Abstract
Background
Obesity is a risk factor for many medical complications; medical research has shown that hemodynamic, morphological and functional abnormalities are correlated with the duration and severity of obesity.
Objective
Present study determined the influence of term of exposure to high-fat diet-induced obesity on myocardial collagen type I and III.
Methods
Thirty-day-old male Wistar rats were randomly distributed into two groups: a control (C) group fed a standard rat chow and an obese (Ob) group alternately fed one of four palatable high-fat diets. Each diet was changed daily, and the rats were maintained on their respective diets for 15 (C15 and Ob15) and 30 (C30 and Ob30) consecutive weeks. Obesity was determined by adiposity index.
Results
The Ob15 group was similar to the C15 group regarding the expression of myocardial collagen type I; however, expression in the Ob30 group was less than C30 group. The time of exposure to obesity was associated with a reduction in collagen type I in Ob30 when compared with Ob15. Obesity did not affect collagen type III expression.
Conclusion
This study showed that the time of exposure to obesity for 30 weeks induced by unsaturated high-fat diet caused a reduction in myocardial collagen type I expression in the obese rats. However, no effect was seen on myocardial collagen type III expression.
Keywords: Obesity, High-fat diet, Collagen Type I, Collagen Type III, Myocardium
Introduction
Obesity is a chronic metabolic disorder characterized by excessive accumulation of adipose tissue in relation to lean tissue. Currently, it is a global epidemic and a major public health problem that affects developed as well as developing countries1,2. Behaviors associated with a modern industrialized society, including a sedentary lifestyle, inadequate eating habits or a combination of both, have led to an increasing prevalence of obesity3.
Obesity is also considered a risk factor for many medical complications, among them cardiovascular diseases4,5. Hemodynamic changes associated with hormonal ones alter myocardial gene expression, promoting myocardial extracellular matrix remodeling6. Studies using either rabbits made obese by a high-fat diet7 or by genetic engineering, Zucker rats8, have reported an increase in myocardial collagen types I and III over 12 and 24 weeks, respectively. In contrast, Carroll et al9, found no change in the fraction of total collagen in obese rats subjected to a high-fat diet for 12 weeks. Previous studies carried out in our laboratory showed that Wistar rats made obese by a 15-week high fat diet10 and Wistar-Kyoto rats made obese by 20-week high fat and carbohydrate diet11 showed increased myocardial total collagen. In these studies, however, the fractions of collagen types I and III were not evaluated.
Medical research has shown that hemodynamic, morphological and functional abnormalities are correlated with the duration and severity of obesity12-14. Because of the scarcity of studies that have evaluated the influence of time of exposure to high fat diet-induced obesity on the fractions of myocardial collagen types I and III, this current study was designed to test the hypothesis that the time of exposure to obesity promotes a progressive increase in the amount of type I and type III myocardial collagen.
Methods
Animals and Experimental Protocol
After a 7-day period for acclimatization, 30-day-old male Wistar rats were randomly assigned to one of two groups: control (C) and obese (Ob). The C group was fed a standard rat chow (RC Focus 1765, Agroceres®, Rio Claro, SP, Brazil) containing 12.3% of kilocalories from fat, 57.9% from carbohydrates, and 29.8% from protein, whereas the Ob group were fed one of four alternating high-fat diets (RC Focus 2413, 2414, 2415, and 2416, Agroceres®, Rio Claro, SP, Brazil) containing 49.2% of kilocalories from fat, 28.9% from carbohydrates, and 21.9% from protein. Each diet was changed daily, and the rats were maintained on their respective diets for 15 (C15 and Ob15; n = 22) and 30 (C30 and Ob30; n = 25) consecutive weeks. The high-fat diet was calorically rich compared to the standard diet (3.65 kcal/g vs. 2.95 kcal/g) due to the higher fat composition. The high-fat diet consisted of saturated and unsaturated fatty acids, which provided 20% and 80% of the fat-derived calories, respectively.
Rats were housed in individual cages in an environmentally-controlled clean-air room at 23 (± 3)ºC with a 12-hour light/dark cycle and 60 (± 5)% relative humidity. All experiments and procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals, published by the National Research Council (1996) and were approved by the Faculdade de Medicina de Botucatu Ethics Committee (UNESP, Botucatu, SP, Brazil).
Nutritional, metabolic and endocrine profiles
Nutritional and metabolic profiles included adiposity index, final body weight (FBW), and glucose tolerance; endocrine profiles included leptin and insulin concentrations. As obesity is defined as an excessive amount of body fat in relation to lean mass15, a criterion based on the adiposity index was used to determine obesity, according to data from earlier studies9,16.
After fasting for 12 to 15 hours, animals were anesthetized (using intraperitoneal sodium pentobarbital 50 mg/kg), decapitated, and thoracotomized; the fat pads of adipose tissue were dissected and weighed. The adiposity index was calculated by the following formula: adiposity index = (body fat [BF]/FBW) × 10017. Body fat was calculated as the sum the weight of the individual fat pads as follows: BF = epididymal fat + retroperitoneal fat + visceral fat.
As obesity can be accompanied by metabolic and endocrine disturbances18, all animals underwent testing for glucose tolerance, leptinemia and insulinemia. After 15 and 30 weeks of treatment, glucose tolerance and insulin resistance were evaluated in all animals through the glucose tolerance test (GTT). After a 4-to 6-hour fast, a blood sample was taken from the tip of the animal's tail and collected in a heparinized tube. The blood glucose (as the basal condition) concentration of each animal was immediately determined using a handheld glucometer (Accuchek Advantage; Roche Diagnostics Co., Indianapolis, IN, USA). Subsequently, 2 g/kg of glucose (Sigma-Aldrich®, St Louis, MO, USA) was given intravenously and blood glucose concentrations were measured after 15, 30, 60, 90, and 120 minutes19. Glucose intolerance was evaluated using the area under the curve (AUC) for glucose.
For hormonal analysis, trunk blood was collected in heparinized tubes and centrifuged at 3000 g for 15 minutes at 4ºC. Serum leptin and insulin concentrations were determined by enzyme-linked immunosorbent assay (ELISA) using commercially available kits (Linco Research Inc., St. Louis, MO, USA).
Cardiovascular profile
As obesity can be associated with cardiovascular comorbidities, the cardiovascular profile of the animals was also assessed, using systolic blood pressure, cardiac tissue morphology and left ventricular collagen types I and III protein expression.
Systolic blood pressure
At the end of the experiment, the systolic blood pressure was assessed using the non-invasive tail-cuff method with a Narco BioSystems®Electro-Sphygmomanometer (International Biomedical, Austin, TX, USA)20. The average of two readings was recorded for each measurement.
Morphological studies
The heart was removed and dissected at the time of euthanasia. LV weights, as well as their respective ratios with the tibia were determined as indexes of cardiac remodeling.
Myocardial collagen types I and III protein expression
Left ventricular tissue was analyzed by Western Blot7 to quantify collagen types I and III protein expression. Briefly, ventricles isolated from control (C15 and C30; n = 6 each group) and obese (Ob15 and Ob30; n = 6 each group) rats were frozen with liquid nitrogen and homogenized in a buffer containing 10 mM Tris (pH 7.4), 100 Mm NaCl, 1 mM EDTA, 1 Mm EGTA, 1% Triton X-100, 10% glycerol, 0.1% sodium dodecyl sulfate (SDS), and 0.5% deoxycholate. The homogenate was centrifuged at 4ºC for 20 minutes at 12000 rpm. The supernatant was collected and total protein content was determined by the Bradford Method (Bradford 1976). Samples were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) in polyacrylamide gels (6% or 10% depending on protein molecular weight). After electrophoresis, proteins were electro-transferred to nitrocellulose membrane (BioRad Biosciences; NJ, USC). Sample weights (50 µg) and transfers were monitored for equality and efficiency, respectively, with the use of 0.5% Ponceau S staining of the blot membrane. The blotted membrane was then blocked (using 5% nonfat dry milk, 10 mmol/L Tris-HCl [pH 7.6], 150 mmol/L NaCl, and 0.1% Tween 20) for 2 hours at room temperature and incubated with specific antibodies overnight at 4ºC. Binding of the primary antibody was detected with the use of peroxidase-conjugated secondary antibodies (anti-rabbit or anti-mouse depending on the protein at a 1:10000 dilution and incubated for 1.5 hours at room temperature), developed by enhanced chemiluminescence (Amersham Biosciences, NJ, USA) and detected by autoradiography. Quantification analysis of the blots was performed with use of Scion Image software (Scion, based on NIH Image). Mouse monoclonal antibodies to collagen types I and III (1:10000) and mouse monoclonal antibodies to β-actin (1:1000) were obtained from Abcam (UK, Cambridge) and Santa Cruz Biotechnology (CA, USA), respectively. Targeted bands were normalized to the expression of cardiac β-actin.
Statistical analysis
All results were reported as mean ± standard deviation and groups were evaluated using two-way analysis of variance (ANOVA) for independent samples. When significant differences were found (p < 0.05), Bonferroni post hoc test for multiple comparisons was carried out21. The level of significance considered was 5% (α = 0.05).
Results
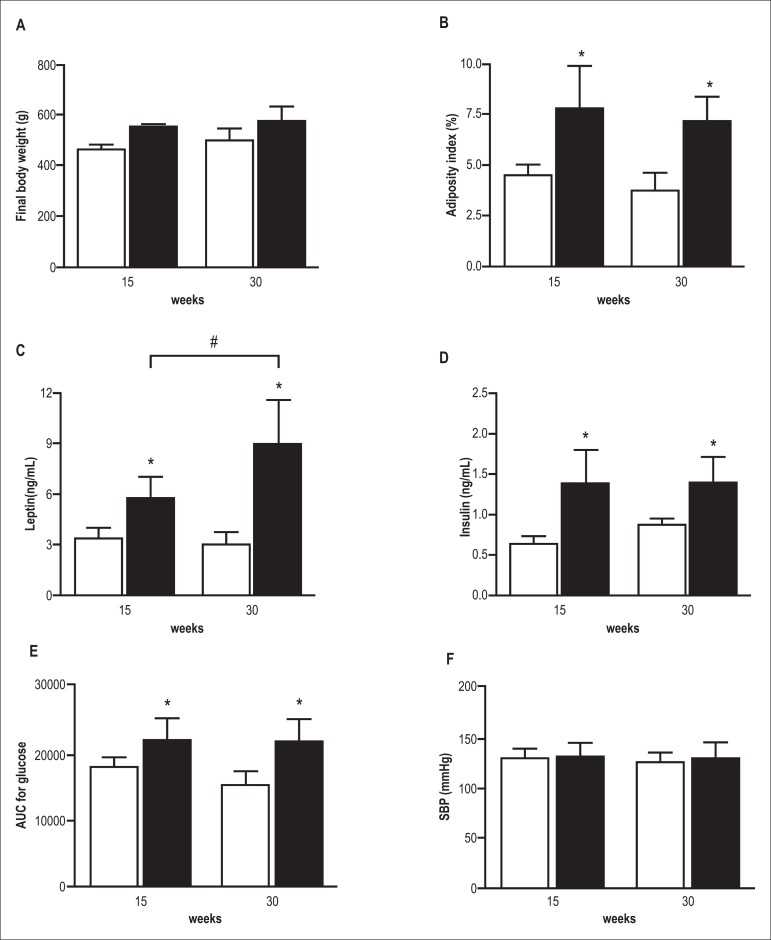
Figure 1 shows the general characteristics of animals in groups C15, C30, Ob15 and Ob30. The high-fat diet caused an increase in FBW and in the index of adiposity in obese animals compared to respective controls at all times evaluated. The duration of exposure to obesity did not result in a significant difference in FBW and adiposity index between the C15 and C30 groups and between the Ob15 and Ob30 groups. There was no difference in systolic blood pressure between the C and Ob groups in the two study periods and that did not change with the time of exposure to obesity. The glucose AUC was higher in Ob groups compared with controls; the time of exposure did not affect glucose levels. Obesity promoted an increase in insulin and leptin levels in Ob groups compared to respective controls. Insulin levels did not change with the time of exposure to obesity; leptin levels were higher in the Ob30 group compared with the Ob15 groups.
Figure 1.
Final body weight (A), adiposity index (B), leptin (C), insulin (D), area under curve (AUC) of intraperitoneal glucose tolerance test (E), systolic blood pressure (SBP) (F) in control (white bars) and obese rats (black bars) after 15 and 30 weeks of treatment. Date are mean ± SD; two-way ANOVA and Bonferroni post hoc test. *p < 0.05 vs control group; # p < 0,05 Ob15 vs Ob30
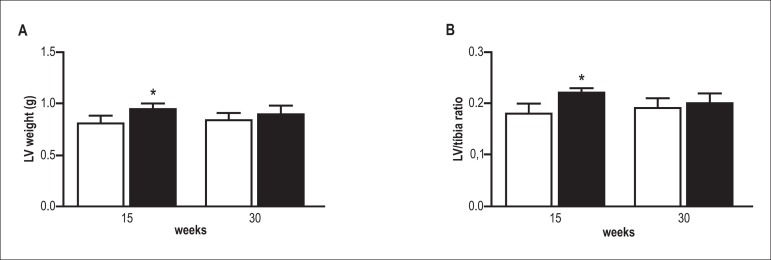
As shown in Figure 2, the presence of obesity increased the weight of the left ventricle and as well as LV/tibia length ratio in the Ob15 group as compared to the C15 group. However, the LV/tibia length ratio was similar in the Ob30 and C30 groups, suggesting that exposure to obesity did not have any effect on this variable.
Figure 2.
Left ventricle dimensions. Left ventricle (LV) weight (A), and left ventricle weight/tíbia ratio (B) in control (white bars) and obese rats (black bars) after 15 and 30 weeks of treatment. Data are mean ± SD; two-way ANOVA and Bonferroni post hoc test. *p < 0.05 vs control group.
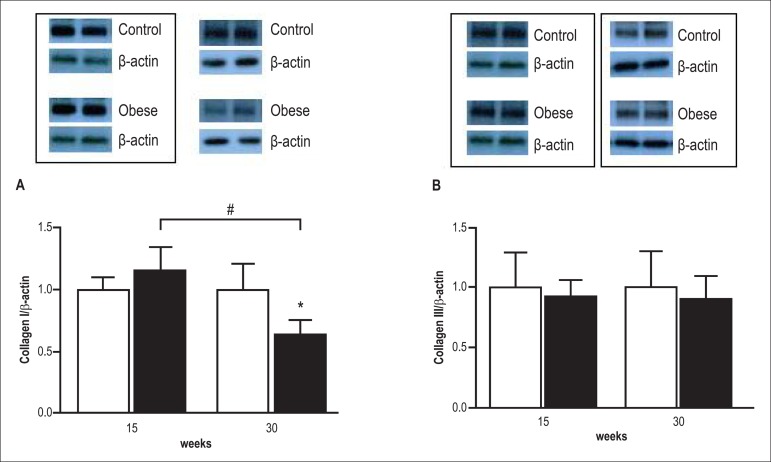
There was no difference in myocardial type I collagen expression between the groups Ob15 and C15 (Figure 3); however, protein expression was lower in the Ob30 group compared with the C30 group. The time of exposure to obesity also resulted in a reduction of collagen type I expression in the Ob30 group as compared with the Ob15 group. Obesity did not alter collagen type III expression, and the time of exposure to obesity did not influence expression of this collagen.
Figure 3.
Westem bolt analysis of collagen I and III in the heart of control (white bars) and obese rats (black bars) after 15 and 30 weeks of treatment. Blots were scanned. Histographic presentation of collagen I/β-actin (A) and collagen III/β-actin ratios. (B). Data are mean ± SD from different animals per group; two-way ANOVA and Bonferroni post hoc test. *p < 0.05 vc control group; # p < 0,05 Ob15 vs Ob30.
Discussion
The main finding of this study was that the time of exposure to obesity induced by an unsaturated high-fat diet affected the expression of myocardial collagen type I, but had no effect on myocardial collagen type III. Obesity that is experimentally induced by diet most closely resembles obesity found in the human population and it has been frequently used to reproduce possible morphological, molecular, biochemical and functional changes in different organs of the human body9,16,22.
The high-calorie diet used in this experiment contained enough calories and was of sufficient duration to promote obesity in rats; it was likely due to the high unsaturated fat content of the diet, which has a higher energy density and greater storage capacity in the body. This study showed that the final body weight and the index of adiposity of obese animals were increased as compared to respective controls at all times evaluated, and these data are consistent with the literature22. However, the time of exposure to the diet did not alter these two variables, which were similar in the two time periods studied - 15 and 30 weeks.
The glucose load in the obese groups resulted in glucose intolerance during the two treatment periods, 15 and 30 weeks. Another important aspect is that time of exposure to obesity did not influence the glycemic profile between obese animals, as glucose intolerance seen in the first 15 weeks remained stable until the 30th week of the experimental protocol. Impaired glucose tolerance, an intermediate stage between normal glucose homeostasis and type 2 diabetes mellitus23, may be related to the development of insulin resistance in obese animals. In support of this assumption, our data show that obesity led to hyperinsulinemia at both evaluations. The results of this study are in agreement with authors who observed that obesity induced by a diet high in unsaturated fat promotes glucose intolerance, hyperinsulinemia and insulin resistance in a short period of time24-27 and these effects are prolonged28.
An increase in leptin serum concentrations after 15 and 30 weeks was seen in obese animals compared with their controls. The time of exposure to obesity influenced the level of this hormone, with increases seen from 15 weeks onward. Leptin concentrations are correlated with body fat, thereby influencing both lipogenesis and lipolysis29. In this study, although the index of adiposity remained the same in the Ob15 and Ob30 groups, leptin concentrations were higher in the Ob30 compared with the Ob15. This fact was likely due to the emergence of resistance to the action of this hormone after the 30th week. Other studies have shown that long periods of obesity promote leptin resistance, which consequently leads to an increase in this hormone14,24,30.
Although the increase in adipose tissue led to metabolic and hormonal alterations, obesity did not result in an increase in blood pressure after 15 and 30 weeks, which remained stable over the two periods studied. The mechanisms responsible for altering blood pressure include hyperactivity of the sympathetic nervous system31, increased activity of the renin-angiotensin-aldosterone system (RAAS)16,32,33 and oxidative stress31, which can result in peripheral vasoconstriction and increased renal sodium reabsorption. The lack of effect on blood pressure suggests that obesity does not alter those factors involved in blood pressure control. These results are in agreement with some authors who observed no change in blood pressure in obese animals9; but differ from studies that showed elevated levels of this parameter32-34. However, although obesity did not alter blood pressure, there was a slight remodeling of the left ventricle, probably due to an increase in the neurohormonal factors mentioned above.
The most important finding of this study was that the time of exposure to obesity caused a reduction in myocardial collagen type I expression in the Ob30 group compared with the Ob15 group and, in contrast, did not modify the expression of myocardial collagen type III. We found no studies that investigated the association between the duration of obesity and expression of myocardial collagen types I and III. The mechanisms responsible for the decrease in collagen type I expression remain unclear. A possible explanation for this reduction may be due to the decrease in synthesis and/or an increase in degradation of collagen. Adipose tissue secretes several substances that are involved in the regulation of myocardial collagen, including the hormone leptin, which is produced mainly by adipocytes and is also synthesized by various tissues including the heart35. The effects of leptin on the heart have not been fully elucidated, but it has been thought to influence cardiomyocyte hypertrophy and regulate production of various components of the extracellular matrix of the myocardium that act on cardiac fibroblasts35. Although there is controversy about the association between leptin and myocardial collagen type I, such as increased expression of procollagen35-37 and decreased synthesis38, there is an agreement among authors that leptin increases the activity of metalloproteinases (MMP-2) 235-38 and mRNA expression of MMP-939,40, participants in myocardial collagen type I degradation. Therefore, it is possible that increased activity of both MMP-2 and MMP-9 is responsible for the reduction in myocardial collagen type I. As stated above, myocardial collagen type III did not change in obesity. No information was found in the literature as a possible explanation for this finding.
Importantly, our finding may have clinical relevance, as it shows that long-term obesity, common in patients, not accompanied by arterial hypertension, can cause a decrease in ventricular compliance due to the reduction in collagen type I. This phenomenon may result in a better adaptation of the heart to obesity because this pathology is associated with increased blood volume.
In conclusion, this study shows that the time of exposure to obesity induced by an unsaturated high-fat diet causes a reduction in myocardial collagen type I expression after 30 weeks, but no changes were seen in myocardial collagen type III. Future studies are needed to determine the mechanism responsible for the decrease in myocardial collagen type I and the lack of effect on collagen type III.
Sources of Funding
FAPESP - process n. 07/53267-3 and 08/50172-4.
Acknowledgments
We are grateful to José C. Georgette, Mário B. Bruno, Sandra A. Fabio, Elenize J. Pereira, Sueli A. Clara, Vitor M. Souza, Camila R. C. Camacho and Corina J. Correa for their technical assistance. This manuscript has been proofread and edited by native English speakers with a related biomedical background in BioMed Proofreading (Cleveland, Ohio, USA).
Footnotes
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
This study was funded by FAPESP.
Study Association
This study is not associated with any thesis or dissertation work.
Author contributions
Conception and design of the research: da Silva DCT, Padovani CR, Cicogna AC; Acquisition of data: da Silva DCT, Lima-Leopoldo AP, Leopoldo AS, de Campos DHS; Analysis and interpretation of the data: da Silva DCT, Lima-Leopoldo AP, Leopoldo AS, de Campos DHS, Nascimento AF, Oliveira Junior SA, Padovani CR, Cicogna AC; Statistical analysis: da Silva DCT, Padovani CR; Obtaining funding: Cicogna AC; Writing of the manuscript: da Silva DCT; Critical revision of the manuscript for intellectual content: da Silva DCT, Lima-Leopoldo AP, Nascimento AF, Oliveira Junior SA, Cicogna AC.
References
- 1.Eckel RH, Barouch WW, Ershow AG. Report of the National Heart, Lung, and Blood Institute-National of Diabetes and Digestive and Kidney Diseases Working Group on the pathophysiology of obesity-associated cardiovascular disease. Circulation. 2002;105(24):2923–2928. doi: 10.1161/01.cir.0000017823.53114.4c. [DOI] [PubMed] [Google Scholar]
- 2.Wong CY, O' Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110(19):3081–3087. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 3.Stein CJ, Colditz GA. The epidemic of obesity. J Clin Endocrinol Metab. 2004;89(6):2522–2525. doi: 10.1210/jc.2004-0288. [DOI] [PubMed] [Google Scholar]
- 4.Malnick SD, Knobler H. The medical complications of obesity. QJM. 2006;99(9):565–579. doi: 10.1093/qjmed/hcl085. [DOI] [PubMed] [Google Scholar]
- 5.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer X, et al. American Heart Association. Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 6.Galinier M, Pathak A, Roncalli J, Massabuau P. Obesity and cardiac failure. Arch Mal Coeur Vaiss. 2005;98(1):39–45. [PubMed] [Google Scholar]
- 7.Carroll JF, Tyagi SC. Extracellular matrix remodelling in the heart of the homocysteinemic obese rabbit. Pt 1Am J Hypertens. 2005;18(5):692–698. doi: 10.1016/j.amjhyper.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 8.Toblli JE, Cao G, DeRosa G, Forcada P. Reduced cardiac expression of plasminogen activator inhibitor 1 and transforming growth factor b1 in obese Zucker rats by perindopril. Heart. 2005;91(1):80–86. doi: 10.1136/hrt.2003.022707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll JF, Zenebe WJ, Strange TB. Cardiovascular function in a rat model of diet-induced obesity. Hypertension. 2006;48(1):65–72. doi: 10.1161/01.HYP.0000224147.01024.77. [DOI] [PubMed] [Google Scholar]
- 10.Leopoldo AS, Sugizaki MM, Lima-Leopoldo AP, do Nascimento AF, Luvizotto Rde A, de Campos DH, et al. Cardiac remodeling in a rat model of diet-induced obesity. Can J Cardiol. 2010;26(8):423–429. doi: 10.1016/s0828-282x(10)70440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliveira SA, Jr, Okoshi K, Lima-Leopoldo AP, Leopoldo AS, Campos DH, Martinez PF, et al. Nutritional and cardiovascular profiles of normotensive and hypertensive rats kept on a high fat diet. Arq Bras Cardiol. 2009;93(5):526–533. doi: 10.1590/s0066-782x2009001100014. [DOI] [PubMed] [Google Scholar]
- 12.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome . Am J Med Sci. 2001;321(4):225–236. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Scaglione R, Dichiara A, Indovina A, Lipari R, Ganguzza A, Parrinello G, et al. Left ventricular diastolic and systolic function in normotensive obese subjects: influence of degree and duration of obesity. Eur Heart J. 1992;13(6):738–742. doi: 10.1093/oxfordjournals.eurheartj.a060249. [DOI] [PubMed] [Google Scholar]
- 14.Nascimento AF, Luvizotto RA, Leopoldo AS, Lima-Leopoldo AP, Seiva FR, Justulin LA, Jr, et al. Long-term high-fat diet-induced obesity decreases the cardiac leptin receptor without apparent lipotoxicity. Life Sci. 2011;88(23-24):1031–1038. doi: 10.1016/j.lfs.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Nascimento TB, Baptista Rde F, Pereira PC, Campos DH, Leopoldo AS, Leopoldo AP, et al. Vascular alterations in high-fat diet-obese rats: role of endothelial L-arginine/NO pathway . Arq Bras Cardiol. 2011;97(1):40–45. doi: 10.1590/s0066-782x2011005000063. [DOI] [PubMed] [Google Scholar]
- 16.Boustany-Kari CM, Gong M, Akers WS, Guo Z, Cassis LA. Enhanced vascular contractility and diminished coronary artery flow in rats made hypertensive from diet-induced obesity. Int J Obes (Lond) 2007;31(11):1652–1659. doi: 10.1038/sj.ijo.0803426. [DOI] [PubMed] [Google Scholar]
- 17.Taylor BA, Phillips SJ. Detection of obesity QTLs on mouse chromosomes 1 and 7 by selective DNA pooling. Genomics. 1996;34(3):389–398. doi: 10.1006/geno.1996.0302. [DOI] [PubMed] [Google Scholar]
- 18.Kopelman PG. Obesity as a medical problem. Nature. 2000;404(6778):635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 19.Ouwens DM, Boer C, Fodor M, de Galan P, Heine RJ, Maassen JA, et al. Cardiac dysfunction induced by high-fat diet is associated with altered myocardial insulin signalling in rats. Diabetologia. 2005;48(6):1229–1237. doi: 10.1007/s00125-005-1755-x. [DOI] [PubMed] [Google Scholar]
- 20.Pfeffer JM, Pfeffer MA, Frohlich ED. Validity of an indirect tail-cuff method for determining systolic arterial pressure in unanesthetized normotensive and spontaneously hypertensive rats. J Lab Clin Med. 1971;78(6):957–962. [PubMed] [Google Scholar]
- 21.Bayley BJ. Tables of the Bonferroni "t" statistic. J Am Stat Assoc. 1977;72:469–478. [Google Scholar]
- 22.Ghibaudi L, Cook J, Farley C, van Heek M, Hwa JJ. Fat intake affects adiposity, comorbidity factors, and energy metabolism of sprague-dawley rats. Obes Res. 2002;10(9):956–963. doi: 10.1038/oby.2002.130. [DOI] [PubMed] [Google Scholar]
- 23.Pereira LO, de Francischi RP, Lancha AH., Jr Obesidade: hábitos nutricionais, sedentarismo e resistência à insulina . Arq Bras Endocrinol Metab. 2003;47(2):111–127. [Google Scholar]
- 24.Woods SC, Seeley RJ, Rushing PA, D'Alessio DA, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003;133(4):1081–1087. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]
- 25.Huang BW, Chiang MT, Yao HT, Chiang W. The effect of high-fat and high-fructose diets on glucose tolerance and plasma lipid and leptin levels in rats. Diabetes Obes Metab. 2004;6(2):120–126. doi: 10.1111/j.1462-8902.2004.00323.x. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Yang G, Li Q, Tang Y, Li K. High-fat- and Lipid-induced insulin resistance in rats: the comparison of glucose metabolism, plasm resistin and adiponectin levels . Ann Nutr Metab. 2006;50(6):499–505. doi: 10.1159/000098141. [DOI] [PubMed] [Google Scholar]
- 27.Winzell MS, Ahrén B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 28.Nivoit P, Morens C, Van Assche FA, Jansen E, Poston L, Remacle C, et al. Established diet-induced obesity in female rats leads to offspring hyperphagia, adiposity and insulin resistance. Diabetologia. 2009;52(6):1133–1142. doi: 10.1007/s00125-009-1316-9. [DOI] [PubMed] [Google Scholar]
- 29.Ainslie DA, Proietto J, Fam BC, Thorburn AW. Short-term, high-fat diets lower circulating leptin concentrations in rats. Am J Clin Nutr. 2000;71(2):438–442. doi: 10.1093/ajcn/71.2.438. [DOI] [PubMed] [Google Scholar]
- 30.Ren J, Zhu BH, Relling DP, Esberg LB, Ceylan-Isik AF. High-fat diet-induced obesity leads to resistance to leptin-induced cardiomyocyte contractile response. Obesity (Silver Spring) 2008;16(11):2417–2423. doi: 10.1038/oby.2008.381. [DOI] [PubMed] [Google Scholar]
- 31.Pausova Z. From big fat cells to high blood pressure: a pathway to obesity-associated hypertension. Curr Opin Nephrol Hypertens. 2006;15(2):173–178. doi: 10.1097/01.mnh.0000214775.42103.a5. [DOI] [PubMed] [Google Scholar]
- 32.Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;287(4):R943–R949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- 33.Smith AD, Brands MW, Wang MH, Dorrance AM. Obesity-induced hypertension develops in young rats independently of the renin-angiotensin-aldosterone system. Exp Biol Med. 2006;231(3):282–287. doi: 10.1177/153537020623100307. [DOI] [PubMed] [Google Scholar]
- 34.Dobrian AD, Davies MJ, Prewitt RL, Lauterio TJ. Development of hypertension in a rat model of diet-induced obesity. Hypertension. 2000;35(4):1009–1015. doi: 10.1161/01.hyp.35.4.1009. [DOI] [PubMed] [Google Scholar]
- 35.Schram K, De Girolamo S, Madani S, Munoz D, Thong F, Sweeney G. Leptin regulates MMP-2, TIMP-1 and collagen synthesis via p38 MAPK in HL-1 murine cardiomyocytes. Cell Mol Biol Lett. 2010;15(4):551–563. doi: 10.2478/s11658-010-0027-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schram K, Wong MM, Palanivel R, No EK, Dixon IM, Sweeney G. Increased expression and cell surface localization of MT1-MMP plays a role in stimulation of MMP-2 activity by leptin in neonatal rat cardiac myofibroblasts. J Mol Cell Cardiol. 2008;44(5):874–881. doi: 10.1016/j.yjmcc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Madani S, De Girolamo S, Muñoz DM, Li RK, Sweeney G. Direct effects of leptin on size and extracellular matrix components of human pediatric ventricular myocytes. Cardiovasc Res. 2006;69(3):716–725. doi: 10.1016/j.cardiores.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 38.Schram K, Ganguly R, No EK, Fang X, Thong FS, Sweeney G. Regulation of MT1-MMP and MMP-2 by leptin in cardiac fibroblasts involves Rho/ROCK-dependent actin cytoskeletal reorganization and leads to enhanced cell migration. Endocrinology. 2011;152(5):2037–2047. doi: 10.1210/en.2010-1166. [DOI] [PubMed] [Google Scholar]
- 39.Tao M, Yu P, Nguyen BT, Mizrahi B, Savion N, Kolodgie FD, et al. Locally applied leptin induces regional aortic wall degeneration preceding aneurysm formation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2013;33(2):311–320. doi: 10.1161/ATVBAHA.112.300543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schroeter MR, Stein S, Heida NM, Leifheit-Nestler M, Cheng IF, Gogiraju R, et al. Leptin promotes the mobilization of vascular progenitor cells and neovascularization by NOX2-mediated activation of MMP9. Cardiovasc Res. 2012;93(1):170–180. doi: 10.1093/cvr/cvr275. [DOI] [PubMed] [Google Scholar]