Abstract
Problem statement: Pelvic girdle pain (PGP) is a common condition during or after pregnancy with pain and disability as most important symptoms. These symptoms have a wide range of clinical presentation. Most doctors perceive pregnancy related pelvic girdle pain (PPGP) as ‘physiologic’ or ‘expected during pregnancy’, where no treatment is needed. As such women with PPGP mostly experience little recognition. However, many scientific literature describes PPGP as being severe with considerable levels of pain and disability and socio-economic consequences in about 20% of the cases.
Objectives: We aimed to (1) inform the gynecologist/obstetrician about the etiology, diagnosis, risk factors, and treatment options of PPGP and (2) to make a proposition for an adequate clinical care path.
Methods: A systematic search of electronic databases and a check of reference lists for recent researches about the diagnosis, etiology, risk factors and treatment of PPGP.
Results: Adequate treatment is based on classification in subgroups according to the different etiologic factors. The various diagnostic tests can help to make a differentiation in the several pelvic girdle pain syndromes and possibly reveal the underlying biomechanical problem. This classification can guide appropriate multidimensional and multidisciplinary management. A proposal for a clinical care path starts with recognition of gynecologist and midwife for this disorder. Both care takers can make a preliminary diagnosis of PPGP and should refer to a physiatrist, who can make a definite diagnosis. Together with a physiotherapist, the latter can determine an individual tailored exercise program based on the influencing bio-psycho-social factors.
Keywords: Clinical care path, etiology, pelvic girdle pain, pregnancy, review, treatment
Introduction
Pelvic girdle pain is often perceived as a modern condition and a normal discomfort of pregnancy where no treatment is needed (Wu et al., 2004; Frederiksen et al., 2008; Vermani et al., 2009; Kanakaris et al., 2011; Pierce, 2012). Recent research is trying to uniform the terminology and is focusing on etiological factors, severity and possible pain locations. European guidelines are published and a definition is made in consensus: Pelvic girdle pain (PGP) generally arises in relation to pregnancy, trauma, arthritis and osteoarthritis. Pain is experienced between the posterior iliac crest and the gluteal fold, particularly in the vicinity of the sacroiliac joints. The pain may radiate in the posterior thigh and can also occur in conjunction with/or separately in the symphysis. The endurance capacity for standing, walking, and sitting is diminished. The diagnosis of PGP can be reached after exclusion of lumbar causes. The pain or functional disturbances in relation to PGP must be reproducible by specific clinical tests. (Vleeming et al., 2008).
The first aim of this paper is to inform the gynecologist/obstetrician and midwife about the etiology, diagnostic tests, risk factors and treatment options of this condition. It is important that these care providers recognize the disorder and give correct information. The second aim is to make a proposition for an appropriate clinical care path.
Methods
Because of the objective to review recent evidence and due to the important impact of the European Guidelines, published in 2008, only articles are include which are:
1. Published from 2000 until now and refer to these European Guidelines or use a similar description of PPGP as stated in the European Guidelines
2. Executed in Western countries
Databases PubMed, Web of Science, Cochrane library are consulted by one reviewer using following search terms:
Fig. 1. Flowchart search strategy of databases .
#1: Pregnancy related pelvic girdle pain OR pregnancy related lumbopelvic pain
#2: #1 AND (classification OR diagnostic test OR etiology OR risk factor OR treatment)
#3: Pregnancy AND (lumbopelvic pain OR pelvic girdle pain)
The reference lists of the retrieved articles are reviewed for possible missing researches.
Furthermore there is a search for non-published proceedings papers in the congress book of the Seventh Interdisciplinary World Congress for Low Back and Pelvic Pain (2010). Three proceedings are selected.
Results
Etiology
Several etiologic factors are proposed in the development of PPGP: degenerative, metabolic, genetic, hormonal, and biomechanical factors/non-optimal stability (Wu et al. 2004; O’Sullivan and Beales, 2007; Vleeming et al. 2008; Vermani et al., 2009; Kanakaris et al., 2011; Katonis et al., 2011; Aldabe et al., 2012a). Although the onset of PPGP remains unclear, hormonal factors in combination with non-optimal stability, as a consequence of motor control impairment and/or maladaptive behaviour, is proposed as the most plausible hypothesis (O’Sullivan and Beales, 2007; Vleeming et al., 2008; Vermani et al., 2009).
Non-optimal stability and impaired force closure
Vleeming et al. published in 2008 a definition of optimal stability: “The effective accommodation of the joints to each specific load demand through an adequately tailored joint compression, as a function of gravity, coordinated muscle and ligament forces, to produce effective joint reaction forces under changing conditions” (Vleeming et al., 2008). Optimal stability depends on an efficient force closure, form closure, motor control and is influenced by our emotions. The central nerve system (CNS) chooses a dynamic or static movement strategy, depending on emotions (i.e. fear, anxiety) and perceptions, which results in specific muscle forces and in coordinated muscle activity. A topic in etiologic research is the impaired force closure of the myofascial structures of the abdominal canister (Figure 2). Bracing the sacroiliac joints (SIJ) by adequate force closure of lumbopelvic muscles and nutation of the ilium, is essential for an efficient load transfer to the legs (Vleeming et al., 2002; O’Sullivan and Beales, 2007; Lee et al., 2008; Arumugam et al., 2012).
Fig. 2. Abdominal canister/intern stabilizing unit.
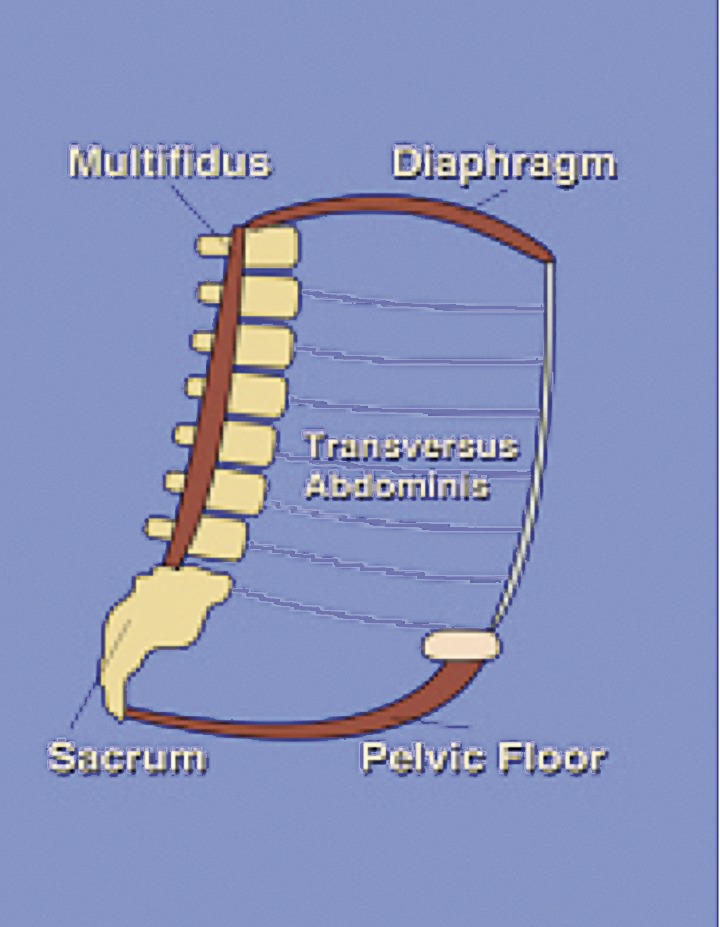
Significant reduced strength of the transversus abdominis (TrA), internal oblique, pelvic floor, lumbar multifidus and inadequate coordination of all lumbopelvic muscles is often observed in patients with PPGP (Gutke et al., 2008a; O’Sullivan, 2010; Aldabe et al., 2012a; Arumugam et al., 2012). When the onset of PGP is in the 2nd and 3th trimester of pregnancy, abdominal stretching and a shift of body gravity centre can possibly cause this muscle impairment. The reduced force closure can lead to neuromuscular compensatory strategies (Lee et al., 2008; Katonis et al., 2011). Lee and colleagues (2008) described two common compensating strategies, namely the butt-gripping and the chest-gripping strategy. In the butt-gripping strategy there is an overuse of the posterior buttock muscles. In the chest-gripping strategy the external oblique is in overdrive and is compensatory for the underuse of the TrA. These strategies may increase sheared forces in the SIJ, which might be responsible for pain (Mens et al., 2009; Vermani et al., 2009). In response to the pain, O’Sullivan and Beales (2007) described two maladaptive forms of behaviour, pain avoidance and pain provocation behaviour, of which both can increase pain and disability.
Hormonal factors
The level of evidence is low with regard to the possible role of higher relaxin levels and its association with more pelvic joint laxity (Björklund et al., 2000; Vleeming et al., 2008; Katonis et al., 2011; Aldabe et al., 2012b; Vøllestad et al., 2012). Moreover, there is evidence that hormonal changes due to pregnancy are compensated by an adequate sacroiliac force closure (Vleeming et al., 2008; Aldabe et al., 2012a). Furthermore, the widening of the symphysis can be seen as physiological when it does not exceed 9,5mm (Mens et al., 2009; Kanarakis et al., 2011). A greater gap can be defined as pathological and is a result of inadequate sacroiliac force closure (Björklund et al., 2000; Mens et al., 2009).
The actual role of hormonal factors can be translated in several propositions. Changing hormones have influence on pain modulation, collagen synthesis and inflammatory processes (O’Sullivan and Beales, 2007). Bjelland et al. (2011) found a link between early menarche and PPGP. They concluded that this link is suggestive for influence of pre-pregnancy hormonal factors rather than changing hormones during pregnancy. Nielsen (2010) conducted a research with cases with persistent PPGP 6 till 12 months postpartum and found that the symptoms are negatively influenced during the menstruation and/or ovulation period. A case report of Sverdrup et al. (2004) mentioned a dramatic exacerbation of PGP when a levonorgestrel intrauterine system is inserted.
Metabolic factors
Eberhard-Gran and Eskild (2008) reported an association with metabolic comorbidities such as diabetes, but the underlying etiologic mechanism is not clear.
Genetic factors
Epidemiologic research elucidate that cases with PGP are more likely to have a mother or sister with PGP (O’Sullivan and Beales, 2007; Kanakaris et al., 2011). A predisposition for PPGP could be associated with changes in action of relaxin (O’Sullivan and Beales, 2007).
Parity-related factors
In a large cohort study of Bjelland et al. (2010), the impact of increased parity is significantly associated with pregnancy related pelvic girdle syndrome (PPGS) (anterior and bilateral posterior pain). The association with parity for severe PPGS is even stronger. Greater odds ratios (OR) are reported for PPGS versus (vs.) severe PPGS: respectively 2,0 vs. 2,6 (parity 1); 2,6 vs. 3,8 (parity 2); 2,6 vs. 3,6 (parity 3 or more). Their conclusion is that parity-related factors may play a causal role in the development of PPGP.
Diagnosis and classification of PPGP
PGP is a specific form of Low Back Pain (LBP) which can occur separately or in conjunction with LBP (Vleeming et al, 2008). There is an overall agreement that PGP results in more affliction of pain and functional disturbances than LBP (Gutke et al., 2006, 2010; Robinson et al., 2010a; Malmqvist et al., 2012).
Specific PGP: trauma and reduced force closure
A rupture of the symphysis is mostly associated with childbirth and the prevalence is estimated around 1 out of 300-30000 births (Dunivan et al., 2009; Najabi et al., 2010). The degree of horizontal separation and vertical instability can be measured through radiographic exploration, (Najabi et al., 2010; Kanakaris et al., 2011; Katonis et al., 2011). When the symphysis is ruptured a pop can be heard and the symptoms of symphyseal and sacroiliac pain start after the pop or delivery. The women are unable to move their legs or bear weight. A vertical translation of more than 5 mm is defined as a symphyseal instability (Najabi et al., 2010).
Non-specific PGP: reduced or excessive force closure
A wide range of pain and functional disturbances is observed, most probably as a result of the multifactorial etiology. Several clinical tests must be performed, because different aspects of PGP are tested (Table I). There are pain provocation and functional ability tests (Vleeming et al., 2008; Vermani et al., 2009; Kanakaris et al., 2011). The combined use can minimize the false negative cases (Albert et al., 2000; Ronchetti et al., 2008; Kanakaris et al., 2011).
Table I. PGP: Pain and functional ability tests.
| TEST NAME | IS EXAMINING |
| Posterior Pelvic Pain Provocation (P4) | SIJ pain and pelvis load capacity |
| Patrick’s = FABER (flexion, abduction, external rotation) | SIJ pain |
| Long Dorsal Sacroiliac Ligament (LDL) (2 versions: during or after delivery) | SIJ pain and pelvis load capacity |
| Gaenslen’s | SIJ pain |
| Symphysis Pain Palpation (SPP) | Symphyseal pain |
| Modified Trendelenburg | Symphyseal pain |
| Active Straight Leg Raise (ASLR) + pelvic compression just below anterior superior iliac spine | Pelvic load capacity |
| Sacroiliac force closure |
Vleeming et al. (2002) found a low correlation between the P4 and LDL test. They attent for the influence of the P4 on the LDL test. It is possible that the LDL test is one of the provoked structures of the P4 test. His team is suggesting that palpation of the LDL is referring to localized pain. Long time straining of the LDL results in a positive P4 test, which means that the pelvic system has been previously overloaded. The ASLR test, is the golden standard for testing the functional ability of the pelvis (Mens et al., 2001; Mens et al., 2002; Vleeming et al. 2008; Vermani et al., 2009; Robinson et al., 2010a). In a recent research of Mens et al. (2012b) on a pregnant population, the specificity of this test is 88% and the sensitivity is moderate (54%) when a cut-off score is used between 0 and 1. Together with the P4 test the sensitivity increases till 68%. Both tests are not suitable for the diagnosis of LBP only, isolated coccyx and symphyseal pain (Mens et al., 2012b). The ASLR test has the ability to distinguish the two most common forms of PGP, the reduced and the excessive form of sacroiliac force closure (O’Sullivan and Beales, 2007). A positive ASLR test is suggestive for loss of functional control due to loss of co-contraction of the lumbopelvic muscles. A negative ASLR test, means high levels of co-contraction of lumbopelvic muscles and excessive force closure.
Validated scales
Not only clinical tests are proposed in literature to make the diagnosis of PGP, also important is a thorough anamnesis (Kanakaris et al., 2011; Stuge et al., 2011). The use of questionnaires and scales can hereby be helpful. The pelvic girdle questionnaire (PGQ) is a condition-specific questionnaire for patients with PGP during or after pregnancy, which evaluate the limitation in activities and the symptoms (Stuge et al., 2011). The PGQ has a good discriminant validity and can be recommended in the assessment of symptoms and disability (Grotle et al. 2012). The Quebec Back Pain Disability Scale (QBPDS) and Visual Analogue Scale (VAS) are commonly used in the evaluation of functional status and pain (Mens et al., 2002; Ronchetti et al., 2008; Mens et al., 2012a).
Classification of severity
Diagnostic tests and pain and disability scales are also useful in classification for severity (Damen et al., 2002b; Gutke et al, 2008b; Ronchetti et al., 2008; van Kessel-Cobelens et al.,2008; Robinson et al., 2010b; Mens et al., 2012a). Characteristics for severity are described in Table II.
Table II. Characteristics for severe PPGP.
| SCALE or TEST | FEATURE FOR SEVERE PGP |
| QBPDS | > 40 |
| VAS for pain | > 5 |
| ASLR test | ≥ 4 |
| Location of pain | Pain in all pelvic joints |
| Thumb-posterior superior iliac spine test | Asymmetric laxity |
| Heel-bank test | |
| Abduction test | |
| ASLR + P4 + LDL test | 3 positive tests + ↓ hip abduction and hip adduction |
Classification of pain location and prognosis
PPGP can be divided in 5 subgroups: symphysiolysis, one- sided SIJ syndrome, double-side SIJ syndrome, the pelvic girdle syndrome (PGS) of which all 3 pelvic joints are affected and a miscellaneous group (Albert et al., 2001, 2006; Vleeming et al., 2008; Vermani et al., 2009; Kanakaris, 2011). The miscellaneous group is defined as inconsistent objective findings of daily pain in ≤ 1 pelvic joint (Albert et al., 2006). According to Albert and colleagues (2001) the PGS group has the worst prognosis: 21% continue to have pain 2 years after delivery. The symphysiolysis group have a 100% change for full recovery.
Risk factors
Latest research topic is the classification of PGP on etiologic basis. As such the suggestion is made that the different forms of PGP will have its own specific risk factors (O’Sullivan and Beales, 2007).Various risk factors (RF) are listed in Table III.
Table III. A description of various risk factors (RF) for PPGP.
| RF (consistent findings) | History of low back pain (LBP) | Wu et al. 2004; Bastiaanssen et al. 2005b; Vleeming et al. 2008; Vermani et al. 2009; Robinson et al. 2010a; Kanakaris et al. 2011; Pierce et al. 2012 |
| Previous PPGP | ||
| Previous trauma of pelvis | ||
| Probable RF (inconsistent findings) | ↑ Workload/physical demanding job, pluripara, parity, ↑ BMI, stress | Wu et al. 2004; Röst et al. 2006; Vleeming et al. 2008; Bjelland et al. 2010, 2011; Katonis et al. 2011 |
| No RF (consistent findings) | Smoking, contraceptive pills, age, interval during following pregnancy | Wu et al. 2004; Vleeming et al. 2008 |
| RF for persistence 3 months after delivery (consistent findings) | ↑ Disability scores, > 1 positive pain provocation tests (PPPT), combined LBP & PGP, PGS, ↑ symphyseal distention, asymmetric laxity of the SIJ, hypermobility and previous LBP | Björklund et al. 2000; Damen et al. 2002b; Mogren 2006; Gutke et al. 2008b; Ronchetti et al. 2008; Vermani et al. 2009; Robinson et al. 2010b |
| RF for specific PPGP | Increased intra-abdominal pressure | Mens et al. 2006b |
Treatment during pregnancy
Complexity of this disorder drives the need for early recognition and effective management during pregnancy (Frederiksen et al., 2008; Pierce et al., 2012).
Informative strategies
Information about the disorder itself and the different possible contributing factors, but also practical and anatomical information can be important in reducing pain, fear and bad postures (Depledge et al., 2005; Frederiksen et al., 2008; Vleeming et al., 2008; Katonis et al., 2011; Pierce et al., 2012). Bed rest during painful periods and minimizing activities which exacerbate pain, are strategies to decrease pain and prevent maladaptive behaviour (O’Sullivan and Beales, 2007; Kanakaris et al., 2011; Katonis et al., 2011).
Pharmacological strategies
Paracetamol as pain relief drug therapy is considered ineffective, but is the only save option during pregnancy (Vleeming et al., 2008; Vermani et al., 2009; Kanakaris et al., 2011). Non-steroidal anti-inflammatory drugs (NSAID’s) are more effective in pain relief and are only advised before 30th pregnancy week (Vermani et al., 2009).
Acupuncture (AP)
The effect of AP on pain and disability is reviewed by Ee et al. (2008) and Vermani et al. (2009). They concluded that one trial provides good evidence for effectiveness of AP as adjunct therapy and more large trials are needed. Elden et al. (2008a, 2008b) conducted 2 RCT’s (Table IV).
Table IV. Comparison of two RCT’s on acupuncture (AP) as an adjunct treatment option for PGP.
| Author | Elden et al. 2008a | Elden et al. 2008b |
| Methods | Single blind RCT | Double blind RCT |
| 6 weeks of treatment (2x/week) | 8 weeks of treatment (12 sessions) | |
| 386 ♀ (2nd trim. pregn.) | 115 ♀ (2nd trim. pregn.; VAS for pain > 5) | |
| Control Group (CG) | Information on pelvic anatomy; pelvic belt exercise of abdominal-, back-, gluteal- and shoulder muscles (= standard treatment ST) n = 130 | ST + non-penetrating sham AP n = 57 |
| Intervention Group (IG) | ST + AP (n = 125) | ST + AP |
| ST + stabilizing exercise (n = 131) | n = 58 | |
| Outcome | Neonatal and maternal adverse events measured by: CTG, birth weight, cord-blood gas, Apgar, gestational age, duration labour, analgesia during labour, use of oxytocin, caesarian | Pain, sick leave, discomfort of PGP, health-related quality of live, recovery, functional status |
| Results | No neonatal or maternal adverse events | No sign. ↓ pain in both groups Sign. |
| Minor adverse effects (headache, drowsiness, rash, pain from needles, unpleasantness, severe nausea, sweating and dizziness) | ↓ number of sick leave in IG Sign. | |
| Interventions are sign. ↑ rated as ‘helpful’ | ↑ ability to do daily activities in IG | |
| No sign. difference between 1 or 2 | No sign. differences in quality of life, discomfort of PGP and recovery |
Pelvic belt (PB)
The use of a PB during pregnancy can help women with reduced force closure. (O’Sullivan and Beales, 2007; Vleeming et al., 2008; Vermani et al., 2009; Katonis et al., 2011; Arumugam et al., 2012). To our knowledge, no research investigated the use of a pelvic belt as a single treatment option. The belt can be used for symptomatic relief and should only be applied for short periods (Nilsson-Wikmar et al., 2005; Vleeming et al., 2008; Vermani et al., 2009).
Physical exercise
Stabilizing training and/or muscle strengthening exercise can reduce pain and enhance functional abilities, but the training program must be individual tailored by the physiotherapist according to the results of the clinical assessment and patients anamnesis (Stuge et al., 2003, 2004; Vleeming et al., 2008). Stabilizing exercises are in favor of muscle strengthening. When the exercises are to heavy or performed to many times, pain can increase (Stuge et al., 2003; O’Sullivan and Beales, 2007; Elden et al., 2008a).
Other treatment options
Supervised group exercise was the research intervention of Eggen et al. (2012). In their single blinded RCT this intervention could not reduce the prevalence of PGP at 36 weeks of pregnancy. Haugland and colleagues (2006) also conducted a RCT with a group exercise program as intervention. They reported a higher self-evaluated utility, measured by a VAS, in the intervention group, but no significant difference in pain between both groups.
Water gymnastics is advised only for cases with low back pain (Granath et al., 2006; Vermani et al. 2009).
Management during labour and delivery
There are recommendations for reducing hip abduction, minimizing the duration of the lithotomy position and propositions for using all-four position or lateral positions during delivery (Vermani et al., 2009; Kanakaris et al., 2011).
Treatment after pregnancy
Non-surgical treatment options for non-specific PPGP
The management of persistent PGP after delivery should focus on multidimensional strategies, since single treatment strategies are generally not found to be effective (Mens et al., 2000; Stuge et al., 2003; Nilsson-Wikmar et al, 2005; Bastiaenen et al., 2006; Penninck and Young, 2007; Gutke et al., 2010). An individualized treatment program is suggested, executed by a multidisciplinary care team and based on an etiologic classification of potential physical, psychosocial, patho-anatomical, hormonal and neurophysiological influencing factors (Stuge et al., 2006; O’Sullivan and Beales, 2007; Vleeming et al., 2008; Hodges, 2010; O’Sullivan, 2010; Kanakaris et al., 2012). General, specific and low evidence treatment options are listed in Table V.
Table V. Non-surgical treatment options for non-specific PPGP.
| Treatment options | What | Author |
| General | Rest, minimizing activities which exacerbate pain, information, education, stabilizing exercises (lumbopelvic & spinal), balance between rest and exercise, pain relief drug therapy | Stuge et al. 2004; Bastiaenen et al. 2006; Stuge et al. 2006 O’Sullivan and Beales 2007; Vleeming et al. 2008; Vermani et al. 2009; Katonis et al. 2011 |
| Reduced force closure | Pelvic belt: just below the anterior superior iliac spine with a tension of 50N | Damen et al. 2002a; Mens et al. 2006a; O’Sullivan and Beales 2007; Lee et al. 2008; Beales et al. 2010; Arumugam et al. 2012 |
| Physical exercise: based on specific lumbopelvic motor control deficit | ||
| Relaxation of thoracopelvic muscles | ||
| Excessive force closure | Breathing techniques, hydrotherapy, relaxation, enhancing passive or relaxing spinal postures, cardiovascular exercise, ceasing stabilizing exercises and pacing strategies | O’Sullivan and Beales 2007 |
| Low evidence | Massage, manual therapy, local cold/hot application, transcutaneous electrical nerve stimulation (TENS) | Vleeming et al. 2008; Vermani et al. 2009; Kanakaris et al. 2011; Katonis et al. 2011 |
Non-surgical treatment options for specific PGP
Bed rest, pelvic binders/castings/braces, bilateral traction, TENS, intra-symphyseal injections, physical and occupational therapy are suggested by Najabi et al. (2010). They mention that a persistent symptomatic diastasis and residual sacroiliac symptoms are often observed with this non-invasive treatment options. Also major complications are common: pressure necrosis, femoral neuropathy, lumbosacral neuropraxia, weakness of lower extremities, difficulties with ambulation and persistent residual PGP.
Surgical treatment options for specific and non-specific PGP
Findings are described in Table VI.
Table VI. Surgical treatment options for specific and non-specific PGP.
| Author | Method | Patients | Surgical procedure | Result |
| van Zwienen et al. 2004 | Prospective 24 months follow-up (FU) | Severe specific and non-specific PPGP (n = 58) | Triple fusion after failure conservative treatment min. 12 months postpartum | Pain relief Improvement in ADL functions |
| Dunivan et al. 2009 | Case report 6 weeks FU | Diastasis 62 mm | External fixation of open book pelvis for 6 weeks | Short hospitalization Rapid ambulation; ↓ Pain |
| Najabi et al. 2010 | Retrospective 1 year FU | (1) Acute (n = 4) (2) Sub-acute (n = 3) (3) Chronic (n = 3) Diastasis [6-70 mm] | Symphyseal fixation within 2 weeks OR (1) 2 weeks until 6 months (2) OR > 6 months (3) | ↓ Pain & early ambulation for surgery within 2 weeks |
| Sturesson 2010 | Review of 10 trials 18 years FU | Severe & long-term (> 2 y) specific & non-specific PGP (n = 45: 36 ♀ + 9 ♂) | Sacroiliac fusion + preceding external fixator test with Hoffman-Slätis frame | 42 cases improved |
| → 25 go back to work | ||||
| 0 cases deteriorated | ||||
| 7 cases re-operated | ||||
| → 5 with success + 2 no further data |
Discussion
A review of recent literature is a prerequisite to reach the postulated goals. In spite of important new evidence, the onset of PPGP remains unclear and there is no guarantee for full recovery.
Firstly, PGP is a specific subgroup of LBP, which has a unique clinical presentation and needs specific management. Therefore, studies that made no distinction between LBP and PGP, are not included in this review.
Furthermore there is evidence of a multifactorial etiology. Hormonal factors in combination with non-optimal stability as a consequence of motor control impairment is proposed as the most plausible hypothesis, though this hypothesis is probably only applicable for a certain subgroup. The onset of PPGP is an important fact which may not be forgotten. When PPGP occur in the first trimester, it is more than likely that hormonal changes are of etiologic value. Their manipulative capacity may influence the motor control. Moreover, there is the observation that the signs and symptoms disappear in most cases within 3 months postpartum. Nevertheless, still 7% suffer from PGP 3 months postpartum, when the hormonal state is considered to return back to normal (Vleeming et al., 2008). It can be important to underline that PPGP does not disappear spontaneously in some cases. Special attention need to be made for women with high scores of pain and disability, high number of positive diagnostic tests, higher scores on the ASLR and P4 test, pain in more than one location and asymmetric laxity of SIJ. These features may be predictive for persistence. Further research is recommended to find out the role of pregnancy hormones, but also to explore the role of pre-pregnancy hormones such as oestrogen. An interesting observation is found by Nielsen and colleagues (2010). They report an increased pain intensity for women with postpartum PGP during menstruation and ovulation. This event reveals that pain intensity is not solely depending on activity level and maladaptive behaviour.
There is evidence that non-optimal stability can be the driver for pain and disability. In response to the pain women with PPGP can exhibit maladaptive behaviour. For this reason it may be beneficial to start as soon as possible with a cognitive approach; information, body awareness, body knowledge and postural advice can possibly reduce fear, faulty believes and pain (Frederiksen et al., 2008; Vleeming et al., 2008; Ryan, 2009; Pierce et al., 2012). O’Sullivan and Beales (2007) discovered that in most cases, psychological factors are associated but not dominant in driving the pain, so treatment should focus on physical exercise. For a small group of women, of whom psycho-social factors are dominant, cognitive behaviour therapy and psychiatric management is suggested (O’Sullivan and Beales, 2007; O’Sullivan, 2010; Carpenter et al., 2012). In this case, and if considered necessary for other cases too, a psychologist and/or psychiatrist should join the treatment team.
The use of an information leaflet can be useful and is already part of the standard treatment in some studies. Written information is additional to oral information and can be read over as many times as needed. All involved care takers can use the brochure so univocal information is given. Furthermore, such a brochure could guide the communication between patient and care taker (Harvey and Fleming, 2005).
A recommendation is made for pharmacological pain treatment during or after pregnancy. This management strategy should follow the guidelines for acute non-specific LBP and take the duration of pregnancy into account (Vleeming et al., 2008; Vermani et al., 2009; Kanakaris et al., 2011). Only O’Sullivan and Beales (2007) made a distinction between peripherally or centrally driven pain. An appropriate goal in pain management could be avoiding that peripherally driven pain becomes centrally driven and so a chronic pain syndrome is induced. When the pain becomes or is originally centrally driven, adequate pharmacological therapy (low dose antidepressant) can be started (Kroenke et al., 2007).
Some studies cannot find a significant decrease in pain and disability due to physical exercise (problem of single treatment strategy or problem of heterogeneity), though there is good evidence that physical therapy (pre- and postpartum) should include stabilizing and general strengthening exercises. The reviewed literature is also indicating that too much exercise may increase pain and disability. This is an important observation which can mean that there is a small boarder between physical ratability and overloading.
Some of the low evidence treatment strategies may possibly be useful in a specific subgroup or individual, because of heterogeneity of the research population due to the multifactorial etiology. The problem of selection bias needs to be kept in mind when reading this article and conducting future trials. Not only the problem of selection bias is a limitation in this review, but also the ‘one-reviewer-only’ fact.
Interestingly are the results of surgical therapy: the evidence to treat a symphyseal disruption is in favour of an early surgical intervention. In the study of Najabi et al. (2010) the acute cases are unable to bear weight and have pain in all pelvic joints. These features are the reason for surgery and not a 9,5 mm diastasis. His team is also describing major complications when a nonsurgical procedure is applied. Complications cannot be underestimated and may have serious psychological, sociological and economic implications. Moreover, a more complex surgical intervention with triple fixation, as suggested by van Zwienen et al. (2004) may possibly be avoided. Further research is needed with extended follow up periods of more than 1 year and with larger study groups, but maybe a new medical era has dawned.
A last recommendation is the use of a care model which can visualize the bio-psycho-social influencing factors, so a correct classification can be made. Such a model can also be useful in inter-professional communication (Steiner et al., 2002; Kroenke et al., 2007). In case of persistence, it could be appropriate to discuss the treatment options during multidisciplinary sessions, so an adequate treatment can be tailored and compliancy can be reached (Kroenke et al., 2007; Ryan, 2009).
Clinical care path proposal
The gynecologist/obstetrician and the consulting midwife can start with global information about the disorder by using an information leaflet. Recognition, empathy and information can reduce anxiety and promote coping and health behaviour change (Frederiksen et al., 2008; Ryan, 2009; Pierce et al., 2012). A preliminary diagnose can be made by both care givers through the Trendelenburg test, symphysis pain palpation test and the LDL test. These tests are not time consuming and easy to perform. When a supine position is possible in the consultation room, the ASLR test can also be executed with and without pelvic compression. As stated earlier, an anamnesis of the women can be very useful in the search for influencing biological and possible influencing psychological and social factors. For a definite diagnosis, it is recommended to refer to a physiatrist. The latter can reproduce the earlier performed diagnostic tests and add more specific tests. The physiatrist has the expertise to exclude lumbar causes and possibly find the underlying mechanism. The use of disability and pain scales can be helpful in the classification according to severity which can be useful for the prognosis and treatment strategies. Together with a specialized physiotherapist, the physiatrist can determine an individual tailored exercise program for simple cases. In cases of severe PGP or when psycho-social factors are dominant in driving the pain, a multidisciplinary care team (gynecologist, midwife, physiatrist, physiotherapist, psychiatrist or psychologist) discusses the treatment options.
Conclusion
It is obvious that PPGP is a complex disorder. For now, no treatment option can guarantee full recovery, as such care is our major concern. Various care disciplines are in contact with PPGP and are important links in the multidimensional management. Aim of this multidimensional and multidisciplinary approach is to increase the women’s self-knowledge and self-efficacy, so pain and disability can be minimized. To inform the gynecologist/obstetrician about the severity, etiology and treatment options of PPGP is an important step in recognition. Because of this recognition timely information can be given and a referral to a physiatrist can be made. Information, education and advice are basic strategies and are the responsibility of all involved care takers. When a higher compliance can be reached through appropriate holistic management the odds for favorable recovery can be optimized and perhaps reduce the prevalence rates of chronic PPGP.
References
- Albert HB, Godkesen M, Westergaard J. Evaluation of clinical tests used classification procedures in pregnancy-related pelvic joint pain. Eur Spine J. 2000;9:161–166. doi: 10.1007/s005860050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert HB, Godskesen M, Westergaard J. Prognosis in four syndromes of pregnancy related pelvic pain. Acta Obstet Gyn Scan. 2001;80:505–510. [PubMed] [Google Scholar]
- Albert HB, Godskesen M, Korsholm L, et al. Risk factors in developing pregnancy-related pelvic girdle pain. Acta Obstet Gyn Scan. 2006;85:539–544. doi: 10.1080/00016340600578415. [DOI] [PubMed] [Google Scholar]
- Aldabe D, Milosavljevic S, Bussey MD. s pregnancy related pelvic girdle pain associatedwith altered kinematic, kinetic and motor control of the pelvis? A systematic review. Eur Spine J. 2012;(DOI 10.1007/s00586-012-2401-1) doi: 10.1007/s00586-012-2401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldabe D, Ribeiro DC, Milosavljevic S, et al. Pregnancy-related pelvic girdle pain and its relationship with relaxin levels during pregnancy: a systematic review. Eur Spine J. 2012;(DOI 10.1007/s00586-012-2162-x) doi: 10.1007/s00586-012-2162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam A, Milosavljevic S, Woodley S, et al. Effects of external pelvic compression on form closure, force closure, and neuromotor control of the lumbopelvic spine e A systematic review. Manual Ther. 2012;17:275–284. doi: 10.1016/j.math.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Bastiaanssen JM, de Bie RA, Bastiaenen CHG, et al. Etiology and prognosis of pregnancy-related pelvic girdle pain; design of a longitudinal study. BMC Public Health. 2005;5(1):1–8. doi: 10.1186/1471-2458-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaenen C, de Bie RA, Wolters PMJC, et al. Effectiveness of a tailor-made intervention for pregnancy-related pelvic girdle and/or low back pain after delivery: Short-term results of a randomized clinical trial. BMC Musculoskel Dis. 2006;27:7–19. doi: 10.1186/1471-2474-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales DJ, Sullivan PB, Briffa NK. The effects of manual pelvic compression on trunk motor control during an active straight leg raise in chronic pelvic girdle pain subjects. Manual Ther. 2010;15:190–199. doi: 10.1016/j.math.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Bjelland EK, Eskild A, Johansen R, et al. Pelvic girdle pain in pregnancy: the impact of parity. Am J Obstet Gynecol. 2010;203:146.e1–6. doi: 10.1016/j.ajog.2010.03.040. [DOI] [PubMed] [Google Scholar]
- Bjelland EK, Eberhard-Gran M, Nielsen CS, et al. Age at menarche and pelvic girdle syndrome in pregnancy: a population study of 74 973 women. BJOG. 2011;118:1646–1652. doi: 10.1111/j.1471-0528.2011.03099.x. [DOI] [PubMed] [Google Scholar]
- Björklund K, Bergström S, Nordström ML, et al. Symphyseal distention in relation to serum relaxin levels and pelvic pain in pregnancy. Acta Obstet Gyn Scan. 2000;79:269–275. doi: 10.1080/j.1600-0412.2000.079004269.x. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, Stoner SA, Mundt JM, et al. An online self-help CBT intervention for chronic lower back pain. Clin J Pain. 2012;28(1):14–22. doi: 10.1097/AJP.0b013e31822363db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damen L, Buyruk HM, Guler-Uysal F, et al. The prognostic value of asymmetric laxity of the sacroiliac joints in pregnancy-related pelvic pain. Spine. 2002;27(24):2820–2824. doi: 10.1097/00007632-200212150-00018. [DOI] [PubMed] [Google Scholar]
- Damen L, Spoor CW, Snijders CJ, et al. Does a pelvic belt influence sacroiliac joint laxity? Clin Biomech. 2002;17:495–498. doi: 10.1016/s0268-0033(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Depledge J, McNair PJ, Keal-Smith C, et al. Management of symphysis pubis dysfunction during pregnancy using exercise and pelvic support belts. Phys Ther. 2005;85(12) [PubMed] [Google Scholar]
- Dunivan GC, Hickman AM, Connolly A. Severe separation of the pubic symphysis and prompt orthopedic surgical intervention. Obstet Gynecol. 2009;114(2):473–475. doi: 10.1097/AOG.0b013e3181998bd1. [DOI] [PubMed] [Google Scholar]
- Eberhard-Gran M, Eskild A. Diabetes mellitus and pelvic girdle syndrome in pregnancy. Is there an association? Acta Obstet Gyn Scan. 2008;87(10):1015–1019. doi: 10.1080/00016340802345944. [DOI] [PubMed] [Google Scholar]
- Ee CC, Manheimer E, Pirotta MV. Acupuncture for pelvic and back pain in pregnancy: a systematic review. AJOG. 2007;(doi: 10.1016/j.ajog.2007.11.008.) doi: 10.1016/j.ajog.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Eggen MH, Stuge B, Mowinckel P, et al. Can supervised group exercises including ergonomic advice reduce the prevalence and severity of low back pain and pelvic girdle pain in pregnancy? A randomized controlled trial. Phys Ther. 2012;92:781–790. doi: 10.2522/ptj.20110119. [DOI] [PubMed] [Google Scholar]
- Elden H, Ostgaard HC, Fagevik-Olsen M, et al. Treatments of pelvic girdle pain in pregnant women: adverse effects of standard treatment, acupuncture and stabilizing exercises on the pregnancy, mother, delivery and the fetus/neonate. BMC Complem Altern M. 2008;8(doi:10.1186/1472-6882- 8-34) doi: 10.1186/1472-6882-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elden H, Fagevik-Olsen M, Ostgaard HC, et al. Acupuncture as an adjunct to standard treatment for pelvic girdle pain in pregnant women: randomised double-blinded controlled trial comparing acupuncture with non-penetrating sham acupuncture. BJOG. 2008;115:1655–1668. doi: 10.1111/j.1471-0528.2008.01904.x. [DOI] [PubMed] [Google Scholar]
- Fredriksen EH, Moland KM, Sundby J. “Listen to your body”. A qualitative text analysis of internet discussions related to pregnancy health and pelvic girdle pain in pregnancy. Patient Educ Couns. 2008;73(2):294–299. doi: 10.1016/j.pec.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Granath AB, Hellgren MSE, Gunnarsson RK. Water aerobics reduces sick leave due to low back pain during pregnancy. JOGNN. 2006;35:465–471. doi: 10.1111/j.1552-6909.2006.00066.x. [DOI] [PubMed] [Google Scholar]
- Grotle M, Garratt AM, Jenssen HK, et al. Reliability and construct validity of self-report questionnaires for patients with pelvic girdle pain. Phys Ther. 2012;92(1) doi: 10.2522/ptj.20110076. [DOI] [PubMed] [Google Scholar]
- Gutke A, Östgaard HC, Öberg B. Pelvic girdle pain and lumbar pain in pregnancy: a cohort study of the consequences in terms of health and functioning. Spine. 2006;31(5):149–155. doi: 10.1097/01.brs.0000201259.63363.e1. [DOI] [PubMed] [Google Scholar]
- Gutke A, Östgaard HC, Öberg B. Association between muscle function and low back pain in relation to pregnancy. J Rehabil Med. 2008;40:304–311. doi: 10.2340/16501977-0170. [DOI] [PubMed] [Google Scholar]
- Gutke A, Östgaard HC, Öberg B. Predicting persistent pregnancy-related low back pain. Spine. 2008;33(12):386–393. doi: 10.1097/BRS.0b013e31817331a4. [DOI] [PubMed] [Google Scholar]
- Gutke A, Sjôdalh J, Öberg B. Specific muscle stabilizing as home exercises for persistent pelvic girdle pain after pregnancy: a randomized controlled trial. J Rehabil Med. 2010;42:929–935. doi: 10.2340/16501977-0615. [DOI] [PubMed] [Google Scholar]
- Harvey HD, Fleming P. The readability and audience acceptance of printed health promotion materials used by environmental health departments. J Environ Health. 2005;65(6):22–28. [PubMed] [Google Scholar]
- Haugland KS, Rasmussen S, Daltveit AK. Group intervention for women with pelvic girdle pain in pregnancy. A randomized controlled trial. Acta Obstet Gyn Scan. 2006;85:1320–1326. doi: 10.1080/00016340600780458. [DOI] [PubMed] [Google Scholar]
- Hodges PW. Vleeming A and Fitzgerald C (eds) 7th Interdisciplinary World Congress on Low Back and Pelvic Pain. Los Angeles, US: Proxomed; 2010. Strategies for motor control of the spine and changes in pain: the deep vs. superficial muscle debate; pp. 414–419. [Google Scholar]
- Kanakaris NK, Roberts GS, Giannoudis PV. Pregnancy-related pelvic girdle pain: an update. BMC Med. 2011;9:15. doi: 10.1186/1741-7015-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katonis P, Kampouroglou A, Aggelopoulos A, et al. Pregnancy-related low back pain. Hippokratia. 2011;15(3):205–210. [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Bair M, Damush T, et al. Stepped care for affective disorders and musculoskeletal pain (SCAMP) study design and practical implications of an intervention for comorbid pain and depression. Gen Hosp Psychiat. 2007;29:506–17. doi: 10.1016/j.genhosppsych.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lee DG, Lee LJ, McLaughlin L. Stability, continence and breathing: The role of fascia following pregnancy and delivery. JBMT. 2008;12:333–348. doi: 10.1016/j.jbmt.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Malmqvist S, Kjaermann I, Andersen K, et al. Prevalence of low back and pelvic pain during pregnancy in a Norwegian population. J Manip Physiol Ther. 2012;35(4) doi: 10.1016/j.jmpt.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Mens JMA, Damen L, Snijders CJ, et al. The mechanical effect of a pelvic belt in patients with pregnancy-related pelvic pain. Clin Biomech. 2006;21:122–7. doi: 10.1016/j.clinbiomech.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Mens JMA, Hoek van Dijke A, Goudzwaard EH, et al. Possible harmful effects of high intra-abdominal pressure on the pelvic girdle. J Biomech. 2006;39:627–635. doi: 10.1016/j.jbiomech.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Mens JMA, Huis in 't Veld' YH, Pool-Goudzwaard A. Severity of signs and symptoms in lumbopelvic pain during pregnancy. Manual Ther. 2012;17:175–179. doi: 10.1016/j.math.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Mens JMA, Huis in 't Veld' YH, Pool-Goudzwaard A, et al. The active straight leg raise test in lumbopelvic pain during pregnancy. Manual Ther. 2012;17:364–368. doi: 10.1016/j.math.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Mens JMA, Snijders CJ, Stam HJ. Diagonal trunk muscle exercises for pelvic girdle pain after pregnancy: a randomized controlled trial. Phys Ther. 2000;80:1164–1173. [PubMed] [Google Scholar]
- Mens JMA, Vleeming A, Snijders CJ, et al. Reliability and validity of the active straight leg raise test in posterior pelvic pain since pregnancy. Spine. 2001;26(10):1167–1171. doi: 10.1097/00007632-200105150-00015. [DOI] [PubMed] [Google Scholar]
- Mens JMA, Vleeming A, Snijders CJ. Validity of the active straight leg raise test for measuring disease severity in patients with posterior pelvic pain after pregnancy. Spine. 2002;27(2):196–200. doi: 10.1097/00007632-200201150-00015. [DOI] [PubMed] [Google Scholar]
- Mens JMA, Pool-Goudzwaard A, Stam HJ. Mobility of the pelvic joints in pregnancy-related lumbopelvic pain: a systematic review. Obstet Gynecol Surv. 2009;64(3):200–208. doi: 10.1097/OGX.0b013e3181950f1b. [DOI] [PubMed] [Google Scholar]
- Mogren IM. BMI, pain and hyper-mobility are determinants of long-term outcome for women with low back pain and pelvic pain during pregnancy. Eur Spine J. 2006;15:1093–1102. doi: 10.1007/s00586-005-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najabi S, Tannast M, Klenck RE, et al. Internal fixation of symphyseal disruption resulting from childbirth. J Orthop Trauma. 2010;24:732–739. doi: 10.1097/BOT.0b013e3181d70259. [DOI] [PubMed] [Google Scholar]
- Nielsen LL. Clinical findings, pain descriptions and physical complaints reported by women with post-natal pregnancy-related pelvic girdle pain. Acta Obstet Gyn Scan. 2010;89:1187–1191. doi: 10.3109/00016349.2010.501853. [DOI] [PubMed] [Google Scholar]
- Nilsson-Wikmar L, Holm K, Oijerstedt R, et al. Effect of three different physical therapy treatments on pain and activity in pregnant women with pelvic girdle pain: a randomized clinical trials with 3, 6, and 12 months follow-up postpartum. Spine. 2005;30:850–856. doi: 10.1097/01.brs.0000158870.68159.d9. [DOI] [PubMed] [Google Scholar]
- O'Sullivan PB. Vleeming A and Fitzgerald C (eds) 7th Interdisciplinary World Congress on Low Back and Pelvic Pain. Los Angeles, US: Proxomed; 2010. Diagnosis and classification of chronic low back pain disorders: maladaptive movement and motor control impairments as underlying mechanism; pp. 160–177. [DOI] [PubMed] [Google Scholar]
- O'Sullivan PB, Beales BJ. Diagnosis and classification of pelvic girdle pain disorders—Part 1: A mechanism based approach within a biopsychosocial framework. Manual Ther. 2007;12:86–97. doi: 10.1016/j.math.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Penninck VE, Young G. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane DB Syst Rev. doi: 10.1002/14651858.CD001139.pub2. 2007;(2) doi: 10.1002/14651858.CD001139.pub2. [DOI] [PubMed] [Google Scholar]
- Pierce H, Homer HSE, Dahlen HG, et al. Pregnancy-related lumbopelvic pain: listening to Australian women. Nurs Res. 2012;(doi:10.1155/2012/387428) doi: 10.1155/2012/387428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson HS, Mengshoel AM, Bjelland EK, et al. Pelvic girdle pain, clinical tests and disability in late pregnancy. Manual Ther. 2010;15:280–285. doi: 10.1016/j.math.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Robinson HS, Mengshoel AM, Veierød MB, et al. Pelvic girdle pain: potential risk factors in pregnancy in relation to disability and pain intensity three months postpartum. Manual Ther. 2010;15:522–528. doi: 10.1016/j.math.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Ronchetti I, Vleeming A, van Wingerden JP. Physical characteristics of women with severe pelvic girdle pain after pregnancy. A descriptive cohort study. Spine. 2008;33(5):145–151. doi: 10.1097/BRS.0b013e3181657f03. [DOI] [PubMed] [Google Scholar]
- Röst CC, Jacqueline J, Kaiser A, et al. Prognosis of women with pelvic pain during pregnancy: a long-term follow-up study. Acta Obstet Gyn Scan. 2006;85:771–777. doi: 10.1080/00016340600626982. [DOI] [PubMed] [Google Scholar]
- Ryan P. Integrated Theory of Health Behaviour Change: Background and Intervention Development. Clin Nurse Spec. 2009;23(3):161–172. doi: 10.1097/NUR.0b013e3181a42373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner WA, Ryser L, Huber E, et al. Use of the ICF model as a clinical problem-solving tool in physical therapy and rehabilitation medicine. Phys Ther. 2002;82(11):1098–1107. [PubMed] [Google Scholar]
- Stuge B, Garratt A, Jenssen HK, et al. The pelvic girdle questionnaire: a condition-specific instrument for assessing activity limitations and symptoms in people with pelvic girdle pain. Phys Ther. 2011;91:1096–1108. doi: 10.2522/ptj.20100357. [DOI] [PubMed] [Google Scholar]
- Stuge B, Gunvor H, Vøllestad N. Physical therapy for pregnancy-related low back and pelvic pain: a systematic review. Acta Obstet Gyn Scan. 2003;82:983–990. doi: 10.1034/j.1600-0412.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- Stuge B, Holm I, Vøllestad N. To treat or not to treat postpartum pelvic girdle pain with stabilizing exercises. Manual Ther. 2006;11:337–343. doi: 10.1016/j.math.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Stuge B, Veierød MB, Laerum E, et al, et al. The efficacy of a treatment program focusing on specific stabilizing exercises for pelvic girdle pain after pregnancy: a two-year follow-up of a randomized controlled trial. Spine. 2004;29(10):197–203. doi: 10.1097/00007632-200405150-00021. [DOI] [PubMed] [Google Scholar]
- Sturesson B. Vleeming A, Fitzgerald C (eds), 7th Interdisciplinary World Congress on Low Back and Pelvic Pain. Los Angeles, US: Proxomed; 2010. New insights in diagnostics and treatment of pelvic girdle pain; pp. 253–260. [Google Scholar]
- Sverdrup B, Kristiansson P. Pelvic girdle pain may be an overlooked hormone adverse effect. Acta Obstet Gyn Scan. 2004;83:316–317. doi: 10.1111/j.0001-6349.2004.0089d.x. [DOI] [PubMed] [Google Scholar]
- van Kessel, Verhagen AP, Mens JMA, et al. Pregnancy-related pelvic girdle pain: intertester reliability of 3 tests to determine asymmetric mobility of the sacroiliac joints. J Manip Physiol Ther. 2008;31:130–136. doi: 10.1016/j.jmpt.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Van Zwienen CMA, van den Bosch EW, Snijders CJ, et al. Triple pelvic ring fixation in patients with severe pregnancy-related low back and pelvic pain. Spine. 2004;29(4):478–484. doi: 10.1097/01.brs.0000092367.25951.4a. [DOI] [PubMed] [Google Scholar]
- Vermani E, Mittal R, Weeks A. Pelvic girdle pain and low back pain in pregnancy: a review. Pain Pract. 2009;10(1):60–71.. doi: 10.1111/j.1533-2500.2009.00327.x. [DOI] [PubMed] [Google Scholar]
- Vleeming A, Albert HB, Östgaard HC, et al. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur Spine J. 2008;17(6):794–819. doi: 10.1007/s00586-008-0602-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleeming A, de Vries HJ, Mens JMA, et al. Possible role of the long dorsal sacroiliac ligament in women with peripartum pelvic pain. Acta Obstet Gyn Scan. 2002;81:430–436. doi: 10.1034/j.1600-0412.2002.810510.x. [DOI] [PubMed] [Google Scholar]
- Vøllestad NK, Torjesen PA, Robinson HS. Association between the serum levels of relaxin and responses to the active straight leg raise test in pregnancy. Manual Ther. 2012;17:225–230. doi: 10.1016/j.math.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Wu WH, Meijer OG, Uegaki K, et al. Pregnancy-related pelvic girdle pain (PGP): terminology, clinical presentation, and prevalence. Eur Spine J. 2004;13(7):575–589. doi: 10.1007/s00586-003-0615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]



