Malafa MP, Lalla J. Chronic alcohol-induced oxidative endothelial injury relates to angiotensin II levels in the rat. Mol Cell Biochem. 2008;307(1-2):51–58. doi: 10.1007/s11010-007-9583-6. [DOI] [PubMed] [Google Scholar]
- 14.Utkan T, Yildiz F, Ilbay G, Ozdemirci S, Erden BF, Gacar N, Ulak G. Blood pressure and vascular reactivity to endothelin-1, phenylephrine, serotonin, KCI and acetylcholine following chronic alcohol consumption in vitro. Fundam Clin Pharmacol. 2001;15(3):157–165. doi: 10.1046/j.1472-8206.2001.00024.x. [DOI] [PubMed] [Google Scholar]
- 15.Tirapelli CR, Al-khoury J, Bkaily G, D'Orléans-juste P, Lanchote VL, Uyemura SA, et al. Chronic ethanol consumption enhances phenylephrine-induced contraction in the isolated rat aorta. J Exp Pharmacol Ther. 2006;316(1):233–241. doi: 10.1124/jpet.105.092999. [DOI] [PubMed] [Google Scholar]
- 16.Guide for the Care and Use of Laboratory Animals. Washington (DC): Institute of Laboratory Animal Resources. National Academy of Sciences; 1996. [Google Scholar]
- 17.Toda N, Ayajiki K. Vascular actions of nitric oxide as affected by exposure to alcohol. Alcohol Alcohol. 2010;45(4):347–355. doi: 10.1093/alcalc/agq028. [DOI] [PubMed] [Google Scholar]
- 18.Husain K, Ferder L, Ansari RA, Lalla J. Chronic ethanol ingestion induces aortic inflammation/oxidative endothelial injury and hypertension in rats. Hum Exp Toxicol. 2010;30(8):930–939. doi: 10.1177/0960327110384520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okruhlicová L, Dlugosová K, Mitasíková M, Bernátová I. Ultrastructural characteristics of aortic endothelial cells in borderline hypertensive rats exposed to chronic social stress. Physiol Res. 2008;57(2):31–37. doi: 10.33549/physiolres.931549. [DOI] [PubMed] [Google Scholar]
- 20.Loria AS, Kang KT, Pollock DM, Pollock JS. Early life stress enhances angiotensin II-mediated vasoconstriction by reduced endothelial nitric oxide buffering capacity. Hypertension. 2011;58(4):619–626. doi: 10.1161/HYPERTENSIONAHA.110.168674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milakofsky L, Harris N, Vogel WH. Effects of repeated stress on plasma arginine levels in young and old rats. Physiol Behav. 1993;54(4):725–728. doi: 10.1016/0031-9384(93)90083-r. [DOI] [PubMed] [Google Scholar]
- 22.Júnior UL, Cordellini S. Differential vascular adaptive response to stress exposure in male and female rats: role of gonadal hormones and endothelial cells. Stress. 2007;10(1):27–36. doi: 10.1080/10253890601135426. [DOI] [PubMed] [Google Scholar]
- 23.Cordellini S. Endothelial dysfunction in DOCA-salt hypertension: possible involvement of prostaglandin endoperoxides. Gen Pharmacol. 1999;32(3):315–320. doi: 10.1016/s0306-3623(98)00188-8. [DOI] [PubMed] [Google Scholar]
- 24.Cordellini S, Carvalho MH, Scivoletto R, Fortes ZB, Nigro D. Indirect evidence for an endothelium-derived contracting factor release in aorta of deoxycorticosterone acetate-sah hypertensive rats. J Hypertens. 1990;8(1):53–60. doi: 10.1097/00004872-199001000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Takase H, Dohi Y, Kojima M, Sato K. Changes in the endothelial cyclooxygenase pathway in resistance arteries of spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1994;23(2):326–330. [PubMed] [Google Scholar]
- 26.Ruffolo RR, Jr, Waddell JE, Yaden EL. Postsynaptic alpha adrenergic receptor subtypes differentiated by yohimbine in tissues from the rat. Existence of alpha-2 adrenergic receptors in rat aorta. J Pharmacol Exp Ther. 1981;217(2):235–240. [PubMed] [Google Scholar]
- 27.Ohyanagi M, Nishigaki K, Faber JE. Interaction between microvascular alpha 1- and alpha 2-adrenoceptors and endothelium-derived relaxing factor. Circ Res. 1992;(1):188–200. doi: 10.1161/01.res.71.1.188. [DOI] [PubMed] [Google Scholar]
- 28.Moina MJ, Bardan B, Campos Toimil M, Alzueta AF, Gil-Longo J, Orallo F. Effects of hydralazine on contractile responses to alpha 1 and alpha 2-adrenoceptor agonists in isolated rubbed rat aorta. Gen Pharmacol. 1994;25(1):165–172. doi: 10.1016/0306-3623(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 29.Ferrer M, Osol G. Estrogen replacement modulates resistance artery smooth muscle and endothelial alpha2-adrenoceptor reactivity. Endothelium. 1998;6(2):133–141. doi: 10.3109/10623329809072200. [DOI] [PubMed] [Google Scholar]
- 30.Tschudi M, Richard V, Bühler FR, Lüscher TF. Importance of endothelium-derived nitric oxide in porcine coronary resistance arteries. Pt 2Am J Physiol. 1991;260(1):H13–H20. doi: 10.1152/ajpheart.1991.260.1.H13. [DOI] [PubMed] [Google Scholar]
- 31.Tejera N, Balfagón G, Marín J, Ferrer M. Gender differences in the endothelial regulation of alpha2-adrenoceptor-mediated contraction in the rat aorta. Clin Sci (Lond) 1999;97(1):19–25. [PubMed] [Google Scholar]
- 32.Van der Voode J, Leusen I. Endothelium-dependent and independent relaxation of aortic ring from hypertensive rats. Pt 2Am J Physiol. 1986;250(5):H711–H717. doi: 10.1152/ajpheart.1986.250.5.H711. [DOI] [PubMed] [Google Scholar]