Abstract
Background
Patients with heart failure (HF) have left ventricular dysfunction and reduced mean arterial pressure (MAP). Increased adrenergic drive causes vasoconstriction and vessel resistance maintaining MAP, while increasing peripheral vascular resistance and conduit vessel stiffness. Increased pulse pressure (PP) reflects a complex interaction of the heart with the arterial and venous systems. Increased PP is an important risk marker in patients with chronic HF (CHF). Non-invasive ventilation (NIV) has been used for acute decompensated HF, to improve congestion and ventilation through both respiratory and hemodynamic effects. However, none of these studies have reported the effect of NIV on PP.
Objective
The objective of this study was to determine the acute effects of NIV with CPAP on PP in outpatients with CHF.
Methods
Following a double-blind, randomized, cross-over, and placebo-controlled protocol, twenty three patients with CHF (17 males; 60 ± 11 years; BMI 29 ± 5 kg/cm2, NYHA class II, III) underwent CPAP via nasal mask for 30 min in a recumbent position. Mask pressure was 6 cmH2O, whereas placebo was fixed at 0-1 cmH2O. PP and other non invasive hemodynamics variables were assessed before, during and after placebo and CPAP mode.
Results
CPAP decreased resting heart rate (Pre: 72 ± 9; vs. Post 5 min: 67 ± 10 bpm; p < 0.01) and MAP (CPAP: 87 ± 11; vs. control 96 ± 11 mmHg; p < 0.05 post 5 min). CPAP decreased PP (CPAP: 47 ± 20 pre to 38 ± 19 mmHg post; vs. control: 42 ± 12 mmHg, pre to 41 ± 18 post p < 0.05 post 5 min).
Conclusion
NIV with CPAP decreased pulse pressure in patients with stable CHF. Future clinical trials should investigate whether this effect is associated with improved clinical outcome.
Keywords: Heart Failure, Pulse Pressure, CPAP
Introduction
Heart failure (HF) is one of the main public burdens in developing countries, and despite medical advances, the mortality of HF remains elevated 1. Neurohumoral activation in HF leads to left ventricular dysfunction and reduced mean arterial pressure (MAP). Compensatory mechanisms to maintain MAP causes vasoconstriction, which increases peripheral vascular resistance and conduit vessel stiffness 2. These effects increase pulse pressure (PP), which reflects a complex interaction of the heart with the arterial and venous systems 3,4. Pulse pressure is determined by two hemodynamic components: a direct component, which is a product of ventricular ejection (stroke volume and ventricular ejection swiftness) and great vessel viscoelastic property interactions, as well as an indirect component resulting from the pulse wave 5,6. As increased PP expresses progression of HF, it is has been related to increased ventricular afterload 7 and myocardial oxygen demand 8, impaired ventricular relaxation 9, and subendocardial ischemia 10. Therefore, increased PP is an important risk marker for subsequent cardiovascular events in patients with chronic HF (CHF)11,12. Previous studies have reported that a PP of 50 mm Hg is the mean normal value for clinic reference in both men and women13, and above 53 mm Hg it increases risk of cardiovascular events 14,15.
Noninvasive ventilation (NIV) has been used in decompensated HF to decrease pulmonary congestion and improve ventilation through both mechanical and hemodynamic effects 16,17. In patients with stable CHF, NIV has not been extensively studied. Naughton et al 18 have shown that the administration of continuous positive airway pressure (CPAP) to patients with stable HF at rest acutely improved cardiac performance and also reduced the work of breathing. Others have shown increases in cardiac output and stroke volume along with decreased systemic vascular resistance among patients with CHF and elevated left ventricular filling pressure 19,20. However, none of these studies has reported the effect of NIV on PP, which is an independent risk marker in patients with stable HF 11,12.
In the present study, we hypothesized that in patients with CHF, NIV would decrease pulse pressure by unloading the ventilatory muscles and improving cardiac performance. Therefore, the aim of the present study was to determine the effects of a single session of NIV with CPAP on pulse pressure in patients with stable CHF.
Methods
The study included patients with systolic CHF from the University Hospital Heart Failure Clinics. The inclusion criteria were: 1) CHF of either ischemic or idiopathic etiology for at least 3 months, 2) left ventricular ejection fraction (LVEF) ≤ 45% within the previous 3 months, documented by echocardiography or radioisotope ventriculography, 3) New York Heart Association class II or III; 4) stable disease with no hospital admission in the previous 3 months. Clinical stability was defined as the absence of change in symptoms, clinical status, or medications in the preceding 3 months. Patients were excluded from the study if they had significant obstructive lung disease (FEV1/FVC < 75% predicted), unstable angina, significant cardiac arrhythmias, or myocardial infarction within the previous 3 months. The subjects completed a screening visit that included a clinical examination and pulmonary function testing (Marquette Hellige, Germany). This protocol was approved by the Human Research Ethics Committee and all patients gave informed consent before entering the study.
The study protocol consisted of a double-blind, randomized, cross-over, and placebo-controlled investigation of the hemodynamic effects of NIV in a controlled environment. Twenty-three patients were recruited and the experiments were performed on two different days with an interval of 3-5 days. The patient had no knowledge of prior randomized ventilation mode and the principal investigator was not present at the scene of NIV. A preliminary NIV session was performed for adaptation and determination of mask size, tolerance to the method and the individual pressure of CPAP to be used in the experiment. The sensation of respiratory discomfort was gauged during all tests by using an arbitrary comfort score (0 = no discomfort, up to 5 = very uncomfortable). This parameter was used to limit the increase or to decrease mask pressure whenever the rating reached 4 or 5 in the NIV adaptation sessions.
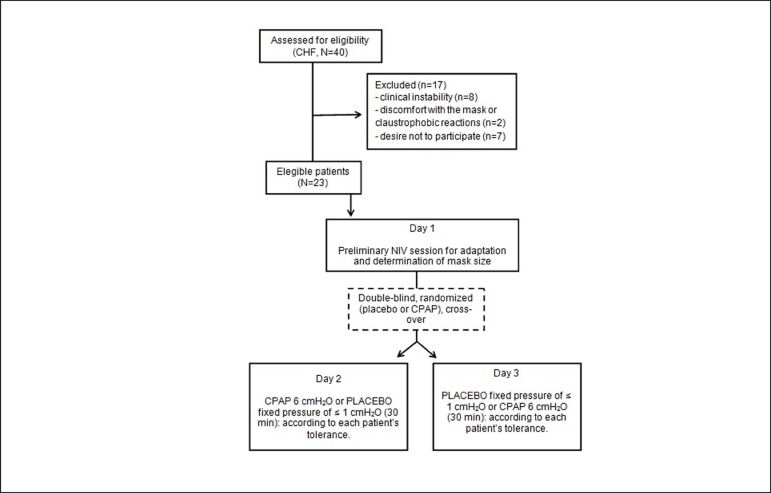
On CPAP day, patients underwent a protocol using the predetermined mask pressure and CPAP time. On the control day, patients underwent placebo CPAP consisting of the application of a fixed pressure of ≤ 1 cmH2O for 30 min by means of a bypass valve (Figure 1).
Figure 1.
Flowchart of the study design. CHF: chronic heart failure; NIV: non-invasive ventilation; CPAP: continuous positive airway pressure.
NIV in the CPAP mode (Tranquility, Healthdyne, USA), was applied via nasal mask (Sealflex, Caradyne, USA) for 30 min in the recumbent position, preceded by a resting steady state period of 15 min of spontaneous breathing. Mask pressure was initially set to 3 cmH2O for 5 min followed to 6 cmH2O, according to each patient's predetermined tolerance.
During NIV, heart rate (HR) and oxygen saturation (SpO2) by pulse oximetry (Healthdyne, Marietta, GA) were continuously monitored, and blood pressure (BP) was measured manually at the end of the resting period and at 5, 10, 20, and 30 min of NIV (auscultatory method obtained by trained researcher using a standard mercury sphygmomanometer on the left arm). Respiratory rate (RR) and SpO2 were recorded, at these same intervals. In order to test whether the effect of CPAP persisted after cessation of NIV, the hemodynamic variables were measured 5 min after discontinuation of NIV (post).
Statistical Analysis
All data were assessed by the Kolmogorov-Smirnov test to determine whether they followed normal distribution. Temporal changes in hemodynamic and respiratory variables (randomized, placebo controlled experiment) were compared during NIV and placebo by two-way ANOVA with repeated measures, where ventilatory mode (NIV or placebo) and time were the main variables. When F values were significant, pairwise comparisons were performed with the Bonferroni post-hoc test. The number of subjects to be studied was calculated from the PP response obtained from previous studies, where the minimal detectable difference in means was 7 mmHg and the expected standard deviation of residuals was 5 mmHg. For this expected size effect and deviation and establishing the statistical power at 0.8 and alpha error at 0.05, the minimum sample size was determined to be of at least 9 subjects. All results are expressed as means ± SEM and p < 0.05 was considered significant.
Results
Forty patients were invited to take part in the study and twenty-three of them agreed to participate and were effectively enrolled in the present study. The characteristics of the subjects are shown in Table 1.
Table 1.
Characteristic of heart failure patients participating in the study
| Sample size | 23 (17M / 6F) |
|---|---|
| Age (years) | 60 ± 10 |
| Weight (kg) | 78 ± 18 |
| Height (cm) | 160 ± 0,1 |
| BMI (kg/cm2) | 29 ± 6 |
| HF Etiology | 12/11 |
| NYHA | 13 class II/ 10 class III |
| Medications | |
| ACEI | 83% |
| Diuretics | 75% |
| Digoxin | 33% |
| Nitrates | 16% |
| β-blockers | 66% |
BMI: body mass index; NYHA: New York Heart Association; M: male; F: female; ACEI: Angiotensin converting enzyme inhibitors.
A controlled experiment was added following a randomized, placebo controlled and cross-over protocol to reduce bias in the analysis of the hemodynamic effects of acute NIV in patients with CHF. Of the 40 patients, 17 had to be excluded from the study due to: 1) clinical instability; 2) discomfort with the mask or claustrophobic reactions; or, 3) unwillingness to participate. Consequently the experiments involved 23 patients, whose characteristics are depicted in Table 1.
The results showed a decrease in HR, systolic blood pressure (SBP), PP, and RR with NIV and CPAP when compared to pre-CPAP values (p < 0.05). Values for SBP, diastolic blood pressure (DBP), and MAP during CPAP were lower than controls (p < 0.05). PP progressively declined, reaching the lowest value at 20 min post CPAP (p < 0.05). On the other hand, HR decreased at 5 min of CPAP and remained lower than pre- values until the end of the analysis (Table 2).
Table 2.
Effects of non-invasive ventilation with continuous positive airway pressure or placebo on hemodynamic variables in heart failure patients (n = 23)
| Moment | HR | SBP | DBP | MAP | PP | RR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPAP | CTRL | CPAP | CTRL | CPAP | CTRL | CPAP | CTRL | CPAP | CTRL | CPAP | CTRL | |
| Pre | 72 ± 9 | 69 ± 9 | 117 ± 17 | 115 ± 17 | 70 ± 12 | 73 ± 10 | 86 ± 10 | 87 ± 10 | 47 ± 20 | 42 ± 18 | 19 ± 4 | 20 ± 3 |
| 5min | 68 ± 11* | 69 ± 11 | 112 ± 16† | 118 ± 19 | 71 ± 12† | 75 ± 10 | 85 ± 11† | 89 ± 12 | 41 ± 17 | 43 ± 16 | 17 ± 3* | 20 ± 3 |
| 10min | 67 ± 11* | 71 ± 12 | 113 ± 17† | 118 ± 20 | 71 ± 11† | 75 ± 11 | 85 ± 11† | 90 ± 13 | 42 ± 18 | 43 ± 16 | 17 ± 3* | 20 ± 3 |
| 20min | 68 ± 11* | 70 ± 12 | 111 ± 17* † | 117 ± 19 | 73 ± 10 | 75 ± 11 | 86 ± 10† | 89 ± 12 | 38±17* † | 42 ± 14 | 17 ± 3* | 19 ± 3 |
| 30min | 67 ± 11* | 70 ± 12 | 112 ± 16* † | 116 ± 20 | 74 ± 9† | 75 ± 9 | 86 ± 9† | 88 ± 11 | 39±16* † | 41 ± 16 | 18 ± 3 | 19 ± 3 |
| Post5min | 67 ± 10* | 72 ± 12 | 113 ± 18* † | 118 ± 20 | 74 ± 10 | 77 ± 12 | 87 ± 10† | 90 ± 12 | 38±19* † | 41 ± 18 | 19 ± 3 | 19 ± 3 |
p < 0.05 vs. Pre for the same mode;
p < 0.05 CPAP vs. Placebo at the same moment; CPAP: continuous positive airway pressure; CTRL: control; HR: heart rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; MAP: mean arterial pressure; PP: pulse pressure; RR: respiratory rate.
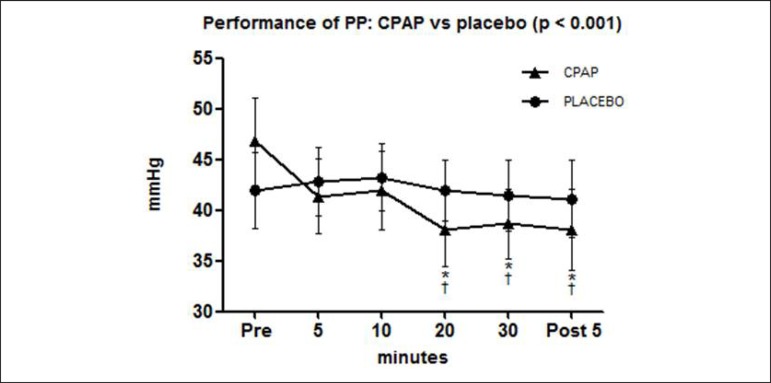
The behavior of pulse pressure during and after CPAP mode against placebo, are shown in Figure 2.
Figure 2.
Pulse pressure (PP), of patients with chronic heart failure (n = 23) measured at 5, 10, 20, and 30 minutes of non-invasive ventilation with continuous positive airway pressure (CPAP; triangle symbols) or Placebo (circle symbols) * p < 0.01 vs. PRE for the same mode of ventilation; † p < 0.001 vs. Placebo at the same moment.
Discussion
Previous studies evaluated the hemodynamic effects of CPAP in patients with acute HF 21,22 while others evaluated the clinical relevance of PP 23,24, but few studies have studied the effects of CPAP in patients with chronic HF 18,25. Therefore, it is not surprising that some of the present findings are similar to previous publications involving patients with decompensated HF 26,27 and may reflect direct heart-lung interactions leading to an overall improvement in cardiac performance and decreased respiratory effort 22. However, the main purpose of the study was to determine specifically the effects of NIV with CPAP on PP since it is a useful hemodynamic indicator of conduit artery vascular stiffness 28,29, has a direct interdependence with key hemodynamic factors, such as stroke volume and peak aortic blood flow 11,30, and carries an independent predictive power for the development of left ventricular dysfunction 31 and CHF in previously healthy subjects 32. In addition, there is a direct relationship between elevated PP and adverse outcome in patients with asymptomatic LV dysfunction 31 and HF 33.
Aronson 11 in his study observed that a lower PP in patients with decompensated HF determined greater risk of death. Also, he concluded that PP depends on the patient's clinical setting, as a high PP in patients with decompensated HF conferred preserved contractility and in outpatients, it confers a higher risk of events. The patients in this study had been in NYHA class II and III for at least 3 months without admission to the emergency room or hospital and therefore, clinically stable.
Verdecchia et al 14 found a higher risk for cardiovascular events when pulse pressure was greater than 53 mmHg. In this study, patients showed an initial PP close to 50 mmHg and CPAP reduced this parameter continuously, which persisted after the withdrawal of NIV.
The present study showed a PP decrease in patients with stable CHF submitted to NIV with CPAP associated with other hemodynamic changes, both in the open study, as well as in the double-blinded controlled cross-over protocol. The patients enrolled in the present study had LVEF ≤45% and high basal PP, which denotes left ventricular dysfunction and a reduction in arterial compliance or distensibility, with an increase in vessel stiffness. These results may have direct clinical implications, as the decrease in PP produced by CPAP may represent improved left ventricular ejection and reduced adverse outcomes.
In addition to the effects on PP, NIV with CPAP also caused a marked effect on other hemodynamic and respiratory variables, which may reflect changes in autonomic modulation rather than or in addition to ventricular loading and venous return 34. The decreased respiratory rate might reflect improved ventilation, explaining the subjective sensation of all the patients who reported greater respiratory comfort. Regarding the hemodynamic variables, there are a number of autonomic reflex links between the pulmonary and circulatory systems that include reflex responses to changes in chest wall and/or respiratory mechanoreceptors, and reflex responses to changes in arterial gas tensions 35. Indeed, lung inflation can lead to systemic vasodilatation via a vagal mediated reflex, which could result in decreases in cardiac volume secondary to decreased LV afterload 36. The blood pressure reduction observed under NIV could be explained by increased airway and intrathoracic pressure leading to increased lung volume and subsequent decreases in transmural left ventricular pressure and afterload 36.
NIV is a safe and feasible ventilation method easily applied in the ambulatory setting, but its effectiveness is critically dependent on patient comfort and acceptance 25,27. Therefore, in the present study, a preliminary NIV session (phase 1) for adaptation and determination of the mask was performed. This preliminary session was also employed to determine the individual CPAP pressure to be used in the subsequent experiments, which was determined to be the lowest pressure that resulted in greater hemodynamic responses, while being comfortable for the patient. The CPAP pressure level that resulted in significant hemodynamic responses was close to 6 cmH2O. These values of CPAP pressure were similar to those in previous studies that demonstrated improved cardiac output with low CPAP levels 19,37. As previous publications had shown myocardial ischemia in patients with ischemic heart failure during the administration of bilevel positive airway pressure (Bipap)38,39, the CPAP mode was chosen in order to decrease the occurrence of potential adverse outcomes. Accordingly, there were no events triggered by CPAP in the patients participating in the present study.
Limitations
In our study it was not possible to determine the exact mechanism responsible for the hemodynamic changes; however, it is already known from previous studies that increases in cardiac output with CPAP might be explained by systemic vasodilatation, possibly on a reflex basis, leading to a decrease in the left ventricular afterload and consequent increased stroke volume and cardiac output 35. We observed that the hemodynamic effects of NIV with CPAP remained up to five minutes after CPAP withdrawal; however, we understand that further studies are needed to determine the duration of these effects after CPAP cessation. Although in the present study clinic outcomes were not measured, in a previous study an improvement was observed in the exercise tolerance with CHF patients after NIV with CPAP (placebo vs. CPAP)40.
Conclusions
We conclude that NIV with CPAP is an effective non-pharmacological method to reduce pulse pressure in patients with stable chronic heart failure with potential clinical implications for the management of this group of patients.
Footnotes
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Study Association
This article is part of the thesis of master submitted by Mônica Quintão from Universidade Federal Fluminense.
Author contributions
Conception and design of the research: Quintão M, Chermont S, Mesquita ET, Nóbrega ACL; Acquisition of data: Quintão M, Chermont S, Rocha NN; Analysis and interpretation of the data: Quintão M, Chermont S, Marchese L, Brandão L, Bernardez SP, Mesquita ET, Rocha NN, Nóbrega ACL; Statistical analysis: Quintão M, Chermont S, Nóbrega ACL; Writing of the manuscript: Quintão M, Chermont S, Marchese L, Bernardez SP, Mesquita ET, Nóbrega ACL; Critical revision of the manuscript for intellectual content: Quintão M, Chermont S, Marchese L, Brandão L, Bernardez SP, Mesquita ET, Nóbrega ACL.
Sources of Funding
There were no external funding sources for this study.
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14(8):803–869. doi: 10.1093/eurjhf/hfs105. Erratum in: Eur J Heart Fail. 2013;15(3):361-2. [DOI] [PubMed] [Google Scholar]
- 2.Wilbur J, James P. Diagnosis and management of heart failure in the outpatient setting. Prim Care. 2005;32(4):1115–1129. doi: 10.1016/j.pop.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Stergiopulos N, Meister JJ, Westerhof N. Determinants of stroke volume and systolic and diastolic aortic pressure. Pt 2Am J Physiol. 1996;270(6):H2050–H2059. doi: 10.1152/ajpheart.1996.270.6.H2050. [DOI] [PubMed] [Google Scholar]
- 4.Stergiopulos N, Westerhof N. Determinants of pulse pressure. Hypertension. 1998;32(3):556–559. doi: 10.1161/01.hyp.32.3.556. [DOI] [PubMed] [Google Scholar]
- 5.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107(22):2864–2869. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 6.Dart AM, Kingwell BA. Pulse pressure - a review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001;37(4):975–984. doi: 10.1016/s0735-1097(01)01108-1. [DOI] [PubMed] [Google Scholar]
- 7.Milnor WR. Arterial impedance as ventricular afterload. Circ Res. 1975;36(5):565–570. doi: 10.1161/01.res.36.5.565. [DOI] [PubMed] [Google Scholar]
- 8.Kelly RP, Tunin R, Kass DA. Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circ Res. 1992;71(3):490–502. doi: 10.1161/01.res.71.3.490. [DOI] [PubMed] [Google Scholar]
- 9.Kohno F, Kumada T, Kambayashi M, Hayashida W, Ishikawa N, Sasayama S. Change in aortic end-systolic pressure by alterations in loading sequence and its relation to left ventricular isovolumic relaxation. Circulation. 1996;93(11):2080–2087. doi: 10.1161/01.cir.93.11.2080. [DOI] [PubMed] [Google Scholar]
- 10.Kass DA, Saeki A, Tunin RS, Recchia FA. Adverse influence of systemic vascular stiffening on cardiac dysfunction and adaptation to acute coronary occlusion. Circulation. 1996;93(8):1533–1541. doi: 10.1161/01.cir.93.8.1533. [DOI] [PubMed] [Google Scholar]
- 11.Aronson D, Burger AJ. Relation between pulse pressure and survival in patients with decompensated heart failure. Am J Cardiol. 2004;93(6):785–788. doi: 10.1016/j.amjcard.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Lee TT, Chen J, Cohen DJ, Tsao L. The association between blood pressure and mortality in patients with heart failure. Am Heart J. 2006;151(1):76–83. doi: 10.1016/j.ahj.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Asmar R, Vol S, Brisac AM, Tichet J, Topouchian J. Reference values for clinic pulse pressure in a nonselected population. Pt 1Am J Hypertens. 2001;14(5):415–418. doi: 10.1016/s0895-7061(01)01284-5. [DOI] [PubMed] [Google Scholar]
- 14.Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Pede S, Porcellati C. Ambulatory pulse pressure: a potent predictor of total cardiovascular risk in hypertension. Hypertension. 1998;32(6):983–988. doi: 10.1161/01.hyp.32.6.983. [DOI] [PubMed] [Google Scholar]
- 15.Sociedade Brasileira de Cardiologia. Sociedade Brasileira de Hipertensão. Sociedade Brasileira de Nefrologia V Diretrizes Brasileiras de Monitorização Ambulatorial da Pressão Arterial (MAPA) e III Diretrizes Brasileiras de Monitorização Residencial de Pressão Arterial (MRPA) Arq Bras Cardiol. 2011;97(3) supl.3:1–24. [Google Scholar]
- 16.Mehta S, Hill NS. Noninvasive ventilation. Am J Respir Crit Care Med. 2001;163:540–577. doi: 10.1164/ajrccm.163.2.9906116. [DOI] [PubMed] [Google Scholar]
- 17.Rogers RL, Feller ED, Gottlieb SS. Acute congestive heart failure in the emergency department. Cardiol Clin. 2006;24(1):115–123. doi: 10.1016/j.ccl.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Naughton MT, Rahman MA, Hara K, Floras JS, Bradley TD. Effect of continuous positive airway pressure in intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation. 1995;91(6):1725–1731. doi: 10.1161/01.cir.91.6.1725. [DOI] [PubMed] [Google Scholar]
- 19.Bradley TD, Holloway RM, McLaughlin PR, Ross BL, Walters J, Liu PP. Cardiac output response to continuous positive airway pressure in congestive heart failure. Pt 1Am Rev Respir Dis. 1992;145(2):377–382. doi: 10.1164/ajrccm/145.2_Pt_1.377. [DOI] [PubMed] [Google Scholar]
- 20.De Hoyos A, Liu PP, Benard DC, Bradley TD. Hemodynamic effects of continuous positive airway pressure in humans with normal and impaired left ventricular function. Clin Sci (Lond) 1995;88(2):173–178. doi: 10.1042/cs0880173. [DOI] [PubMed] [Google Scholar]
- 21.Bendjelid K, Schütz N, Suter PM, Fournier G, Jacques D, Fareh S, et al. Does continuous positive airway pressure by face mask improve patients with acute cardiogenic pulmonary edema due to left ventricular diastolic dysfunction? Chest. 2005;127(3):1053–1058. doi: 10.1378/chest.127.3.1053. [DOI] [PubMed] [Google Scholar]
- 22.Pinsky MR. Cardiovascular issues in respiratory care. Chest. 2005;128(5) Suppl 2:592S–597S. doi: 10.1378/chest.128.5_suppl_2.592S. [DOI] [PubMed] [Google Scholar]
- 23.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 24.London GM, Marchais SJ, Guerin AP, Pannier B. Arterial stiffness: pathophysiology and clinical impact. Clin Exp Hypertens. 2004;26(7-8):689–699. doi: 10.1081/ceh-200031982. [DOI] [PubMed] [Google Scholar]
- 25.Nadar S, Prasad N, Taylor RS, Lip GY. Positive pressure ventilation in the management of acute and chronic cardiac failure: a systematic review and meta-analysis. Int J Cardiol. 2005;99(2):171–185. doi: 10.1016/j.ijcard.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 26.Baratz DM, Westbrook PR, Shah PK, Mohsenifar Z. Effect of nasal continuous positive airway pressure on cardiac output and oxygen delivery in patients with congestive heart failure. Chest. 1992;102(5):1397–1401. doi: 10.1378/chest.102.5.1397. [DOI] [PubMed] [Google Scholar]
- 27.Bersten AD, Holt AW, Vedig AE, Skowronki GA, Baggoley CJ. Treatment of severe cardiogenic pulmonary edema with continuous positive airway pressure delivered by face mask. N Engl J Med. 1991;325(26):1825–1830. doi: 10.1056/NEJM199112263252601. [DOI] [PubMed] [Google Scholar]
- 28.Dart AM, Kingwell BA. Pulse pressure-a review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001;37(4):975–984. doi: 10.1016/s0735-1097(01)01108-1. [DOI] [PubMed] [Google Scholar]
- 29.Domanski MJ, Davis BR, Pfeffer MA, Kastantin M, Mitchell GF. Isolated systolic hypertension prognostic information provided by pulse pressure. Hypertension. 1999;34(3):375–380. doi: 10.1161/01.hyp.34.3.375. [DOI] [PubMed] [Google Scholar]
- 30.Milnor WR. Arterial impedance as ventricular afterload. Circ Res. 1975;36(5):565–570. doi: 10.1161/01.res.36.5.565. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell GF, Moyé LA, Braunwald E, Rouleau JL, Bernstein V, Geltman EM, et al. Sphygmomanometric determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. SAVE investigators. Survival and Ventricular Enlargement. Circulation. 1997;96(12):4254–4260. doi: 10.1161/01.cir.96.12.4254. [DOI] [PubMed] [Google Scholar]
- 32.Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH. Increased pulse pressure and risk of heart failure in the elderly. JAMA. 1999;281(7):634–639. doi: 10.1001/jama.281.7.634. [DOI] [PubMed] [Google Scholar]
- 33.Domanski MJ, Mitchell GF, Norman JE, Exner DV, Pitt B, Pfeffer MA. Independent prognostic information provided by sphygmomanometrically determined pulse pressure and mean arterial pressure in patients with left ventricular dysfunction. J Am Coll Cardiol. 1999;33(4):951–958. doi: 10.1016/s0735-1097(98)00679-2. [DOI] [PubMed] [Google Scholar]
- 34.Kaye DM, Mansfield D, Aggarwal A, Naugthton MT, Esler MD. Acute effects of continuous positive airway pressure on cardiac sympathetic tone in congestive heart failure. Circulation. 2001;103(19):2336–2338. doi: 10.1161/01.cir.103.19.2336. [DOI] [PubMed] [Google Scholar]
- 35.Scharf SM. Respiratory-circulatory interactions in health and disease: lung biology in health and disease. New York: NY: Marcel Dekker; 2001. pp. 519–549. [Google Scholar]
- 36.Pinsky MR. Heart-lung interactions in health and disease: lung biology in health and disease. New York, NY: Marcel Dekker; 1989. pp. 839–876. [Google Scholar]
- 37.Pery M, Payen D, Pinsky MR. Monitoring the effect of CPAP on left ventricular function using continuous blood saturation. Chest. 1991;99(2):512–513. doi: 10.1378/chest.99.2.512. [DOI] [PubMed] [Google Scholar]
- 38.Mehta S, Jay GD, Woolard RH, Hipona RA, Connolly EM, Cimini DM, et al. Randomized, prospective trial of bilevel versus continuous positive airway pressure in acute pulmonary edema. Crit Care Med. 1997;25(4):620–628. doi: 10.1097/00003246-199704000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Bellone A, Monari A, Cortellaro F, Vettorello M, Arlati S, Coen D. Myocardial infarction rate in acute pulmonary edema: noninvasive pressure support ventilation versus continuous positive airway pressure. Crit Care Med. 2004;32(9):1860–1865. doi: 10.1097/01.ccm.0000139694.47326.b6. [DOI] [PubMed] [Google Scholar]
- 40.Chermont S, Quintão MM, Mesquita ET, Rocha NN, Nóbrega AC. Non-invasive ventilation with continuous positive airway pressure acutely improves 6-minute walk distance in chronic heart failure. J Cardiopulm Rehabil Prev. 2009;29(1):44–48. doi: 10.1097/HCR.0b013e3181927858. [DOI] [PubMed] [Google Scholar]