Abstract
In pregnancy, both maternal vascular tone and cardiac function are considered key players to reach a normal outcome for both mother and child. This complex story of maternal hemodynamics is intensely discussed in current scientific literature, however the role of the maternal veins has been strongly underestimated.
We developed and evaluated a set of measurable objective parameters which give an indication of venous function, i.e. the venous impedance index and the venous pulse transit time. These parameters turned out to be subject to changes throughout normal pregnancy and in preeclampsia enabling their use in gestational hemodynamic studies.
From our studies, we concluded that the venous system is a crucial determinant of cardiac output, which can be estimated by impedance cardiography. The introduction of these non-invasive techniques in obstetrics enables profiling the maternal cardiovascular system, integrating both arteries and veins, as well as maternal cardiac function.
Studying the cascade of cardiovascular changes throughout pregnancy using such non-invasive, easily applicable, and highly accessible methods opens perspectives to introduce this maternal cardiovascular profile in several clinical settings. The early discrimination between low and high risk patients, together with the classification of different pregnancy disorders may help guiding the clinical work-up of the pregnant population regarding both prevention and treatment, as well as follow-up.
We illustrate that the venous system, being an “ugly duckling” at first neglected by the medical world, transforms and matures into a beautiful swan, accepted by the obstetric world. We are confident that this is the beginning of many other studies regarding the maternal venous system, an important piece of the gestational physiology puzzle.
Keywords: cardiovascular system, Doppler, impedance cardiography, preeclampsia, pregnancy, veins
Introduction
Human pregnancy is characterized by several profound changes at the maternal cardiovascular level; a decrease in peripheral vascular resistance is most likely the first adaptation mechanism in early pregnancy (Duvekot et al., 1993; Carbillon et al., 2000). This systemic decrease in tonicity during pregnancy is thought to be related to uteroplacental-induced vasoactive substances which interact with the inner layer of the blood vessel, i.e. the endothelium (Duvekot and Peeters, 1994a; Carbillon et al., 2000; Hagedorn et al., 2007). This fall in systemic vascular tone affects both the arteries and the veins, and in turn will affect renine-angiotensin and other osmoregulatory systems, therefore facilitating volume retention (Duvekot et al., 1994; Fu and Levine, 2009). Due to this plasma volume expansion, a preload-induced reversible remodelling of the maternal heart, i.e. a left ventricular eccentric hypertrophy, occurs during normal pregnancy (Simmons et al., 2002; Melchiorre et al., 2012).
In this adaptation process, the role of the venous system is still rather underestimated. However, together with the observed plasma volume expansion, the increase in venous capacitance, due to enhanced venous distensibility, provides a larger buffer to control and regulate cardiac output in pregnancy (Sakai et al., 1994; van Oppen et al., 1996a; van Oppen et al., 1996b; Krabbendam and Spaanderman, 2007).
Preeclampsia, defined as pregnancy-induced hypertension with de novo proteinuria, is a serious pregnancy disorder affecting 7 to 10% of all pregnancies together with a high morbidity and mortality for both mother and child (Sibai and Stella, 2009; Reece, 2010). The pathophysiological mechanism behind this disorder is suggested to be related to endothelial dysfunction caused by intolerance to the normal plasma volume expansion (Bernstein et al., 1998). This indicates the importance of the increase in venous distensibility in this adaptive mechanism, which in turn is necessary for the shift between unstressed and stressed volumes to regulate maternal cardiac output (Gelman, 2008). Maternal cardiac output is reported to be related to foetal growth (Desai et al., 2004; Jia et al., 2010).
However, several reports show the existence of preeclampsia with either a low or a high maternal cardiac output, and thus the association with both foetal growth restriction and macrosomic infants respectively, suggesting several types of preeclampsia (Easterling et al., 1991; Valensise et al., 2008). These different cardiac output states are possibly related to different characteristics of the maternal heart, arteries and veins.
Hypothesis and strategies
We hypothesize that global cardiovascular profiling in pregnancy, including heart, arteries and veins, might allow (a) defining a risk profile for gestational disorders with placental dysfunction as common denominator and (b) guiding the clinical work-up of this high-risk population. The main research question in this thesis was: Does including the concomitant information of the dynamics of the maternal venous system ameliorate cardiovascular profiling in pregnancy? To evaluate this, we divided our study into several subquestions based on three main methodological strategies: 1) Doppler ultrasonography, 2) impedance cardiography, and 3) the maternal cardiovascular profile integrating both techniques.
1. Doppler ultrasonography
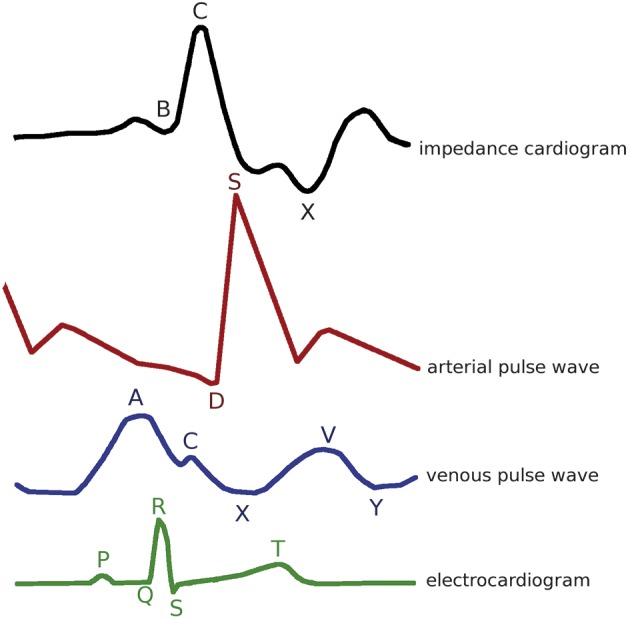
Doppler ultrasonography has been shown to be an appropriate tool to study the circulatory system. Using the scattered reflection of ultrasound waves against red blood cells allows Doppler to image and register blood flow (Nelson and Pretorius, 1988). Moreover, its use is widely accepted in obstetrics as it is non-invasive and safe in pregnancy, enabling to visualize both maternal and foetal circulation. However, most gestational Doppler studies focus either on the foetal (Malcus, 2004) or the uteroplacental circulation, especially on the uterine artery waveforms (Papageorghiou and Leslie, 2007; Abramowicz and Sheiner, 2008; Cnossen et al., 2008) whereas Doppler studies on the veins are scarce (Roobottom et al., 1995; Bateman and Cuganesan, 2002; Karabulut et al., 2003; Gyselaers, 2008). The application of Doppler ultrasonography to study the maternal venous system was extensively explored by the group of Gyselaers et al. The venous pulse wave is a representative of changes in right atrial pressure (Figure 1). The A-wave represents transient venous distension caused by retrograde pressure from the contraction of the right atrium by lack of a valve mechanism between the right atrium and the vena cava. The X-descent, which represents the fast filling of the right atrium during atrial relaxation and the downward movement of the tricuspid valve during ventricular systole, may be interrupted by a small upward deflection (C-wave) at the time of tricuspid valve closure. The V-wave corresponds to passive filling of the right atrium from the systemic veins, when the tricuspid valve is closed. Opening of the tricuspid valve in early diastole allows blood to flow rapidly from the right atrium into the right ventricle; that fall in right atrial pressure corresponds to the Y-descent. After this Y-minimum, venous return slows down again, thus increasing the venous pressure, due to the reduced ventricular filling phase (Boulpaep, 2003; Martin and Lilly, 2007).
Fig. 1. Flowchart search strategy of databases .
1.1. The maternal venous impedance index (Gyselaers et al., 2009; Gyselaers et al., 2010; Mesens et al., 2010)
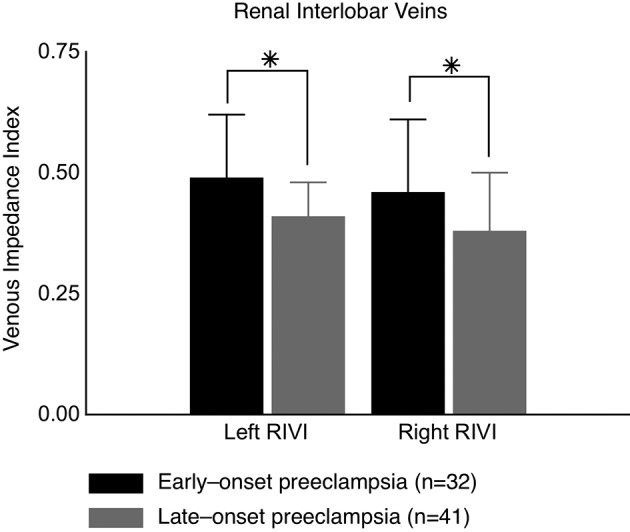
In our research group, venous compliance was studied by use of the venous Doppler equivalent of the arterial resistivity index, i.e. the venous impedance index derived from the venous Doppler waveform velocities at the level of both renal interlobar veins and hepatic veins. Both kidneys and liver play important roles in the volume homeostasis, and thus, the regulation of CO in the gestational physiology (Pang, 2001; Segal, 2003; Gelman, 2008). As such, the renal interlobar venous impedance index (RIVI = (X-A)/X) turned out to be increased in preeclampsia when compared to uncomplicated pregnancy, which can be linked to known features of gestational cardiovascular physiology, i.e. the X- and A-velocities mimic changes in stroke volume and renal glomerular filtration rate (Gyselaers et al., 2009). On top of this, it became clear that RIVI might also be associated with foetal growth and renal function; RIVI was higher in early-onset than in late-onset preeclampsia (Figure 2) in which early-onset preeclampsia presented with lower birth weight percentiles (22.5 (15.0-35.0) vs. 40.0 (12.0-55.0); P = 0.01) and higher proteinuria (4131 ± 3885 vs. 1190 ± 1133 mg/24 h; P < 0.0001) (Gyselaers et al., 2010). However, when calculating mean values of three consecutive Doppler measurements rather than relying on a single value, Doppler velocimetry in the hepatic veins seemed to remain much more variable than in the renal interlobar veins, which showed good reproducibility using this protocol in pregnancy with intra- and inter-observer intra-class correlations of ≥ 0.66 (Mesens et al., 2010). This can be explained by the morphology of the hepatic venous tree and thus the appearance of atypical waves troubling the interpretation of its velocity characteristics. Therefore, we introduced the maternal electrocardiogram (ECG) as part of the venous Doppler wave analysis.
Fig. 2. Flowchart search strategy of databases .
1.2. The maternal venous pulse transit time (Tomsin et al., 2012b; Tomsin et al., 2012c)
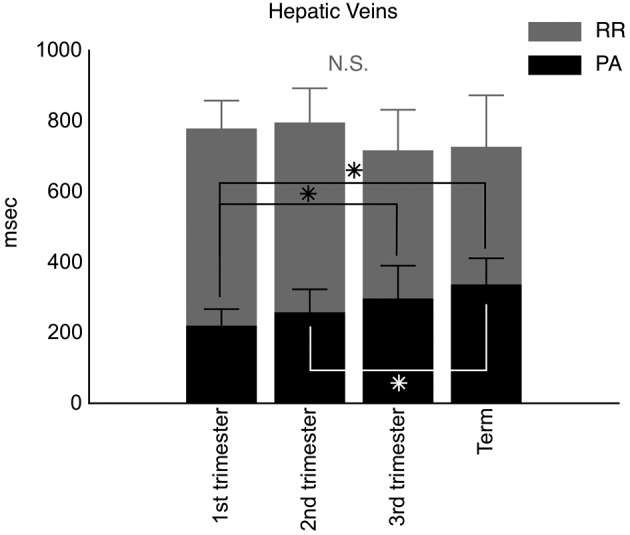
Next to the improved reproducibility, the maternal ECG enabled the correct identification of the different venous Doppler flow characteristics, i.e. the Doppler A-wave represents right atrial contraction which corresponds to the P-wave of the ECG (Tomsin et al., 2012c) (Figure 1). From this, it became clear that the time interval between these corresponding ECG- and Doppler waves was subject to change throughout normal pregnancy and could be used to study characteristics of venous tone, e.g. the PA time interval increased gradually from 12 ± 1, 21 ± 1, 30 ± 0 to 38 ± 1 weeks of gestation at both the level of the hepatic veins (Figure 3) as the renal interlobar veins, while heart rate (RR time interval) remained constant (P ≥ 0.09) (Tomsin et al., 2012c). Moreover, the assessment of this pulse transit time, at both the arterial (QD time interval) and the venous side (PA time interval), corrected for heart rate turned out to be an easily and highly accessible measure for vascular reactivity (Tomsin et al., 2012b). This pulse transit time can be considered the venous equivalent of the arterial pulse transit time, which is indirectly related to arterial wall stiffness (Allen and Murray, 2002; Sharwood-Smith et al., 2006). In uncomplicated pregnancy, all pulse transit times increased throughout pregnancy (P ≤ 0.01) with median values ranging from 0.38 to 0.51 at the level of the left kidney, for instance. In preeclampsia, the median values of this pulse transit time were significantly shorter than controls at corresponding gestational age (P < 0.0001), i.e. 0.35 (with an interquartile range of 0.31-0.41) for early-onset preeclampsia and 0.40 (0.36-0.43) for late-onset preeclampsia using a non-parametric F-test on two degrees of freedom. Again, these observations correlated well with known gestational cardiovascular adaptation mechanisms, i.e. a vascular hypertonia in preeclampsia (Tomsin et al., 2012b).
Fig. 3. Flowchart search strategy of databases .
1.3. Three-dimensional ultrasonography (Claeskens et al., 2013)
Next to this, we considered the use of three-dimensional ultrasonography to visualize changes in hepatic vascularity as it is becoming a popular method in obstetrics. Here, the intrahepatic ultrasonographic approach to visualize the hepatic tree seemed more reliable for clinical application than the subhepatic approach which resulted in 30% failure of re-identification of the user-defined anatomic reference point. With intraclass correlation coefficients of more than 0.60 when analysing sample volumes of approximately 0.1 cm³, this technique is considered reproducible between two separate occasions. Therefore, three-dimensional ultrasonography might be feasible in the study of the gestational evolution of hepatic vascularity. However, more research is needed to optimize this type of hepatic venous evaluation in pregnancy (Claeskens et al., 2013).
1.4. Doppler ultrasonography of the maternal venous system (Gyselaers et al., 2011)
From our studies, we concluded that the venous system is indeed a crucial determinant of cardiac output, and that this can possibly be translated into measurable objective changes in venous Doppler waveforms, and thus impedance indices and venous pulse transit times (VPTT). On top of this, these parameters are subject to changes in uncomplicated pregnancy and preeclampsia enabling their use in gestational hemodynamic studies (Gyselaers et al., 2011).
2. Impedance cardiography
In order to further investigate the role of the maternal venous system in gestational hemodynamics, changes in maternal cardiac output needed to be evaluated in parallel to the venous Doppler velocimetry. However, the invasive standard approaches to obtain information on the heart and arteries are not justified in non-critically ill pregnant women. Therefore, impedance cardiography (ICG) is proposed to be a good non-invasive and safe alternative for invasive methods (Critchley, 1998; Mitchell and Palta, 2004), as it correlates highly with the invasive standard methods, such as thermodilution (Summers et al., 2003). ICG is based on the change in thoracic impedance over time to an alternating high frequent electrical current with very low amplitude between four electrodes (Figure 1). From these measurements, a series of cardiovascular characteristics can be calculated, such as stroke volume, cardiac output, velocity index and aortic compliance.
2.1. Reliability of ICG in non-pregnant and pregnant individuals (Tomsin et al., 2011; Tomsin et al., 2012a)
Results of ICG-measurements are often debated by others (Drazner et al., 2002; Engoren and Barbee, 2005; Sodolski and Kutarski, 2007; de Waal et al., 2008; Mathews and Singh, 2008). Therefore, two reliability studies were conducted to ensure that the ICG-measurements could be reproduced in our specific population. As cardiovascular measurements are subject to variation caused by orthostasis and the hemodynamic response to postural challenge is altered in human pregnancy (Heiskanen et al., 2008), our protocol included changes in position, i.e. supine, standing and sitting positions. We concluded that the reproducibility of such ICG-measurements is much better when mean values of multiple measurements over time are used for each ICG-parameter, instead of individual beat-to-beat measurements. Intra- and intersession intraclass correlation for individual measurements varied between 0.02 and 1.00 for all parameters, however intra-and inter-session correlation coefficients for mean values were consistently higher than 0.80 for e.g. stroke volume, cardiac output, velocity index and thoracic fluid content (Tomsin et al., 2011). Even though postural challenges in pregnancy have a significant influence on cardiovascular parameters, e.g. the vena cava syndrome (Kinsella and Lohmann, 1994), good correlation was found for the ICG-measurements under these standardized conditions, and this was especially true for the aortic flow parameters acceleration-, velocity- and Heather index with correlation coefficients of ≥ 0.80 in uncomplicated pregnancy and preeclampsia (Tomsin et al., 2012a). From a longitudinal study, it became clear that the ICG-observations throughout uncomplicated pregnancy are comparable with known gestational changes in heart and arteries (Duvekot and Peeters, 1994b; Del Bene et al., 2001; Melchiorre et al., 2012). Therefore, it is considered a good technique to study these characteristics in pregnancy. We learned that measurements during the standing position were the most accurate and remained stable throughout gestation, as in this position the vena cava syndrome is likely to be absent. In a single session, parameters of pressure, left ventricular output, cardiac cycle time intervals, thoracic fluid, and aortic flow are continuously registered (Kim, 1989; Woltjer et al., 1997; Sodolski and Kutarski, 2007). Therefore, ICG allows for a global impression of cardiac and arterial function, which together with the combined ECG-Doppler ultrasonographic evaluation of venous function, enables creating a generalized maternal cardiovascular profile based on non-invasive, easily applicable, and highly accessible methods.
3. Maternal cardiovascular profile (Tomsin et al., 2013a; Tomsin et al., 2013b)
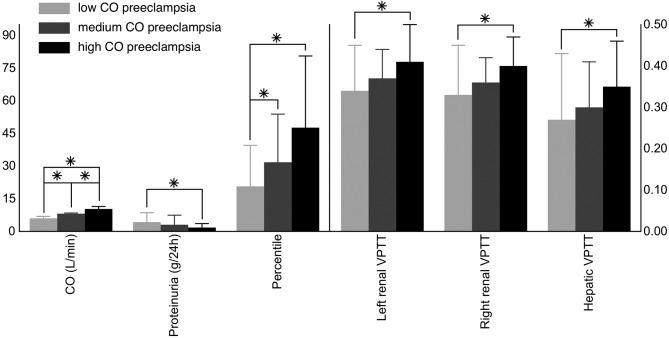
As both combined ECG-Doppler and ICG are proven to be feasible in the study of gestational hemodynamics, we wondered whether the combination of both could give us valuable information on the global maternal cardiovascular state. To this end, this cardiovascular profiling was tested in an experimental setting in which one hemodynamic parameter was changed in a non-pregnant healthy female population (n = 14), i.e. an acute increase of intravenous volume. From this, one can conclude that minor changes, such as an increase of 12 ± 4% in stroke volume (P = 0.001) and a decrease of 16 ± 4% (P = 0.007) in right renal VPTT after an intravenous administration of 500 mL saline solution (NaCl 0.9%) at 999 mL/h, can be registered with this type of cardiovascular profiling, which is based on both the combined ECG-Doppler and the ICG-examinations (Tomsin et al., 2013b). Following this observation, the maternal cardiovascular profile was compared between uncomplicated pregnancy and maternal hypertensive disorders revealing differences in a large set of cardiovascular parameters including all three entities, i.e. the heart, arteries, and veins. From this, the development of proteinuria in preeclampsia was found to be related to an increase in RIVI, discriminating preeclampsia from gestational hypertension in terms of degree in venous dysfunction (Figure 4). Next to this, a correlation between birth weight percentiles and maternal cardiac output and stroke volume (Pearson correlation coefficients of 0.363 and 0.297, respectively; P ≤ 0.008) was observed in this pregnant population (Tomsin et al., 2013a), confirming former observations of other research groups (Nisell et al., 1988; Easterling et al., 1991; Desai et al., 2004). From this correlation, a stratification of the preeclampsia population based on the maternal cardiac output state revealed different maternal cardiovascular profiles. Here, the venous dysfunction was inversely correlated to the cardiac output state, and correlated to maternal and foetal outcome; preeclamptic women with aggravated venous dysfunction (e.g. shorter VPTT) presented with higher proteinuria and delivered neonates with lower birth weight percentiles compared to preeclampsia women with high cardiac output and thus milder venous dysfunction (Figure 5) (Tomsin et al., 2013a). Once again, this confirms the role of the venous system in the regulation of cardiac output, which seems directly related to foetal growth.
Fig. 4. Flowchart search strategy of databases .
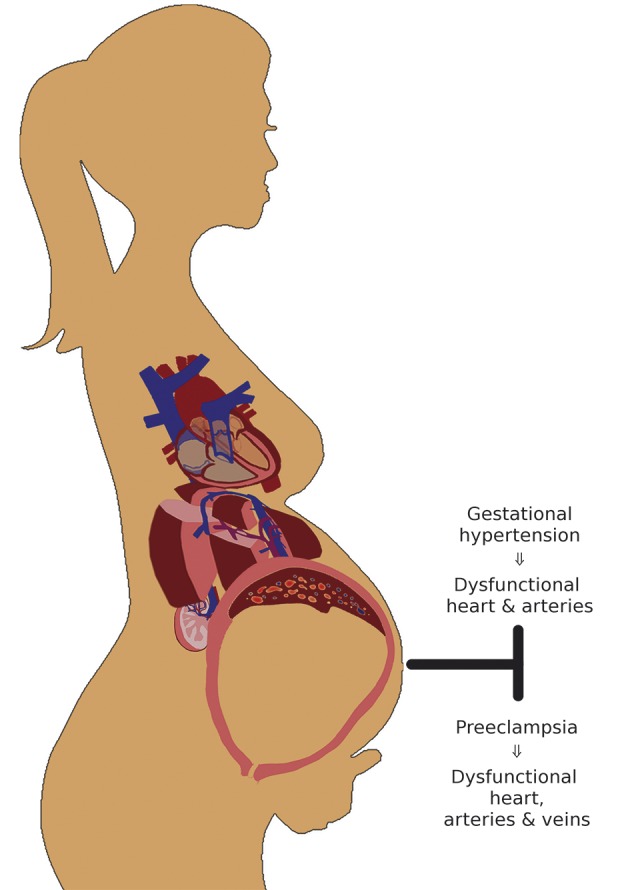
Fig. 5. Flowchart search strategy of databases .
Conclusion
All three strategies helped answering our main research question, i.e. it is possible to include the venous system in a clinically useful maternal cardiovascular profile in pregnancy, and this inclusion seems to be valuable in the study of maternal physiology and pathophysiology. This maternal cardiovascular profile combines the heart, arteries, and veins into one functional unit, and minor changes in these different compartments are able to influence its global functionality. The methodology presented in this thesis enabled classifying women with preeclampsia according to their individual cardiovascular state and this might open perspectives to change today’s standard clinical work-up, including prevention, treatment, and follow-up, towards an individualized type of clinical management for each patient. Future research is needed to shed more light on the hypothesis that maternal cardiovascular profiling, as presented in this thesis, might enable defining a type-specific risk profile for many gestational diseases, which may guide the clinical work-up of and screening for this high-risk population.
What is already known
• Gestational maladaptation of the maternal cardiovascular system occurs before clinical onset of preeclampsia
• Next to heart and arteries, also maternal veins are subject to gestational maladaptation in preeclampsia
• Maternal cardiovascular maladaptation is different between early-onset (severe) preeclampsia and late-onset (mild) preeclampsia.
• The normal physiologic functions of the venous compartment are: (1) regulation of cardiac output, (2) regulation of circulating and stored blood volumes, and (3) control of capillary function. These 3 functions are all disturbed in preeclampsia.
• Venous hemodynamic (dys)function can be measured by combined ECG-Doppler sonography.
What is new from this research
• Vascular pulse transit time can be measured at the arterial but also at the venous compartment
• There is an inverse relation between venous pulse transit time and venous impedance index. To a certain extent, both parameters relate to rigidity of the venous vascular walls.
• In both early- and late-onset preeclampsia, venous impedance index is significantly higher than in gestational hypertension or uncomplicated pregnancy.
• There is a direct relation between renal interlobar vein impedance index and proteinuria
• There is an inverse relation between hepatic vein impedance index and neonatal birth weight percentile
• Low and high matenal cardiac output preeclampsia are associated with respectivley low and high neonatal birth weight percentiles
Future perspectives
• In the maternal cardiovascular assessment during uncomplicated and complicated pregnancy, the venous compartment can become a new target for:
° Exploration of the etiology of preeclampsia and fetal growth restriction
° Screening for preeclampsia and fetal growth restriction
° New diagnositic tools for clinical work-up of preeclampsia
° New treatments for preeclampsia
• Cardiovascular profiling, defined as the combined assessment of maternal heart, arteries and veins, has the potential to define specific subtypes of the human cardiovascular system which may predispose to (1) gestational complications such as preeclampsia or fetal growth restriction, and (2) long term cardiovascular disease.
Promotor: Gyselaers W., Faculty of Medicine & Life Sciences, Hasselt University, Diepenbeek, Belgium; Department of Obstetrics and Gynaecology, Ziekenhuis Oost-Limburg, Genk, Belgium.
Co-promotor: Peeters L., Department of Obstetrics and Gynaecology, University Medical Centre, Utrecht, the Netherlands
References
- Abramowicz JS, Sheiner E. Ultrasound of the placenta: a systematic approach. Part II: functional assessment (Doppler) Placenta. 2008;29:921–929. doi: 10.1016/j.placenta.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Allen J, Murray A. Age-related changes in peripheral pulse timing characteristics at the ears, fingers and toes. J Hum Hypertens. 2002;16:711–717. doi: 10.1038/sj.jhh.1001478. [DOI] [PubMed] [Google Scholar]
- Bateman GA, Cuganesan R. Renal vein Doppler sonography of obstructive uropathy. AJR Am J Roentgenol. 2002;178:921–925. doi: 10.2214/ajr.178.4.1780921. [DOI] [PubMed] [Google Scholar]
- Bernstein IM, Meyer MC, Osol G, et al. Intolerance to volume expansion: a theorized mechanism for the development of preeclampsia. Obstet Gynecol. 1998;92:306–308. doi: 10.1016/s0029-7844(98)00207-5. [DOI] [PubMed] [Google Scholar]
- Boulpaep EL. In Boron W. F. and Boulpaep E. L. (eds) Medical physiology. Philadelphia: Elsevier Inc; 2003. Regulation of arterial pressure and cardiac output; pp. 534–557. [Google Scholar]
- Carbillon L, Uzan M, Uzan S. Pregnancy, vascular tone, and maternal hemodynamics: a crucial adaptation. Obstet Gynecol Surv. 2000;55:574–581. doi: 10.1097/00006254-200009000-00023. [DOI] [PubMed] [Google Scholar]
- Claeskens J, Tomsin K, Molenberghs G, et al. Validation of 3D power Doppler and VOCAL software in the sonographic assessment of hepatic venous flow. F,V&V in ObGyn. 2013;5:7–12. [PMC free article] [PubMed] [Google Scholar]
- Cnossen JS, Morris RK, ter Riet G, et al. Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. CMAJ. 2008;178:701–711. doi: 10.1503/cmaj.070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley LA. Impedance cardiography. The impact of new technology. Anaesthesia. 1998;53:677–684. doi: 10.1046/j.1365-2044.1998.437-az0550.x. [DOI] [PubMed] [Google Scholar]
- de Waal EE, Konings MK, Kalkman CJ, et al. Assessment of stroke volume index with three different bioimpedance algorithms: lack of agreement compared to thermodilution. Intensive Care Med. 2008;34:735–739. doi: 10.1007/s00134-007-0938-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bene R, Barletta G, Mello G, et al. Cardiovascular function in pregnancy: effects of posture. BJOG. 2001;108:344–352. doi: 10.1111/j.1471-0528.2001.00099.x. [DOI] [PubMed] [Google Scholar]
- Desai DK, Moodley J, Naidoo DP. Echocardiographic assessment of cardiovascular hemodynamics in normal pregnancy. Obstet Gynecol. 2004;104:20–29. doi: 10.1097/01.AOG.0000128170.15161.1d. [DOI] [PubMed] [Google Scholar]
- Drazner MH, Thompson B, Rosenberg PB, et al. Comparison of impedance cardiography with invasive hemodynamic measurements in patients with heart failure secondary to ischemic or nonischemic cardiomyopathy. Am J Cardiol. 2002;89:993–995. doi: 10.1016/s0002-9149(02)02257-9. [DOI] [PubMed] [Google Scholar]
- Duvekot JJ, Cheriex EC, Pieters FA, et al. Early pregnancy changes in hemodynamics and volume homeostasis are consecutive adjustments triggered by a primary fall in systemic vascular tone. Am J Obstet Gynecol. 1993;169:1382–1392. doi: 10.1016/0002-9378(93)90405-8. [DOI] [PubMed] [Google Scholar]
- Duvekot JJ, Cheriex EC, Tan WD, et al. Measurement of anterior-posterior diameter of inferior vena cava by ultrasonography: a new non-invasive method to assess acute changes in vascular filling state. Cardiovasc Res. 1994;28:1269–1272. doi: 10.1093/cvr/28.8.1269. [DOI] [PubMed] [Google Scholar]
- Duvekot JJ, Peeters LL. Maternal cardiovascular hemodynamic adaptation to pregnancy. Obstet Gynecol Surv. 1994;49:S1–14. doi: 10.1097/00006254-199412011-00001. [DOI] [PubMed] [Google Scholar]
- Duvekot JJ, Peeters LL. Renal hemodynamics and volume homeostasis in pregnancy. Obstet Gynecol Surv. 1994;49:830–839. doi: 10.1097/00006254-199412000-00007. [DOI] [PubMed] [Google Scholar]
- Easterling TR, Benedetti TJ, Carlson KC, et al. The effect of maternal hemodynamics on fetal growth in hypertensive pregnancies. Am J Obstet Gynecol. 1991;165:902–906. doi: 10.1016/0002-9378(91)90436-u. [DOI] [PubMed] [Google Scholar]
- Engoren M, Barbee D. Comparison of cardiac output determined by bioimpedance, thermodilution, and the Fick method. Am J Crit Care. 2005;14:40–45. [PubMed] [Google Scholar]
- Fu Q, Levine BD. Autonomic circulatory control during pregnancy in humans. Semin Reprod Med. 2009;27:330–337. doi: 10.1055/s-0029-1225261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman S. Venous function and central venous pressure: a physiologic story. Anesthesiology. 2008;108:735–748. doi: 10.1097/ALN.0b013e3181672607. [DOI] [PubMed] [Google Scholar]
- Gyselaers W. Hemodynamics of the maternal venous compartment: a new area to explore in obstetric ultrasound imaging. Ultrasound Obstet Gynecol. 2008;32:716–717. doi: 10.1002/uog.6113. [DOI] [PubMed] [Google Scholar]
- Gyselaers W, Mesens T, Tomsin K, et al. Maternal renal interlobar vein impedance index is higher in early- than in late-onset pre-eclampsia. Ultrasound Obstet Gynecol. 2010;36:69–75. doi: 10.1002/uog.7591. [DOI] [PubMed] [Google Scholar]
- Gyselaers W, Mesens T, Tomsin K, et al. Doppler assessment of maternal central venous hemodynamics in uncomplicated pregnancy: a comprehensive review. F,V&V in ObGyn. 2009;1:171–181. [PMC free article] [PubMed] [Google Scholar]
- Gyselaers W, Mullens W, Tomsin K, et al. Role of dysfunctional maternal venous hemodynamics in the pathophysiology of pre-eclampsia: a review. Ultrasound Obstet Gynecol. 2011;38:123–129. doi: 10.1002/uog.9061. [DOI] [PubMed] [Google Scholar]
- Hagedorn KA, Cooke CL, Falck JR, et al. Regulation of vascular tone during pregnancy: a novel role for the pregnane X receptor. Hypertension. 2007;49:328–333. doi: 10.1161/01.HYP.0000253478.51950.27. [DOI] [PubMed] [Google Scholar]
- Heiskanen N, Saarelainen H, Valtonen P, et al. Blood pressure and heart rate variability analysis of orthostatic challenge in normal human pregnancies. Clin Physiol Funct Imaging. 2008;28:384–390. doi: 10.1111/j.1475-097X.2008.00818.x. [DOI] [PubMed] [Google Scholar]
- Jia RZ, Liu XM, Wang X, et al. Relationship between cardiovascular function and fetal growth restriction in women with pre-eclampsia. Int J Gynecol Obstet. 2010;110:61–63. doi: 10.1016/j.ijgo.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Karabulut N, Baki Yagci A, Karabulut A. Renal vein Doppler ultrasound of maternal kidneys in normal second and third trimester pregnancy. Br J Radiol. 2003;76:444–447. doi: 10.1259/bjr/81976752. [DOI] [PubMed] [Google Scholar]
- Kim DW. Detection of physiological events by impedance. Yonsei Med J. 1989;30:1–11. doi: 10.3349/ymj.1989.30.1.1. [DOI] [PubMed] [Google Scholar]
- Kinsella SM, Lohmann G. Supine hypotensive syndrome. Obstet Gynecol. 1994;83:774–788. [PubMed] [Google Scholar]
- Krabbendam I, Spaanderman ME. Venous adjustments in healthy and hypertensive pregnancy. Expert Rev Obstet Gynecol. 2007;2:671–679. [Google Scholar]
- Malcus P. Antenatal fetal surveillance. Curr Opin Obstet Gynecol. 2004;16:123–128. doi: 10.1097/00001703-200404000-00005. [DOI] [PubMed] [Google Scholar]
- Martin N, Lilly LS. Philadelphia: Lippincott Williams and Wilkins; 2007. The cardiac cycle: Mechanisms of heart sounds and murmurs. In Lilly L. S. (eds) Pathophysiology of heart disease; pp. 29–44. [Google Scholar]
- Mathews L, Singh RK. Cardiac output monitoring. Ann Card Anaesth. 2008;11:56–68. doi: 10.4103/0971-9784.38455. [DOI] [PubMed] [Google Scholar]
- Melchiorre K, Sharma R, Thilaganathan B. Cardiac structure and function in normal pregnancy. Curr Opin Obstet Gynecol. 2012;24:413–421. doi: 10.1097/GCO.0b013e328359826f. [DOI] [PubMed] [Google Scholar]
- Mesens T, Tomsin K, Molenberghs G, et al. Reproducibility and repeatability of maternal venous Doppler flow measurements in renal interlobar and hepatic veins. Ultrasound Obstet Gynecol. 2010;36:120–121. doi: 10.1002/uog.7648. [DOI] [PubMed] [Google Scholar]
- Mitchell JE, Palta S. New diagnostic modalities in the diagnosis of heart failure. J Natl Med Assoc. 2004;96:1424–1430. [PMC free article] [PubMed] [Google Scholar]
- Nelson TR, Pretorius DH. The Doppler signal: where does it come from and what does it mean? AJR Am J Roentgenol. 1988;151:439–447. doi: 10.2214/ajr.151.3.439. [DOI] [PubMed] [Google Scholar]
- Nisell H, Lunell NO, Linde B. Maternal hemodynamics and impaired fetal growth in pregnancy-induced hypertension. Obstet Gynecol. 1988;71:163–166. [PubMed] [Google Scholar]
- Pang CC. Autonomic control of the venous system in health and disease: effects of drugs. Pharmacol Ther. 2001;90:179–230. doi: 10.1016/s0163-7258(01)00138-3. [DOI] [PubMed] [Google Scholar]
- Papageorghiou AT, Leslie K. Uterine artery Doppler in the prediction of adverse pregnancy outcome. Curr Opin Obstet Gynecol. 2007;19:103–109. doi: 10.1097/GCO.0b013e32809bd964. [DOI] [PubMed] [Google Scholar]
- Reece EA. Reece EA and Barbieri RL (eds) Obstetrics and Gynecology: The essentials of clinical care. Stuttgart - New York: Thieme; 2010. Pre-eclampsia-eclampsia syndrome; pp. 149–158. [Google Scholar]
- Roobottom CA, Hunter JD, Weston MJ, et al. Hepatic venous Doppler waveforms: changes in pregnancy. J Clin Ultrasound. 1995;23:477–482. doi: 10.1002/jcu.1870230804. [DOI] [PubMed] [Google Scholar]
- Sakai K, Imaizumi T, Maeda H, et al. Venous distensibility during pregnancy. Comparisons between normal pregnancy and preeclampsia. Hypertension. 1994;24:461–466. doi: 10.1161/01.hyp.24.4.461. [DOI] [PubMed] [Google Scholar]
- Segal SS. Boron W. F. and Boulpaep E. L. (eds) Medical physiology. Philadelphia: Elsevier Inc; 2003. Special circulations; pp. 558–573. [Google Scholar]
- Smith G, Bruce J, Drummond G. Assessment of pulse transit time to indicate cardiovascular changes during obstetric spinal anaesthesia. Br J Anaesth. 2006;96:100–105. doi: 10.1093/bja/aei266. [DOI] [PubMed] [Google Scholar]
- Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol. 2009;200:481. doi: 10.1016/j.ajog.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Simmons LA, Gillin AG, Jeremy RW. Structural and functional changes in left ventricle during normotensive and preeclamptic pregnancy. Am J Physiol Heart Circ Physiol. 2002;283:H1627–H1633. doi: 10.1152/ajpheart.00966.2001. [DOI] [PubMed] [Google Scholar]
- Sodolski T, Kutarski A. Impedance cardiography: A valuable method of evaluating haemodynamic parameters. Cardiol J. 2007;14:115–126. [PubMed] [Google Scholar]
- Summers RL, Shoemaker WC, Peacock WF, et al. Bench to bedside: electrophysiologic and clinical principles of noninvasive hemodynamic monitoring using impedance cardiography. Acad Emerg Med. 2003;10:669–680. doi: 10.1111/j.1553-2712.2003.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Tomsin K, Mesens T, Molenberghs G, et al. Diurnal and position-induced variability of impedance cardiography measurements in healthy subjects. Clin Physiol Funct Imaging. 2011;31:145–150. doi: 10.1111/j.1475-097X.2010.00993.x. [DOI] [PubMed] [Google Scholar]
- Tomsin K, Mesens T, Molenberghs G, et al. Impedance cardiography in uncomplicated pregnancy and pre-eclampsia: A reliability study. J Obstet Gynaecol. 2012;32:630–634. doi: 10.3109/01443615.2012.673036. [DOI] [PubMed] [Google Scholar]
- Tomsin K, Mesens T, Molenberghs G, et al. Venous pulse transit time in normal pregnancy and preeclampsia. Reprod Sci. 2012;19:431–436. doi: 10.1177/1933719111424440. [DOI] [PubMed] [Google Scholar]
- Tomsin K, Mesens T, Molenberghs G, et al. Time interval between maternal electrocardiogram and venous Doppler waves in normal pregnancy and preeclampsia: a pilot study. Ultraschall Med. 2012;33:E119–E125. doi: 10.1055/s-0029-1245698. [DOI] [PubMed] [Google Scholar]
- Tomsin K, Mesens T, Molenberghs G, et al. Characteristics of heart, arteries, and veins in low and high cardiac output preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2013 doi: 10.1016/j.ejogrb.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Tomsin K, Vriens A, Mesens T, et al. Non-invasive cardiovascular profiling using combined electrocardiogram-Doppler ultrasonography and impedance cardiography: an experimental approach. Clin Exp Pharmacol Physiol. 2013 doi: 10.1111/1440-1681.12105. [DOI] [PubMed] [Google Scholar]
- Valensise H, Vasapollo B, Gagliardi G, et al. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension. 2008;52:873–880. doi: 10.1161/HYPERTENSIONAHA.108.117358. [DOI] [PubMed] [Google Scholar]
- van Oppen AC, Stigter RH, Bruinse HW. Cardiac output in normal pregnancy: a critical review. Obstet Gynecol. 1996;87:310–318. doi: 10.1016/0029-7844(95)00348-7. [DOI] [PubMed] [Google Scholar]
- van Oppen AC, van der Tweel I, Alsbach GP, et al. A longitudinal study of maternal hemodynamics during normal pregnancy. Obstet Gynecol. 1996;88:40–46. doi: 10.1016/0029-7844(96)00069-5. [DOI] [PubMed] [Google Scholar]
- Woltjer HH, Bogaard HJ, de Vries PM. The technique of impedance cardiography. Eur Heart J. 1997;18:1396–1403. doi: 10.1093/oxfordjournals.eurheartj.a015464. [DOI] [PubMed] [Google Scholar]