Abstract
Preterm birth (PTB) remains the most common cause of neonatal morbidity and mortality as well as long-term disability. Current strategies to prevent or arrest spontaneous preterm labor (SPTL) have limited success. For almost three decades, there have been no novel pharmacological agents used clinically to address this important obstetrical complication. In this review, we focus on the uterine myocyte as a target for prevention of spontaneous PTB. After presenting an overview of intracellular signaling pathways that are important in regulation of smooth muscle contractility, we discuss previous and current pharmacological approaches to manage SPTL. We also present recent evidence from our own laboratories suggesting a potentially novel and uterine-specific approach to maintain or impose uterine relaxation. Finally, we briefly discuss extrinsic systems that might affect uterine activity and reinforce the concept that SPTL represents a syndrome that is the end result of a variety of pathophysiologic etiologies leading to PTB. We conclude by emphasizing the need for much more research to provide sufficient understanding of the mechanisms of SPTL and to make inroads towards reducing the incidence and adverse consequences of this common and serious syndrome.
Keywords: Prematurity, preterm labour, myosin regulatory light chain, rhoA-associated kinase, tocolysis, tocolysis contractility
Introduction
Preterm birth (PTB) is widely recognized as the major cause of neonatal mortality and long-term disability. There are three broad categories of PTB. Approximately one-third of cases are associated with and result from intrauterine infection. Current obstetrical practice is to terminate these pregnancies immediately, regardless of gestational age, because of potential serious complication to either the baby or the mother. The second and increasingly large category is the iatrogenic group where concerns about maternal or fetal health dictate that the outcome of the fetus would be better with immediate delivery, again regardless of gestational age. The third category, and the one most relevant to this discussion is the group that enters spontaneous preterm labor (SPTL) without evidence of maternal or fetal factors that would preclude attempts to prolong gestation. These are the pregnancies where the prevention of SPTL or treatment to arrest the premature contractions (tocolysis) could logically diminish the occurrence of complications due to PTB and generally enhance neonatal outcomes. This group is the focus of this paper.
Despite increasing awareness within the scientific community of the importance of SPTL (Behrman et al., 2007; Howson et al., 2012), there has been little advancement towards understanding the mechanisms that determine the timing of birth. The result of this relative lack of progress is a dearth of novel strategies to prevent PTB. Indeed, over the past three decades, there have been no new, clinically proven pharmacological approaches to prevent PTB arising from SPTL.
The objective of this paper is to discuss potential new strategies for the prevention of PTB. We will begin by describing the general mechanisms that regulate smooth muscle contractility. We have recently reviewed this topic in more detail than will be presented here (Aguilar and Mitchell, 2010). Then we will consider potential mechanisms that are intrinsic to the smooth muscle myocyte and could act either by inhibition of pro-contractile mechanisms or stimulation of pro-relaxant pathways. We will then discuss the concept of specific regulatory mechanisms intrinsic to uterine myocytes that might support development of targets specifically to affect the uterus but without risk of adverse effects on other smooth muscle beds, particularly the cardiovascular system. Finally, we will discuss the potential of targeting pathways extrinsic to the uterine myocyte that could affect uterine contractility.
Regulation of smooth muscle contractility
The pivotal event for triggering the contractile machinery in smooth muscle is the phosphorylation of myosin regulatory light chains (RLC). As illustrated in Figure 1, this reaction is catalyzed by myosin regulatory light chain kinase (MLCK). The reverse reaction, leading to muscle relaxation, is mediated by myosin regulatory light chain phosphatase (MLCP). Oxytocin (OT) is the best-characterized and most potent uterine agonist. This hormone is produced in the hypothalamus and stored in the neurohypophysis of both mother and fetus. Perhaps more relevant to parturition, OT also is produced in the maternal decidua immediately adjacent to the uterine smooth muscle (myometrium) (Chibbar et al., 1993). Other potent agonists include endothelin-1 and prostaglandin (PG) F2α, which also are produced by maternal decidua (Arthur et al., 2008). Each of these agonists interact with specific membrane receptors linked to signaling pathways through small heterotrimeric GTPase proteins (G-proteins). The Gα subunits are key to determining which pathway will be activated. The Gαq/11 subunit mediates activation of adjacent phospholipase Cβ, which hydrolyses membrane phosphatidylinositol bisphosphate into two small ‘second messengers,’ inositol trisphosphate (IP3) and diacyl glycerol (DAG) (Berridge, 1993). IP3 stimulates the release of Ca2+ from the intracellular sarcoplasmic reticulum stores resulting in increased free intracellular Ca2+. The resultant depolarization activates and opens the voltage-dependent L-type Ca2+ channels on the myocyte membrane. Since the extracellular concentration of Ca2+ is approximately 5 orders of magnitude greater than the intracellular Ca2+ concentration, this results in rapid and massive influx of Ca2+ from the extracellular space to the intracellular space. The free intracellular Ca2+ binds to calmodulin (CaM) and the Ca2+-CaM complex subsequently binds to the activation site on MLCK, resulting in phosphorylation of RLC (here referred to as ‘pRLC’) and activation of the contractile apparatus. Because of this central role for Ca2+ in the initiation of myocyte shortening, this is known as the ‘Ca2+-dependent’ pathway of smooth muscle contraction.
More recently, there has been growing awareness of a ‘Ca2+-independent’ pathway. In the classical description of this pathway (Kozasa et al., 1998), the Gα12-14 subunit family initiates the signaling by activation, through a yet unknown mechanism, of a guanidine nucleotide exchange factor (GEF). This results in activation of the small monomeric G-protein rhoA-GDP by exchanging GTP for GDP. The major target of activated rhoA-GTP is rhoA-associated kinase (ROK), which is the principal effector of this pathway. A major target of ROK is the substrate binding subunit of MLCP. Phosphorylation of the targeting subunit prevents targeting to and dephosphorylation of pRLC by MLCP, thus promoting muscle contraction. This mechanism has been recognized as the explanation of the phenomenon of ‘Ca2+-sensitization,’ which describes a situation where the rise in intracellular Ca2+ concentration produces a larger-than-expected contraction. This effect is thought to be the result of simultaneous MLCP inhibition alongside MLCK activation (Somlyo and Somlyo, 2003). As we will discuss later, we believe this pathway may represent an important target for future tocolytics.
A third potential pathway for uterine myocyte stimulation is mediated by Gαi. This pathway does not appear to be a major regulator of uterine contractility but may be involved in transduction of signals arising from the PGE2 receptor isoform EP3 (Kotani et al., 2000) and OT (Phaneuf et al., 1996).
Heterotrimeric G-proteins also mediate a major mechanism for uterine relaxation. In this instance, the predominant isoform is Gαs. Activation of this subunit causes increased activity of membrane adenylyl cyclase (AC), which converts adenosine trisphosphate (ATP) to cyclic-5’-adenosine monophosphate (cAMP) and this in turn activates protein kinase A (PKA). This pathway is stimulated by endogenous and exogenous β-adrenergic agonists to cause uterine relaxation. Similarly, nitric oxide (NO) can directly activate cytosolic guanylyl cyclase (GC) in uterine myocytes to stimulate production of cyclic-5’-guanosine monophosphate (cGMP) with subsequent activation of PKG. PKA and PKG exhibit their relaxant effects on smooth muscle by acting on at least three targets. PKA mediates phosphorylation of the Ca2+-CaM binding site on MLCK, thereby hindering enzyme activation. PKA and PKG also increase the activity of the Ca2+-ATPases in the plasma membrane (PMCA) and sarcoplasmic and endoplasmic reticulum (SERCA) resulting in active extrusion of Ca2+ from the intracellular compartment and repolarization of the plasma membrane. Lastly, PKA and PKG can phosphorylate the MLCP targeting subunit at a site adjacent to the ROK target site, thus rendering MLCP insensitive to the inhibitory effects of ROK.
Non-specific mechanisms of tocolysis
Essentially all the above pathways regulating uterine contractility have been exploited in attempts to develop a useful tocolytic drug to arrest SPTL. Here, we will provide only a brief overview of these trials. More authoritative and in-depth reviews are available in the Cochrane Collaboration databases. Unfortunately, most of these pathways are active in all types of smooth muscle. Therefore, use of doses that will arrest uterine activity is often associated with serious off-target side effects, most notably hypotension and related cardiovascular complications.
The β-adrenergic agonists are the best-studied tocolytic class of drugs. Multiple good-quality randomized control trials (RCTs) demonstrated efficacy to delay delivery for at least 48-72 hours (King et al., 1988) but there is no compelling evidence of improved infant outcome. The maternal cardiovascular complications, such as hypotension and others noted above, were common and often led to serious adverse outcomes, including mortality. Use of lower doses to avoid these complications has not been shown to produce effective tocolysis. In addition, these drugs mimic the metabolic effects of β-adrenergic agents that can lead to hyperinsulinemia and hypokalemia, further potentiating the cardiovascular complications. These drugs are uncommonly used in current practice, though they have been introduced and are being promoted in developing countries.
Drugs that block Ca2+ channels, for example nifedipine, are among the most commonly used tocolytic agents today (King et al., 2003). Unfortunately, their use is not supported by clinical trial comparison to a placebo. This is an important consideration since, depending on the criteria for diagnosing SPTL and the rigor of the diagnosis, the success rate of placebo treatment might be well over 50%. Since there is no evidence that uterine myoctes in SPTL have any greater sensitivity to Ca2+ channel blockers than do vascular myocytes, it seems likely that concentrations required to provide effective tocolysis would be associated with similar cardiovascular complications as the β-adrenergic drugs. Indeed, safety concerns regarding the use of these drugs in pregnancy is the focus of a recent systematic review (Khan et al., 2010).
Another pharmacological approach has targeted the synthesis of PGF2α, which may activate the uterus, ripen the cervix or directly stimulate contractions. Most studies have used non-specific inhibitors of cyclooxygenase (COX-1 and COX-2), most commonly the non-steroidal anti-inflammatory drug (NSAID) indomethacin. Although the original studies showed dramatic beneficial effects (Zuckerman et al., 1974; Zuckerman et al., 1984), more recent studies have not demonstrated such strong effects. Other studies pointed to major fetal-neonatal side effects including oligohydramnios, patent ductus arteriosus, cerebral hemorrhage and necrotizing enterocolitis (Norton et al., 1993). Again, there has been no evidence suggesting improved outcome for the infant. Other NSAIDs, including COX-2-specific inhibitors have been used but have not been shown to be safer or more effective (Groom et al., 2005). The COX inhibitors block production of all prostanoids, including PGE2 and PGI2. The physiological roles of these hormones on human uterine function are extremely complex and may differ in the upper and lower segments of the uterus. It is likely that they affect uterine myocytes as well as connective tissue cells. When the roles of the various PGs are more clearly understood, it is possible that development of more selective drugs targeting enzymes or receptors in the PG pathway will be useful for clinical control of uterine contractility. At present, the NSAIDs appear not to have fulfilled their initial promise (Mitchell and Olson, 2004).
Another approach to tocolysis involves the use of NO donors to induce activation of PKG, as described above (Smith et al., 2007). Once again, due to the lack of uterine specificity of this approach, it is likely that significant uterine relaxation will be accompanied by vascular relaxation and increased maternal side effects, including the potential for decreased uterine blood flow.
Potential uterine-specific mechanisms of tocolysis
Oxytocin antagonists
The most potent contractile agonist for uterine myocytes appears to be OT. Relative to other tissues, OT receptor (OTR) is most highly expressed in the myometrium, decidua and myoepithelial cells of the breast. Therefore, regarding smooth muscle targets, OTR is relatively tissue specific for the uterus. The development of OTR antagonists began approximately 25 years ago and clinical trials demonstrated their efficacy just over a decade ago. In general, these drugs are short polypeptides that mimic the native molecule and interfere with OT binding to OTR. Not surprisingly, there is overlap with the receptor for vasopressin, which is a hormone with similar structure to OT. These drugs are expensive to produce and, as peptides, need to be administered parenterally. Though there is strong evidence of beneficial effects and few side effects in comparison to other tocolytic drugs, atosiban never received approval for use in the United States, perhaps because of a flaw in the placebo-controlled RCT to demonstrate the efficacy of the drug. In this trial (Romero et al., 2000), there was a very significant imbalance in the number of extremely preterm pregnancies (22-26 weeks) randomized to receive atosiban. The death rate in these babies at the brink of viability was very high. Because of this, when all babies were considered, there was an overall increase in the perinatal mortality rate of the babies receiving the drug compared to the placebo. The drug has been approved and is in common use in Europe. There are non-peptide OTR agonists that can be administered orally but trials of these in humans have not yet been published (Pettibone et al., 1995).
More recently, a different approach has been taken in the development of receptor antagonists. Rather than interfering with ligand binding, these ‘allosteric’ inhibitors interfere with other extracellular components of the seven transmembrane receptors and interfere with the signal transduction mechanisms. Indeed, such an inhibitor has been developed for the PGF2α receptor. Interestingly, this experimental drug (THG113.31) had little effect on PGF2α-induced contractions in human uterine strips ex vivo but was a good inhibitor of OT-induced contractions (Friel et al., 2005). Further studies demonstrated that this inhibitor has an inhibitory effect on the Gα12-14 signaling cascade but appears to enhance the Gαq/11 pathway (Goupil et al., 2010). This emphasizes that uterine contractility is regulated by two distinct and counterbalanced systems – the Ca2+-dependent, MLCK-mediated phosphorylation of RLC and the Ca2+-independent, MLCP-mediated dephosphorylation of pRLC. Development of effective tocolytic drugs requires consideration of both pathways. To date, most drug development has focused on the former. Our recent research has examined regulation of MLCP and, we believe, has uncovered a potentially important and unique feature of uterine myocytes that could provide an effective and specific pharmacological target for tocolysis.
Inhibition of the MLCP pathway
As would be expected from the schematic in Figure 1, inhibitors of ROK reduce the inhibitory restraints on MLCP, promote dephosphorylation of pRLC, and thereby lead to diminished contractility of smooth muscle. Several studies have shown inhibition of basal and OT-stimulated uterine contractions both in vivo and ex vivo in human and rat myometrium (Niiro et al., 1997; Kupittayanant et al., 2001; Moran et al., 2002; Woodcock et al., 2004; Woodcock et al., 2006). Unfortunately, interruption of the ROK pathway also affects vascular smooth muscle so this may represent a non-specific pathway and the potential use of ROK inhibitors for prevention or treatment of SPTL would be complicated by intolerable off-target effects in vascular or other smooth muscle.
Fig. 1.
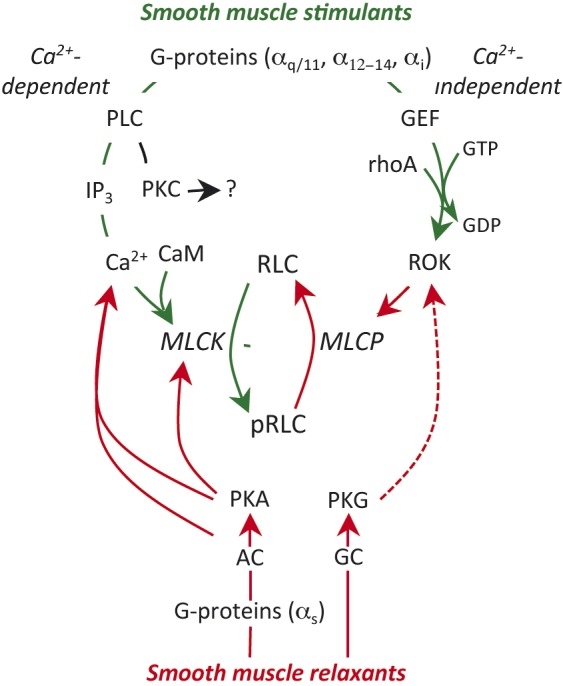
Regulation of smooth muscle contractility. Uterine stimulants generally stimulate specific G-protein coupled receptors (GPCR) on the myocyte membrane. This triggers two stimulatory pathways. The Gαq subunit activates phospholipase C (PLC) in the adjacent membrane and this results in hydrolysis of membrane phosphoinositides to from inositol trisphosphate (IP3) and diacyl glycerol, which can activate protein kinase C (PKC; see text). IP3 stimulates release of Ca2+ from the sarcoplasmic reticulum and the resulting decrease in cell membrane resting potential leads to massive influx of Ca2+ from the extracellular space through voltage–gated L-type Ca2+-channels. Ca2+ binds to calmodulin (CaM). The Ca2+-CaM complex interacts with and activates myosin regulatory light chain kinase (MLCK). MLCK mediates the phosphorylation of myosin regulatory lights chains (RLC) and initiates the contractile apparatus. The second stimulatory pathway is mediated through Gα12-14. This causes activation of a guanidine nucleotide exchange factor (GEF), which activates membrane rhoA-GDP by substituting GTP for the GDP. The rhoA-GTP activates rhoA-associated kinase (ROK). ROK phosphorylates myosin regulatory light chain phosphatase (MLCP), which inhibits its activity. Since MLCP is normally responsible for removing the phosphate from pRLC to cause uterine relaxation, enhanced ROK activity prolongs the effectiveness of pRLC and promotes enhanced contractile activity. The major inhibitory pathways might also be mediated by GPCR, linked through Gαs resulting in activation of adenylate cyclase (AC) or direct activation of guanylate cyclase (GC), which in turn activates protein kinase A (PKA) or PKG. These kinases inhibit contractility through a variety of mechanisms described in the text. The green lines indicate stimulatory activity and the red lines inhibitory. The broken line indicates an indirect effect.
We considered that the logical focus of our approach to modification of uterine myocyte activity would be the reversible phosphorylation of RLC. We developed relatively high throughput assays to assess this reaction using human uterine myocytes (Aguilar et al., 2010; Aguilar., 2011). Since we were primarily interested in uterine contractility during late pregnancy, we obtained uterine biopsies from the upper flap of the lower segment transverse uterine incision at the time of elective cesarean section at term but prior to clinical labor onset. Since it has been suggested that there might be regional differences in physiological function for myocytes located in the upper more contractile part of the uterus compared myocytes located in the lower segment, we isolated myocytes from both uterine regions to validate that the cells from the lower segment were representative of those in the fundus. For these experiments, we obtained paired (upper and lower) uterine biopsies. The lower segment biopsies were taken as described above. The fundal biopsy was obtained by first excising the overlying decidua, then excising a small myometrial biopsy using iris scissors.
For the first step in validation, we used RT-PCR to examine expression of mRNA for OTR in primary cultures of uterine myocytes from the upper (fundal) and lower parts of the uterus at several passages. In Figure 2 it is seen that OTR expression is similar in both regions and is maintained through several passages of cells. A Ca2+ assay was then used to investigate the response of these cells to OT (Fig. 3). Treatment of fundal and lower segment myocytes with OT resulted in a concentration-dependent increase in intracellular Ca2+. There was no difference in the responses between the fundal and lower segment cells.
Fig. 2.
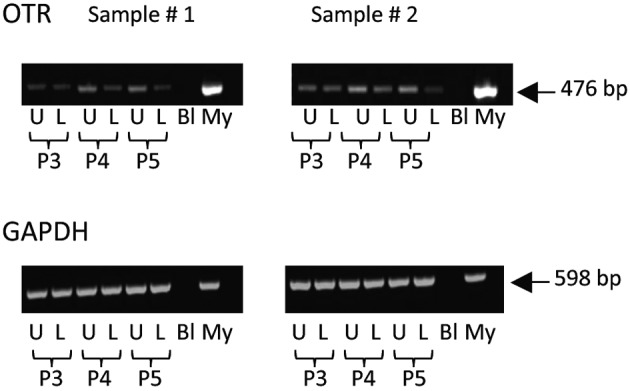
Expression of mRNA for oxytocin receptor in myocytes derived from upper and lower uterine segments. PCR of extracts from primary cultures of uterine myocytes derived from paired samples of uterine muscle (n = 2 patients) obtained from the upper fundal portion of the uterus (U) or the lower uterine segment (L) along with molecular weight markers (M), blanks (Bl) and extracts from myometrial tissues (My). The cells were used at passage 3, 4 and 5 (P3-P5). The upper panels demonstrate expression of OTR (476 bp) and the bottom panels show expression of GAPDH (598 bp) as a control. OTR is expressed in upper and lower segment myocytes and does not change with passage number.
Fig. 3.
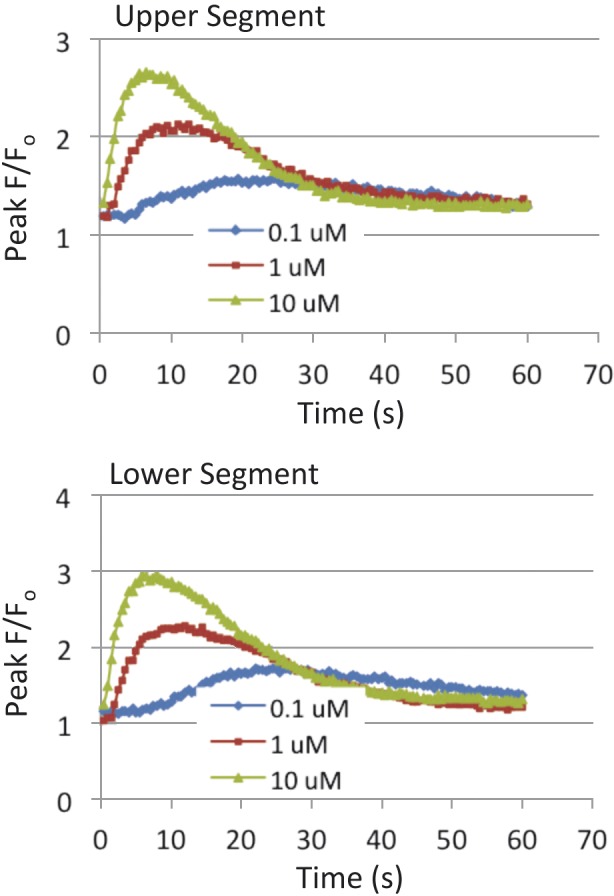
Intracellular concentrations of Ca2+ following stimulation of uterine myocytes with oxytocin. Primary cultures of fundal (upper panel) and lower (lower panel) segment uterine myocytes were plated in Greiner Bio-One black plates (5.0 × 104 cells/ml) and grown for 48 hours. Cells were then loaded with Fluo-4 dye and incubated at 37°C for 30 minutes. OT (0.1 µM, 1 µM, 10 µM) was added to the cells and fluorescence was measured. Data are presented as peak fluorescence over baseline fluorescence (F/F0; mean ± SEM; n = 4 patients).
We then demonstrated (Fig. 4) concentration-dependent, and statistically significant increases in concentrations of pRLC following stimulation with OT. These responses were similar in cells from fundal and lower segment biopsies. The peak responses in phosphorylation of RLC occurred within the first 20 s, in accord with the Ca2+ data in Figure 3. We noted that the potent and specific ROK inhibitor glycyl-H1152 (g-H) caused a 40-50% reduction of resting pRLC concentrations but the response to OT was maintained. A similar response profile was demonstrated using the MLCK inhibitor ML7. Again, these responses were similar in cells from the fundal and lower uterine segments. We also measured responses for diphosphorylated RLC (ppRLC; Fig. 5) and again noted significant responses to OT, which were similar in the cells from fundal and lower segments. Treatment with the inhibitors of ROK or MLCK again caused suppression of basal ppRLC concentrations. However, unlike pRLC, the responses of ppRLC to OT were blocked by either of the inhibitors, again similarly in cells from the fundal and lower segments.
Fig. 4.
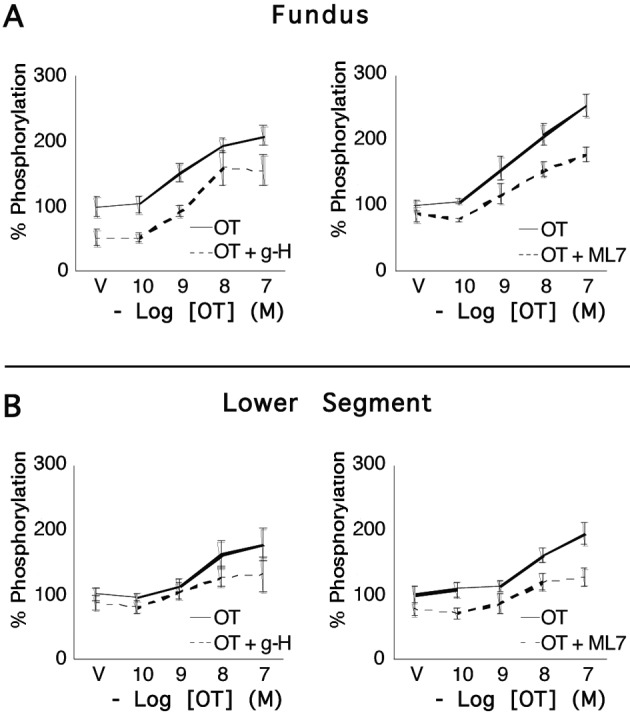
Concentrations of pRLC in uterine myocytes from upper and lower uterine segments following stimulation by oxytocin. Primary cultures of myocytes from the uterine fundus and lower uterine segments (n = 5 patients) were treated with increasing concentrations of OT for 20 s and the response measured using a specific antibody for pRLC phosphorylated at S19 in an in-cell western assay. There were significant, concentration-dependent increases that were similar in cells from upper and lower segments. 15-minute pretreatment with the rho-kinase inhibitor (g-H; 1 µM) or the myosin regulatory light chain kinase inhibitor (ML7; 25 µM) caused approximately 20-30% suppression of basal pRLC but the response to OT was maintained.
Fig. 5.
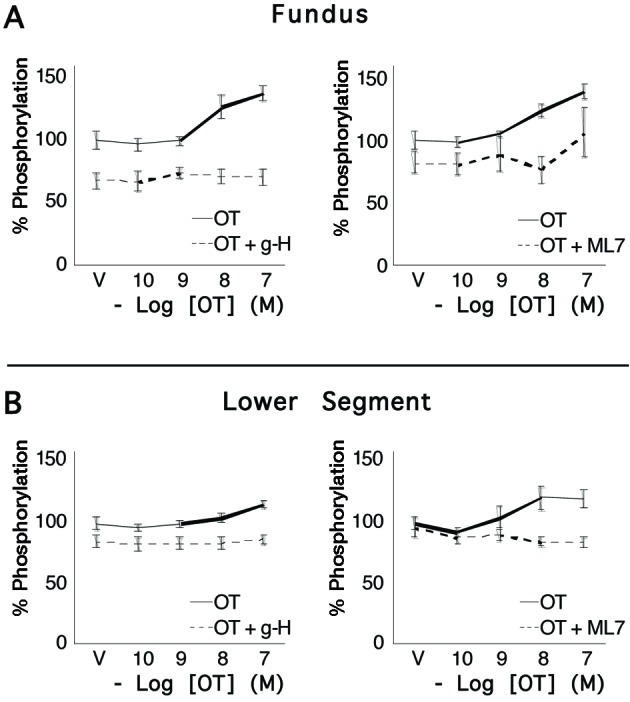
Concentrations of ppRLC in uterine myocytes from upper and lower uterine segments following stimulation by oxytocin. Primary cultures of myocytes from the uterine fundus and lower uterine segments (n = 5 patients) were treated with increasing concentrations of OT for 20 s and the response measured using a specific antibody for ppRLC phosphorylated at T18 and S19 in an in-cell western assay. There were significant, concentration-dependent increases in ppRLC in cells from upper and lower segments. As for pRLC, 15-mimute pretreatment with the rho-kinase inhibitor (g-H; 1 µM) or the myosin regulatory light chain kinase inhibitor (ML7; 25 µM) caused approximately 20-30% suppression of basal ppRLC. However, in contrast to the results for pRLC, the ppRLC responses to OT were completely abolished in the presence of the kinase inhibitors.
We have reported previously on the results of a series of experiments specifically exploring the role of ROK and MLCK in mono- and diphosphorylation of RLC in uterine myocytes following stimulation with physiological uterine agonists (Aguilar et al., 2010; 2011; 2012). We made specific comparisons to the responses of vascular myocytes to physiological agonists. There were two major conclusions from these experiments. The first conclusion was that uterine myocytes respond to stimulation with significant increases in both pRLC and ppRLC whereas the vascular myocytes respond only with pRLC. The responses in pRLC are due to the well-described effects of physiological agonists: increased Ca2+-dependent MLCK-mediated phosphorylation of RLC and increased ROK-mediated inhibition of MLCP.
The second major conclusion of these studies was a novel and potentially important finding. The increased ppRLC was produced by ROK-mediated phosphorylation of pRLC to ppRLC. Since this biochemical conversion of pRLC to ppRLC was not noted in the vascular myocytes, we speculated that it might be a unique feature of smooth muscle with a phasic contractile pattern, such as the uterus. Since ppRLC causes 3-fold greater activation of myosin ATPase, which provides the energy for the contractile apparatus (Ikebe et al., 1986; 1988), we reasoned that diphosphorylation of RLC may represent an evolutionary adaptation of phasic smooth muscle driven by the need to produce periodic but powerful contractions, in contrast to tonic smooth muscle such as vascular, which requires long-term stable tension with relatively little change in amplitude. Importantly, the ROK inhibitor prevented the ppRLC response to OT in uterine myocytes in vitro at concentrations that were 100-fold lower that those required to decrease the concentration of pRLC in vascular myocytes. Thus, inhibition of ROK has potential to be a clinically useful and utero-specific target to mediate uterine relaxation while avoiding vascular compromise. Our current concept of the mechanisms regulating phosphorylation of RLC specifically in uterine myocytes is presented in the schematic in Figure 6.
Fig. 6.
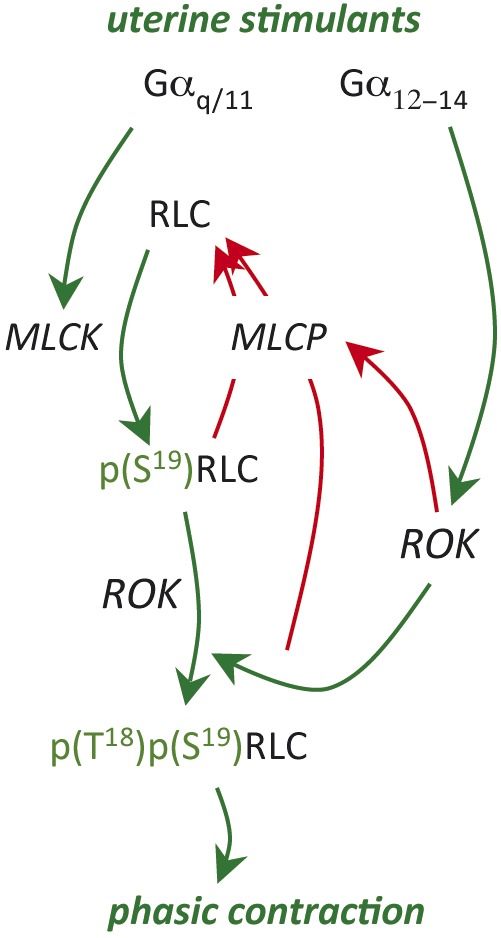
Schematic diagram illustrating the unique pattern of phosphorylation of RLC in uterine myocytes. As in all smooth muscle, myosin regulatory light chain (RLC) is phosphorylated by myosin regulatory light chain kinase (MLCK) at S19. However, in uterine myocytes, pRLC is additionally phosphorylated at T18 to form ppRLC, a more potent activator of the myosin ATPase that supplies energy for the contraction. This diphosphorylation pathway might be a unique adaptive pathway for smooth muscle that contract in a phasic pattern. The green lines indicate stimulatory activity and the red lines inhibitory.
Extrinsic influences on uterine myocytes
The previous discussions have considered mechanisms that are intrinsic to the uterine myocyte. Clearly, there are mechanisms extrinsic to the myocyte that will influence myocyte responses to agonists or antagonists. Indeed, SPTL is likely the end result of many differing pathophysiologic pathways that involve intrinsic and/or extrinsic pathways. As such, SPTL represents a syndrome rather than a specific disease entity. Here, we discuss some of the mechanisms extrinsic to the uterine myocyte that could provide pharmacological targets to influence uterine contractility.
Concept of uterine activation and stimulation
Several years ago, a useful concept was proposed suggesting that the status of uterine contractility during pregnancy could be considered in three categories (Challis et al., 2000). Uterine quiescence was the period that began at implantation and lasted most of pregnancy, during which the uterus remained relatively quiet. Next was the period of uterine activation, which occurred over a few days prior to clinical labor onset. During this time, the density of ‘contraction associated proteins’ (CAPs) increased to transform the uterus into a sensitive contractile organ capable of responding to agonists with powerful, coordinated contractions. Included among the CAPs are the receptors for uterine agonists (OT, PGF2α, etc.), a variety of ion channels, and the major gap junction protein connexin 43. Uterine stimulation occurs due to the actions of endogenous agonists such as OT, PGF2α, etc. There remains only poor understanding of the identity of the key endogenous agonist(s). Regulation of these processes can be considered ‘upstream’ of the uterine myocyte, though the mechanisms are similarly unclear. These mechanisms might represent earlier targets for interruption of the cascade of events leading to parturition. The following sections deal with two examples of such potential extrinsic regulatory systems.
Estrogen/progesterone ratio
Over the past half century, it has been demonstrated that, in most mammalian species, withdrawal of progesterone from the maternal circulation is necessary and sufficient to cause parturition. However, in higher order primates, including humans, there is no such decline in maternal serum progesterone at the time of parturition. It has been proposed that, in these species, progesterone still may be an essential progestational hormone but its withdrawal at the time of parturition occurs on a more local, intrauterine basis that is not reflected in maternal serum. Various mechanisms have been suggested for a ‘functional progesterone withdrawal.’ These include decreased synthesis or increased metabolism within the amnion, chorion and decidua (Mitchell and Powell, 1984). These tissues lay immediately adjacent to the myometrium and thus might have significant influence over progesterone effects on uterine myocytes. Similarly, changes in expression and activity of progesterone receptors within the uterine myocyte could regulate progesterone-dependent transcriptional activity (Mesiano et al., 2002; Condon et al., 2003).
Over the past decade, there has been renewed interest in the use of supplemental progesterone to prevent PTB in women at high-risk. Based on the results of a 30-year old trial (Johnson et al., 1975), an RCT demonstrated some efficacy in preventing PTB using prophylactic progestin supplementation over the last half of gestation (Meis et al., 2003). The drug used was 17-hydroxyprogesterone caproate, which clearly is not progesterone and its mechanism of action is not fully understood. However, the results were supported by an additional RCT using natural progesterone administered by vaginal suppository, which also showed a statistically significant improvement on the rates of PTB (da Fonseca et al., 2003). Though there is increasing use of this prophylactic regimen for woman at high risk of PTB, it remains unclear what the outcomes will be regarding the health of the newborn. Prophylactic use of progestin also has been shown to be efficacious in women with a short cervix demonstrated on routine mid-pregnancy ultrasound (Romero et al., 2012). The cost-effectiveness of this strategy of ultrasound screening and progestin administration and the potential longer-term effects of this approach remain to be determined.
Research from several animal species has also suggested that estrogen plays an important role in the timing of parturition. In general, its effects tend to oppose those of progesterone. This has given rise to the concept of the ratio of estrogen/progesterone as a key factor. In particular, estrogen has been demonstrated to enhance transcription of many of the genes that code for CAP proteins while progesterone can block the increase in CAP mRNA expression (Ou et al., 1997; 1998; 2000). As with progesterone, local estrogen synthesis and metabolism might be of primary importance (Mitchell & Wong, 1993; Romero et al., 1988). Estrogen antagonists will cause delay in rat parturition but this is complicated by decreased growth rate of the pups (Fang et al., 1996). In theory, the potentially important negative effects of administration of estrogen antagonists outweigh their potential to delay birth. In addition, there is no evidence that they are effective in SPTL.
Immune system
It has been known for decades that parturition, whether at term or preterm, is accompanied by an inflammatory response resulting in an invasion of leukocytes into intrauterine tissues (Liggins, 1981). Abundant recent research has extensively characterized the pro-inflammatory responses that accompany parturition, either with or without infection at term or preterm. However, the purpose of this inflammatory response remains unclear. Some have reasoned that this enhanced activity of the immune system is focused on healing the placental site following parturition and mediating the process of uterine involution (Mitchell and Taggart, 2009). Others have suggested that the inflammatory response initiates the process of parturition. It is possible that both lines of thought could be correct. From the evolutionary perspective, activation of the healing response concurrently with the process that leads to increased uterine contractility would be logical and beneficial. Linking the two processes such that they are interdependent would ensure the two distinct and vital activities act together. This might be analogous to the situation in sheep where increased activity of the uterus is linked to the same mechanisms that mature the fetal lungs in preparation for extrauterine existence.
To date, the role of the immune system in the initiation of parturition, though extensively studied, remains unresolved. It is clear that, in the presence of intrauterine infection, this inflammatory reaction is markedly enhanced. There is convincing evidence that a severe intrauterine inflammatory event, such as infection or chemical irritation can provoke parturition at any stage of gestation. However, the role of the pro-inflammatory reactions in regulation of term labor and SPTL require further clarification. Similarly, there has been elegant demonstration of the effects of uterine stretch on pathways thought to be important in regulating parturition, such as the up-regulation of CAPs (Ou et al., 1997; Terzidou et al., 2005). However, again, the role of such mechanisms in determining the occurrence of labor at term or preterm is not well understood.
At present, the research has yet to produce clinically effective therapeutic tools for tocolysis. Historically, the extensive RCTs using large doses of glucocorticoids administered to women with SPTL to accelerate fetal lung maturation have demonstrated clearly that these potent anti-inflammatory agents had no clinically significant effect on the timing of birth. In the cases of infection-associated PTL, where the inflammatory response may be the driving force to PTB, current practice is to hasten delivery and avoid the use of tocolytics. In summary, despite massive research activity, the role of immunomodulators in the management of SPTL has yet to be defined.
Conclusions
It is clear that PTB can arise from many distinct etiologic pathways involving a myriad of physiological and pathophysiological mechanisms. Some of these arise from within the target cells – the uterine myocytes – and some involve systemic extrinsic systems that modulate uterine myocyte activity. The mechanistic pathways concern many membrane proteins of the uterine myocyte including GPCR and ion channels, among many others. Many intracellular signal transduction pathways, including the Ca2+-CaM-MLCK and the rhoA-ROK-MLCP pathways, are likely to be important. Extrinsic influences such as the estrogen/progesterone ratio, uterine stretch and the immune system are likely to play important modulatory or even causative roles. We have considered each of these pathways, and undoubtedly many more, to be modules that affect uterine contractions. Though not included in this discussion, there are many more modules that potentially regulate the process of cervical ripening. When a critical number of modules are activated, labor will occur: this is the concept of modular accumulation of physiological systems (MAPS) that we have proposed earlier (Mitchell and Taggart, 2009).
In view of this probable heterogeneity of pathways contributing to the occurrence of SPTL, future attempts at tocolytic therapy might need to be broad-based and include combinations of targets. Future research using genomic, proteomic and metabolomic technologies holds the promise of predicting women at risk of SPTL and hopefully indicate the modules that are prematurely activated. This will facilitate a preventative rather than a therapeutic (tocolytic) approach, which is likely to have many advantages. Combination pharmacological regimens will necessitate even more attention to uterine-specific therapies to avoid off-target effects that will preclude the ability to use effective doses. Drug interactions will then become even more important considerations and pharmacogenomic approaches may help resolve these issues. Whereas the past has been disappointing with respect to prevention of PTB, the future hold new promise that every baby can be born at its optimal time.
References
- Aguilar HN, Mitchell BF. Physiological Pathways and Molecular Mechanisms Regulating Uterine Contractility. Hum Reprod Update. 2010;16:725–744. doi: 10.1093/humupd/dmq016. [DOI] [PubMed] [Google Scholar]
- Aguilar HN, Tracey CN, Tsang SC, et al. Phos-Tag-Based Analysis of Myosin Regulatory Light Chain Phosphorylation in Human Uterine Myocytes. PLoS One. 6:e20903. doi: 10.1371/journal.pone.0020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar HN, Tracey CN, Zielnik B, Mitchell BF. Quantification of Rapid Myosin Regulatory Light Chain Phosphorylation Using High-Throughput in-Cell Western Assays: Comparison to Western Immunoblots. PLoS One. 2010;5:e9965. doi: 10.1371/journal.pone.0009965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar HN, Tracey CN, Zielnik B, Mitchell BF. Rho-kinase mediates diphosphorylation of myosin regulatory light chain in cultured uterine but not vascular smooth muscle cells. http://onlinelibrary.wiley.com/doi/ 10.1111/j.1582-4934.2012.01625.x/abstract. J Cell Mol Med. 2012 doi: 10.1111/j.1582-4934.2012.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur P, Taggart MJ, Zielnik B, Mitchell BF. Relationship between Gene Expression and Function of Uterotonic Systems in the Rat During Gestation, Uterine Activation and Both Term and Preterm Labour. J Physiol. 2008;586:6063–6076. doi: 10.1113/jphysiol.2008.164004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrman RE, Butler AS. Washington: National Academies Press; 2007. Preterm Birth: Causes, Consequences, and Prevention. [PubMed] [Google Scholar]
- Berridge M J. Inositol Trisphosphate and Calcium Signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Challis JRG, Matthews SG, Gibb W, et al. Endocrine and Paracrine Regulation of Birth at Term and Preterm. Endocr Rev. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- Chibbar R, Miller FD, Mitchell BF. Synthesis of Oxytocin in Amnion, Chorion, and Decidua May Influence the Timing of Human Parturition. J Clin Invest. 1993;91:185–192. doi: 10.1172/JCI116169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon JC, Jeyasuria P, Faust JM, et al. A Decline in the Levels of Progesterone Receptor Coactivators in the Pregnant Uterus at Term May Antagonize Progesterone Receptor Function and Contribute to the Initiation of Parturition. Proc Natl Acad Sci USA. 2003;100:9518–9523. doi: 10.1073/pnas.1633616100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Fonseca EB, Bittar RE, Carvalho MH, et al. Prophylactic Administration of Progesterone by Vaginal Suppository to Reduce the Incidence of Spontaneous Preterm Birth in Women at Increased Risk: A Randomized Placebo-Controlled Double-Blind Study. 2003;188:419–424. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- Fang X, Wong S, Mitchell BF. Relationships among Sex Steroids, Oxytocin, and Their Receptors in the Rat Uterus During Late Gestation and at Parturition. Endocrinology. 1996;137 doi: 10.1210/endo.137.8.8754742. [DOI] [PubMed] [Google Scholar]
- Friel AM, O’Reilly MW, Sexton DJ, et al. Specific Pgf(2alpha) Receptor (Fp) Antagonism and Human Uterine Contractility in Vitro. BJOG. 2005;112:1034–1042. doi: 10.1111/j.1471-0528.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- Goupil E, Tassy D, Bourguet C, et al. A Novel Biased Allosteric Compound Inhibitor of Parturition Selectively Impedes the Prostaglandin F2alpha-Mediated Rho/Rock Signaling Pathway. J Biol Chem. 2010;285:25624–25636. doi: 10.1074/jbc.M110.115196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom KM, Shennan AH, Jones BA, et al. Tocox – a Randomised, Double-Blind, Placebo-Controlled Trial of Rofecoxib (a Cox-2-Specific Prostaglandin Inhibitor) for the Prevention of Preterm Delivery in Women at High Risk. BJOG. 2005;112:725–730. doi: 10.1111/j.1471-0528.2005.00539.x. [DOI] [PubMed] [Google Scholar]
- Howson CP, Kinney MV, Lawn JE. Geneva: Save the Children; 2012. Born Too Soon: The Global Action Report on Preterm Birth. March of Dimes, PMNCH. [Google Scholar]
- Ikebe M, Hartshorne DJ, Elzinga M. Identification, Phosphorylation, and Dephosphorylation of a Second Site for Myosin Light Chain Kinase on the 20,000-Dalton Light Chain of Smooth Muscle Myosin. J Biol Chem. 1986;261:36–39. [PubMed] [Google Scholar]
- Ikebe M, Koretz J, Hartshorne DJ. Effects of Phosphorylation of Light Chain Residues Threonine 18 and Serine 19 on the Properties and Conformation of Smooth Muscle Myosin. J Biol Chem. 1988;263:6432–6437. [PubMed] [Google Scholar]
- Johnson JW, Austin KL, Jones GS, et al. Efficacy of 17alpha-Hydroxyprogesterone Caproate in the Prevention of Premature Labor. N Engl J Med. 1975;293:675–680. doi: 10.1056/NEJM197510022931401. [DOI] [PubMed] [Google Scholar]
- Khan K, Zamora J, Lamont RF, et al. Safety concernsfor the use of calcium channel blockersin pregnancy for the treatment of spontaneous preterm labour and hyprtension: a systematic review and regression analysis. J Mat-Fetal Neonatal Med. 2010;23(9):1030–1038. doi: 10.3109/14767050903572182. [DOI] [PubMed] [Google Scholar]
- King JF, Flenady VJ, Papatsonis DN, et al. Calcium Channel Blockers for Inhibiting Preterm Labour. Cochrane Database Syst Rev. 2003;(CD002255) doi: 10.1002/14651858.CD002255. [DOI] [PubMed] [Google Scholar]
- King JF, Grant A, Keirse MJ, et al. Beta-Mimetics in Preterm Labour: An Overview of the Randomized Controlled Trials. Br J Obstet Gynaecol. 1988;95:211–222. doi: 10.1111/j.1471-0528.1988.tb06860.x. [DOI] [PubMed] [Google Scholar]
- Kotani M, Tanaka I, Ogawa Y, et al. Multiple Signal Transduction Pathways through Two Prostaglandin E Receptor Ep3 Subtype Isoforms Expressed in Human Uterus. J Clin Endocrinol Metab. 2000;85:4315–4322. doi: 10.1210/jcem.85.11.6989. [DOI] [PubMed] [Google Scholar]
- Kozasa T, Jiang X, Hart MJ, et al. P115 Rhogef, a Gtpase Activating Protein for Galpha12 and Galpha13. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- Kupittayanant S, Burdyga T, Wray S. The Effects of Inhibiting Rho-Associated Kinase with Y-27632 on Force and Intracellular Calcium in Human Myometrium. Pflugers Arch. 2001;443:112–114. doi: 10.1007/s004240100668. [DOI] [PubMed] [Google Scholar]
- Liggins GC. Edinburgh: Churchill Livigstone; 1981. Cervical Ripening as an Inflammatory Reaction. [Google Scholar]
- Meis PJ, Klebanoff M, Thom E, et al. Prevention of Recurrent Preterm Delivery by 17 Alpha-Hydroxyprogesterone Caproate. N Engl J Med. 2379. 2003;348 doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Chan EC, Fitter JT, et al. Progesterone Withdrawal and Estrogen Activation in Human Parturition Are Coordinated by Progesterone Receptor a Expression in the Myometrium. J Clin Endocrinol Metab. 2002;87:2924–2930. doi: 10.1210/jcem.87.6.8609. [DOI] [PubMed] [Google Scholar]
- Mitchell BF, Olson DM. Prostaglandin Endoperoxide H Synthase Inhibitors and Other Tocolytics in Preterm Labour. Prostaglandins Leukot Essent Fatty Acids. 2004;70 doi: 10.1016/j.plefa.2003.04.006. [DOI] [PubMed] [Google Scholar]
- Mitchell BF, Powell WA. Progesterone Production by Human Fetal Membranes: An in Vitro Incubation System for Studying Hormone Production and Metabolism. Am J Obstet Gynecol. 1984;148:303–309. doi: 10.1016/s0002-9378(84)80073-3. [DOI] [PubMed] [Google Scholar]
- Mitchell BF, Taggart MJ. Are Animal Models Relevant to Key Aspects of Human Parturition? Am J Physiol Regul Integr Comp Physiol. 2009;297:R525–545. doi: 10.1152/ajpregu.00153.2009. [DOI] [PubMed] [Google Scholar]
- Mitchell BF, Wong S. Changes in 17 Beta,20 Alpha-Hydroxysteroid Dehydrogenase Activity Supporting an Increase in the Estrogen/Progesterone Ratio of Human Fetal Membranes at Parturition. Am J Obstet Gynecol. 1993;168:1377–1385. doi: 10.1016/s0002-9378(11)90768-6. [DOI] [PubMed] [Google Scholar]
- Moran CJ, Friel AM, Smith TJ, et al. Expression and Modulation of Rho Kinase in Human Pregnant Myometrium. Mol Hum Reprod. 2002;8:196–200. doi: 10.1093/molehr/8.2.196. [DOI] [PubMed] [Google Scholar]
- Niiro N, Nishimura J, Sakihara C, et al. Up-Regulation of Rho a and Rho-Kinase mRnas in the Rat Myometrium During Pregnancy. Biochem Biophys Res Commun. 1997;230:356–359. doi: 10.1006/bbrc.1996.5960. [DOI] [PubMed] [Google Scholar]
- Norton ME, Merrill J, Cooper BA, et al. Neonatal Complications after the Administration of Indomethacin for Preterm Labor. N Engl J Med. 1993;329:1602–1607. doi: 10.1056/NEJM199311253292202. [DOI] [PubMed] [Google Scholar]
- Ou CW, Chen ZQ, Qi S, et al. Expression and Regulation of the Messenger Ribonucleic Acid Encoding the Prostaglandin F(2alpha) Receptor in the Rat Myometrium During Pregnancy and Labor. Am J Obstet Gynecol. 2000;182:919–925. doi: 10.1016/s0002-9378(00)70347-4. [DOI] [PubMed] [Google Scholar]
- Ou CW, Chen ZQ, Qi S, et al. Increased Expression of the Rat Myometrial Oxytocin Receptor Messenger Ribonucleic Acid During Labor Requires Both Mechanical and Hormonal Signals. Biol Reprod. 1998;59:1055–1061. doi: 10.1095/biolreprod59.5.1055. [DOI] [PubMed] [Google Scholar]
- Ou CW, Orsino A, Lye SJ. Expression of Connexin-43 and Connexin-26 in the Rat Myometrium During Pregnancy and Labor Is Differentially Regulated by Mechanical and Hormonal Signals. Endocrinology. 1997;138:5398–5407. doi: 10.1210/endo.138.12.5624. [DOI] [PubMed] [Google Scholar]
- Pettibone DJ, Guidotti M, Harrell CM, et al. Progress in the Development of Oxytocin Antagonists for Use in Preterm Labor. Adv Exp Med Biol. 1995;395:601–612. [PubMed] [Google Scholar]
- Phaneuf S, Carrasco MP, Europe-Finner GN, et al. Multiple G Proteins and Phospholipase C Isoforms in Human Myometrial Cells: Implication for Oxytocin Action. J Clin Endocrinol Metab. 1996;81:2098–2103. doi: 10.1210/jcem.81.6.8964834. [DOI] [PubMed] [Google Scholar]
- Romero R, Nicolaides K, Agudelo A, et al. Vaginal Progesterone in Women with an Asymptomatic Sonographic Short Cervix in the Midtrimester Decreases Preterm Delivery and Neonatal Morbidity: A Systematic Review and Metaanalysis of Individual Patient Data. Am J Obstet Gynecol. 2012;206(124) doi: 10.1016/j.ajog.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Sibai BM, Ramos L, et al. An oxytocin receptor antagonist (atosiban) in the treatment of preterm labor: a randomized, double-blind, placebo-controlled trial with tocolytic rescue. Am J Obstet Gynecol. 2000;182:1173–1183. doi: 10.1067/mob.2000.95834. [DOI] [PubMed] [Google Scholar]
- Romero R, Scoccia B, Mazor M, et al. Evidence for a Local Change in the Progesterone/Estrogen Ratio in Human Parturition at Term. Am J Obstet Gynecol. 1988;159:657–660. doi: 10.1016/s0002-9378(88)80029-2. [DOI] [PubMed] [Google Scholar]
- Smith GN, Walker , MC , Ohlsson A, et al. Randomized Double-Blind Placebo-Controlled Trial of Transdermal Nitroglycerin for Preterm Labor. Am J Obstet Gynecol. 2007;196(37):e31–38. doi: 10.1016/j.ajog.2006.10.868. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ Sensitivity of Smooth Muscle and Nonmuscle Myosin Ii: Modulated by G Proteins, Kinases, and Myosin Phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Terzidou V, Sooranna SR, Kim LU, et al. Mechanical Stretch up-Regulates the Human Oxytocin Receptor in Primary Human Uterine Myocytes. J Clin Endocrinol Metab. 2005;90:237–246. doi: 10.1210/jc.2004-0277. [DOI] [PubMed] [Google Scholar]
- Woodcock NA, Taylor W, Thornton S. Effect of an Oxytocin Receptor Antagonist and Rho Kinase Inhibitor on the [Ca++]I Sensitivity of Human Myometrium. Am J Obstet Gynecol. 2004;190:222–228. doi: 10.1016/s0002-9378(03)00925-6. [DOI] [PubMed] [Google Scholar]
- Woodcock NA, Taylor CW, Thornton S. Prostaglandin F2alpha Increases the Sensitivity of the Contractile Proteins to Ca2+ in Human Myometrium. Am J Obstet Gynecol. 2006;195:1404–1406. doi: 10.1016/j.ajog.2006.03.099. [DOI] [PubMed] [Google Scholar]
- Zuckerman H, Reiss U, Rubinstein I. Inhibition of Human Premature Labor by Indomethacin. Obstet Gynecol. 1974;44:787–792. [PubMed] [Google Scholar]
- Zuckerman H, Shalev E, Gilad G, et al. Further Study of the Inhibition of Premature Labor by Indomethacin. Part I. J Perinat Med. 1984;12:19–23. doi: 10.1515/jpme.1984.12.1.19. [DOI] [PubMed] [Google Scholar]


