Abstract
Childlessness and infertility care are neglected aspects of family planning in resource-poor countries, although the consequences of involuntary childlessness are much more dramatic and can create more wide ranging societal problems compared to Western societies, particularly for women. Because many families in developing countries completely depend on children for economic survival, childlessness has to be regarded as a social and public health issue and not only as an individual medical problem.
In the Walking Egg Project we strive to raise awareness surrounding childlessness in resource-poor countries and to make infertility care in all its aspects, including assisted reproductive technologies, available and accessible for a much larger part of the world population.
We hope to achieve this goal through innovation and research, advocacy and networking, training and capacity building and service delivery. The Walking Egg non-profit organization has chosen a holistic approach of reproductive health and therefore strengthening infertility care should go together with strengthening other aspects of family planning and mother care.
Right from the start The Walking Project has approached the problem of infertility in a multidisciplinary and global manner. It gathers medical, social, ethical, epidemiological, juridical and economical scientists and experts along with artists and philosophers to discuss and work together towards its goal.
We recently developed a simplified tWE lab IVF culture system with excellent results. According to our first cost calculation, the price of a single IVF cycle using the methodologies and protocols we described, seems to be less than 200 Euros.
We realize that universal access to infertility care can only be achieved when good quality but affordable infertility care is linked to effective family planning and safe motherhood programmes. Only a global project with respect to sociocultural, ethical, economical and political differences can be successful.
Keywords: Assisted reproduction, developing countries, infertility care, intrauterine insemination, IVF, medical education, one-step diagnostic phase, resource-poor countries, simplified IVF, sociocultural factors
Rationale - Background
Infertility is a global reproductive health problem: it has been estimated that 8 to 12% of the couples worldwide are infertile. The large majority of childless couples are residents of developing countries (DC). A silent population of more than 180 million couples worldwide is facing the consequences of childlessness day by day (Rutstein and Iqbal, 2004; Boivin et al., 2007).
The infertility experience has significant negative effects on the individual woman and man as well as the couple and the broader family (Greil, 1997). Consequences of infertility are numerous: stress, depression, low-self esteem, guilt, marital problems, and sexual problems. So far infertile people in the Western countries and the resource-poor countries have something in common. However the differences are emerging mainly for two reasons: (a) sociocultural values surrounding procreation and infertility and (b) availability of infertility treatments.
Consequences of involuntary childlessness are usually more dramatic in DC when compared to Western societies, particularly for women. Often, the woman is blamed for the infertility, even when a male factor is involved. Negative psychosocial consequences are severe: childless women are frequently stigmatised, isolated, ostracized, disinherited and neglected by the entire family and even the local community (Papreen et al., 2000; Van Balen and Gerrits, 2001; Daar and Merali, 2002; Dyer et al., 2002a, 2002b, 2004, 2005; Van Balen and Bos, 2009; Gerrits and Shaw, 2010). This may lead to physical and psychological violence, polygamy, and even into suicide. Infertile women - and men - are marginalized, disadvantaged and disempowered. As many families – and elderly people in particular - in DC completely depend on children for economic survival, childlessness in DC becomes an important social and public health issue and not only an individual medical problem (Ombelet et al., 2008; Dhont, 2011a, 2011b; Ombelet, 2011). In the last two decades the manifold consequences of infertility, most significant within DC, at personal, conjugal, family and community levels, and financially, have been well documented (Inhorn, 2003; Van Balen and Bos, 2009; Van Balen and Gerrits, 2001). These studies have also shown that the way people experience, explain and deal with infertility is strongly related with their sociocultural and economic life circumstances as well with the availability and non-availability of health care options.
The most important reasons for infertility in DC are (1) the high incidence of sexually transmitted diseases (STDs) - which affects both men and women - and (2) pregnancy-related infections, due to unsafe abortions and home deliveries in unhygienic circumstances, mainly in rural areas.
The high prevalence of genital infections in developing countries is commonly compounded by a complete lack of diagnosis together with incomplete, inappropriate or no intervention at all. Yet, severe male infertility due to STDs and female infertility due to tubal block can only be treated by “expensive” assisted reproductive technologies (ART), which are not available at all or only within reach of those (the happy few) who can afford it, mostly only in private settings (Ombelet et al., 2008).
Reduced fecundity in HIV-infected individuals has been described before (Brocklehurst and French, 1998; Glynn et al., 2000). Marital instability and polygamy, as a reaction to infertility and childlessness within the conjugal relationship, may in turn increase the spread of HIV-1 infection (and STD’s) (Dhont, 2011a, 2011c). Studies have shown that HIV was up to 3 times more prevalent in childless couples compared with fertile couples in the same population (Nabaitu et al., 1994). Moreover, expanded access to antiretroviral therapy (ARVs) implies that HIV+ people have increased hopes to live longer and healthier lives, which also has been associated with an increased chance to bear children. HIV+ males could have a child that is HIV- with the assistance of sperm washing procedures commonly used in assisted reproductive procedures such as intrauterine insemination (IUI) and in-vitro fertilization (IVF).
Despite the high infertility prevalence and the severe economical consequences of childlessness in DC, infertility care remains a low priority area for local health care providers and community leaders. It has been marginalized and neglected by health care authorities despite its high prevalence and unmet need (Fathalla et al., 2006; Ombelet, 2011).
According to MDG5 (Millennium Development Goal 5) universal access to reproductive care, including both contraceptive and infertility care, should be adopted by the year 2015. Until today, nothing has been done to help childless couples in developing countries and according to a recent questionnaire none of the international organizations, NGOs and foundations is planning to do so in the forthcoming years (Ombelet, 2011).
Till now, infertility care in most DC has been fragmented between public and private spheres. Inadequate or complete lack of rules and regulations concerning treatment conditions and commercial interests may lead to unethical practices (Guilhem, 2001). Overall, very little is known about actual practices and results within clinics providing infertility services in DC.
The Walking Egg non-profit organization
The Walking Egg npo has opted for a multidisciplinary and global approach towards the problem of infertility (Dhont, 2011b). The main goal of the Walking Egg Project is to raise global awareness surrounding childlessness, and to make infertility care in all its aspects universally available and accessible. Therefore we need to change and optimise the whole set-up of infertility care in terms of availability, affordability and effectiveness (Ombelet and Campo, 2007).
To realize this objective a number of actions are planned including the following: (1) to raise awareness surrounding the problem of childlessness within (a) the donor community, politicians, funding agencies and research organisations through lobbying and publishing, (b) the general population through information, education and counselling on infertility and its consequences, (2) to study the ethical, sociocultural and economical aspects surrounding the issue of childlessness and infertility care in resource-poor countries, (3) to develop new methods to make infertility diagnosis and infertility treatment including ART accessible for a much larger part of the population, by (a) simplifying the diagnostic procedures, (b) simplifying the IVF laboratory procedures and (c) modifying the ovarian stimulation protocols for IVF and last but not least (4) to work together with other organisations and societies working in the field of reproductive health to reach the goal of “universal access to infertility care”
Research and Innovation
Non-medical
There is an urgent need for more research on sociocultural, ethical, religious and juridical aspects of infertility in poor-resource countries. Reliable data on economical consequences of childlessness in DC are lacking. Ethical considerations and debates on this subject are scarce (Pennings et al., 2008, 2009). Legal aspects and rights dealing with the severe consequences of childlessness for women in DC are almost never mentioned in the literature, and what to say about the legal right to have access to infertility care, agreed upon and mentioned in so many political international statements and commitments (Ombelet et al., 2010).
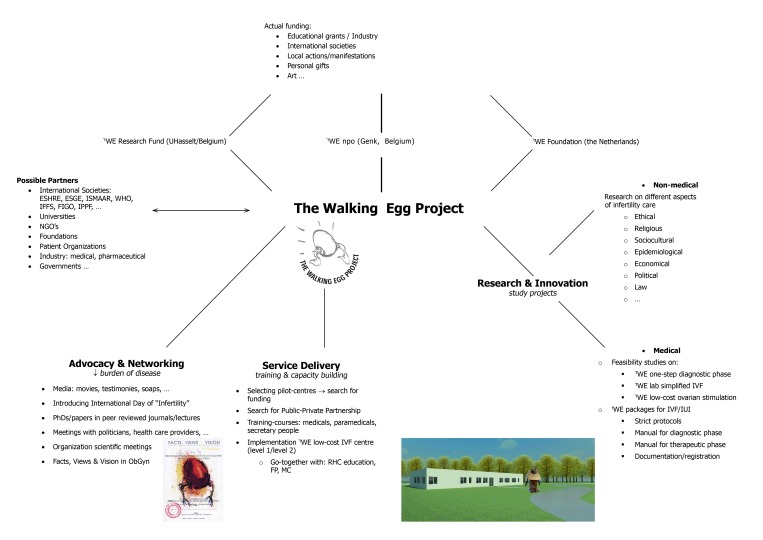
In the Walking Egg Project we aim to initiate and expand an international network of social science research (in broad sense) in these fields. The first expert-meeting on the “Socio-cultural implications of childlessness in developing countries” was organized by The Walking Egg npo in 2009, in cooperation with ESHRE (European Society of Human Reproduction and Embryology) and WHO (World Health Organization). A Monograph with articles of most experts was published in “Facts, Views & Vision in ObGyn” (www.fvvo.eu). This Monograph was distributed to 9000 participants of the annual ESHRE meeting in Rome. A second expert-meeting on “Barriers, Access and Ethics of biomedical care in resource-poor countries” was held in 2011 and followed by the publication of another Monograph distributed at the Annual ESHRE meeting in Istanbul in 2012 (www.fvvo.eu). Participants at the 2011 Expert Meeting highlighted the importance of studies addressing barriers to infertility care, and studies to prepare, assess and follow up the supply and use of accessible and affordable infertility care in different low-resource contexts. They concluded that, to be successful, the project has to be global with a strong sociocultural, ethical and economical component. It will need the support of a reliable network of social scientists supporting the project by discussing the various sociocultural, psychological and ethical aspects of biomedical infertility care in different DC. This network will be crucial in the introduction and follow-up of accessible infertility care services in resource-poor countries.
Medical
From a pure medical point of view, our first objective is the establishment of low-cost “one-stop clinics” for the diagnosis of infertility. Simplification of the ART procedures without loss of quality is our second objective. Our final goal is the implementation of “accessible” infertility services, if possible integrated within health care facilities, providing good quality family planning services, reproductive health education and high-standard mother care.
The one-stop diagnostic phase
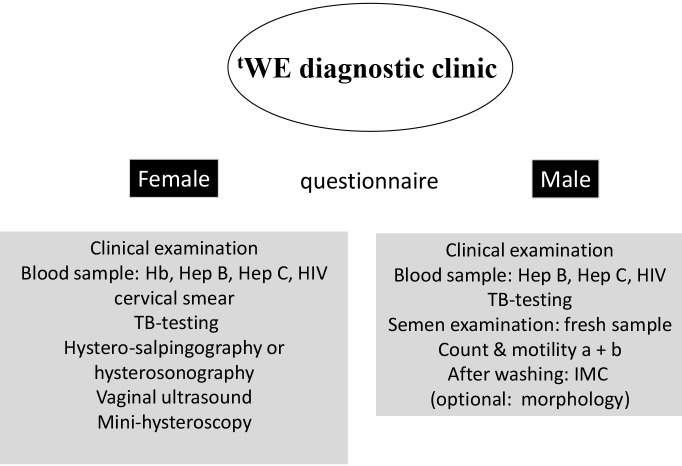
Standardized investigation of the couple at minimal costs is possible and undoubtedly will enhance the likelihood that infertile couples, both men and woman, will come to the infertility centres. How to organize a one-stop diagnostic clinic has been described before (Ombelet et al., 2012) and is shown in Figure 1.
Fig. 1. tWE diagnostic clinic for infertility work-up in a resource-poor setting (tWE = the Walking Egg).
A questionnaire will be provided to both partners. This questionnaire can be adapted to the local situation in the specific locations and countries. Screening for infections and STDs can be done by using low-cost affordable screening tests which became available recently (Huyser and Fourie, 2010).
Since tubal obstruction associated with previous pelvic infections is the most important reason for infertility in some developing regions, hysterosalpingography and/or hystero-salpingo-contrast-sonography are affordable techniques to detect this problem, easy to perform and without major costs. Combining these techniques with an accurate medical history will identify the majority of women’s infertility causes, such as ovulatory disorders, uterine malformations and tubal infertility. A standard gynaecological and fertility ultrasound scanning of the uterus and the ovaries can easily be done.
Male factor infertility can be evaluated by a simple semen analysis (WHO, 2010). Semen analyses can also be performed by well-trained paramedicals, another important advantage for developing countries. It is also important to calculate the IMC (inseminating motile count). The IMC is the total number of motile spermatozoa after sperm wash procedure. The IMC is very crucial in selecting patients for either IUI (intrauterine inseminations), IVF (in-vitro fertilization) or ICSI (Intra-Cytoplasmic Sperm Injection) (Ombelet et al., 1997, 2012).
Office mini-hysteroscopy to investigate intrauterine abnormalities has been simplified in its instrumentation and technique, so that it can become a non-expensive diagnostic technique accessible for every gynaecologist, provided there has been appropriate training (Campo et al., 2005; Ombelet and Campo, 2007).
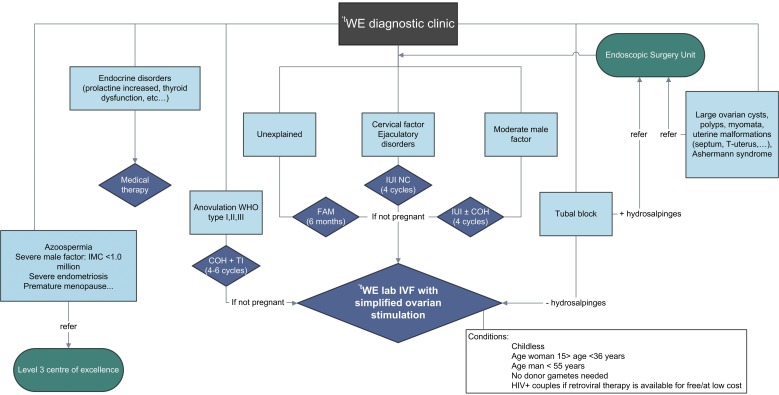
All the procedures of the one-day diagnostic clinic can be performed by a small team of health care providers within a short period of time in an inexpensive setting (Ombelet and Campo, 2007). A flowchart for the tWE (The Walking Egg) diagnostic clinic is shown in Figure 2.
2. Proposed flowchart for the tWE diagnostic clinic (tWE = the Walking Egg, FAM = Fertility Awareness Methods, IUI = Intrauterine insemination, NC =Natural cycle, COH = Controlled Ovarian Hyperstimulation, TI = Timed Intercourse, IMC = Inseminating Motile Count).
Future studies are planned to assess the reproducibility of ‘one-stop infertility clinics’ in different developing countries.
Simplified infertility treatment and non-IVF assisted reproduction
If tubal patency is demonstrated in ovulatory women and if severe male factor subfertility has been excluded, fertility awareness programmes are an inexpensive and efficient first line approach to infertility management (Gnoth et al., 2002, 2003). Fertility awareness counselling to couples about the meaning and detection of cervical mucus secretion can be given by nurses and paramedical staff working in existing reproductive health care centres.
For ovulatory dysfunction, representing almost 20 % of female infertility, clomiphene citrate (CC) is a very cheap and rewarding option. In case of resistance to CC, a low dose ovarian stimulation regimen with gonadotrophins aimed at monofollicular growth is advisable, although this medication is more expensive.
In case of unexplained and moderate male factor infertility and provided tubal patency has been documented, intrauterine insemination (IUI) with husband’s semen in natural cycles or after mild stimulation is a excellent first-line treatment without major costs and without expensive infrastructure (Ombelet et al., 2003; Verhulst et al., 2006). IUI programmes can be runned by well-trained paramedical staff, another advantage for resource-poor countries. Controlled ovarian hyperstimulation (COH), with or without IUI, is associated with the risk of multiple gestations, especially when gonadotrophins are used (Gleicher et al., 2000). Appropriate standardized protocols are available to minimize the risk for multiple pregnancies which is even more important in developing countries because the consequences of multiple pregnancies can be devastating.
Simplified IVF laboratory procedures
Another major challenge is to reduce costs of laboratory procedures, namely fertilization and culture of eggs and embryos for IVF. Different options and approaches have been developed or are presently being field- tested with promising results.
Intravaginal fertilization and culturing has been used since many years for low cost IVF (Frydman and Ranoux, 2008). A tube filled with culture medium containing the oocytes and washed spermatozoa is hermetically closed and placed in the vagina. It is held intravaginally by a diaphragm for incubation for 44 to 50 hours. Over 800 cycles cycles have been published worldwide with a very reasonable clinical pregnancy rate of almost 20 % (Frydman and Ranoux, 2008).
As part of the Walking Egg Project and based on previous findings and experience (Van Blerkom and Manes, 1974; Swain, 2011) we developed a new simplified method of IVF culturing, called the tWE lab method. With this new system, specifically designed for low resource settings, we can avoid the high costs of medical gases, complex incubation equipment and infrastructure typical of IVF laboratories in high resource settings.
For insemination of the eggs, we only use 1000- 5000 motile washed spermatozoa per oocyte, with very promising results, which makes this technique usable for more the 70% of the actual IVF/ICSI population (Genk data, not published).
Since development from insemination to transfer is undisturbed and in the same tube until embryo transfer, we can avoid many problems frequently occurring in regular IVF laboratories, such as unwanted temperature changes, air quality problems etc.
Up to April 2013 twelve healthy babies have been born after using this technique while a prospective study comparing the embryo quality after using tWE lab versus regular IVF procedures is still ongoing.
Low-cost ovarian stimulation protocols for IVF
In order to make infertility care more affordable in developing countries, effective, cheap and safe stimulation schemes for intrauterine insemination (IUI) and in-vitro fertilization (IVF) need to be established. A review of the literature clearly shows the value and effectiveness of mild ovarian stimulation protocols in ART settings (Verberg et al., 2009). The success rates of natural cycle IVF can be low per cycle due to high cancellation rates because of premature LH rise and premature ovulation. But the use of indomethacin to block ovulation helps to reduce cancellations. Cumulative pregnancy and live birth rates after four consecutive cycles could reach 46 % and 32% respectively making it a cost-effective, safe and patient-friendly option (Nargund et al., 2001). The use of clomiphene citrate (CC), a very cheap oral drug, has been proven in many studies to be an optimal alternative with acceptable results, minimal side effects and a very low complication rate (Ingerslev et al., 2001; Nargund et al., 2007; Verberg et al., 2009; Kato et al., 2012).
Monitoring of follicular development in an IVF cycle, as well as the timing of the hCG administration can be done solely on sonographic criteria with basic inexpensive ultrasound equipment thereby avoiding the need of expensive endocrine investigations (Rojanasakul et al., 1994).
Nevertheless, although very promising results concerning the different steps of IVF are described, we still have to perform a lot of feasibility studies to examine the value of a one-stop diagnostic phase and to study the value of the simplified tWE lab system and different low-cost ovarian stimulation protocols in resource-poor settings.
Service delivery: The implementation of tWE pilot-centres in DC
The ultimate aim of the Walking Egg project is the implementation of good quality but low-cost infertility centres in DC, if possible and preferable integrated into existing Reproductive Health Care Centres. Diagnostic and therapeutic procedures and protocols should be affordable, effective, safe and standardized. Ideally, infertility management should be integrated into sexual and reproductive health care programmes.
As developing countries differ in their status of development, three levels of assistance are suggested (Sallam, 2008). A level 1 infertility clinic is a basic infertility clinic capable of offering the following services: basic infertility workout including semen analysis, hormonal assays, follicular scanning, ovulation induction and IUI. In Level 2 infertility clinics IVF can be performed as well.
During many expert meetings it was decided that Level 3 infertility clinics capable of offering ICSI, cryopreservation and operative endoscopy are not part of the Walking Egg Project in the initial phase. Therefore our first target is the implementation of good quality level 2 centres.
Implementation of level 2 services entails the following activities (Sallam, 2008):
1. Equipping the clinics: Infertility clinics in developing countries should be provided with low-cost and easy serviceable equipment taking into consideration the local problems often encountered (e.g. fluctuating voltage, frequent power cuts, unavailability of servicing facilities, irregular supply of consumables, etc...). This may require negotiations with various manufacturers to supply these tools at affordable prices, particularly if large quantities are ordered.
2. Training the staff: This includes the training of the medical, paramedical as well as the administrative staff. Training courses should tailor to the local conditions and the possible difficulties encountered in developing countries. Table I gives an overview of the key topics covered in the training courses. Training, quality control, regular audit and systems of accreditation and registration should be implemented in order to maintain appropriate standards of care. Our objective is to organize a one week course for all members of the team who are involved in the set-up of a pilot-centre, part of the Walking Egg Project. This training will need the support of experts in the field, who are capable to tutor the training courses at the highest level in a very short time, taking into account the experience of the trainees and the quality of facilities that can be expected in the new pilot-centres.
Table I. Key categories of the training courses (Ombelet et al., 2012).
| • Reproductive health care education basic course ➛ Target group: nurses, midwifes |
| • A general and medical history of and basic clinical examination both partners ➛ clinician (medical) |
| • Screening for infections and STDs ➛ clinician (medical, paramedical) |
| • How to perform and evaluate a hysterosalpingography and/or hystero-salpingo-contrast-sonography ➛ clinician (medical, paramedical) |
| • Standard Operational Procedures for the gynaecological and fertility ultrasound scan ➛ clinician (medical, paramedical) |
| • Basic semenology training course according to WHO 2010 manual ➛ laboratory staff (paramedical) |
| • Sperm washing procedures ➛ laboratory staff (paramedical) |
| • Mini-hysteroscopy ➛ clinician (medical) |
| • Documentation and registration ➛ administrative staff (clerical) |
3. Educating the public: This necessitates establishing contacts and working relationships with schools, community leaders, traditional healers as well as the media, producing and distributing educational materials (brochures, posters and audio-visual material) etc.
4. Running the services: This should take into consideration staff salaries, regular purchasing of consumables, cost of equipment maintenance, cost of investigations, cost of medical interventions and the cost of medication. Special servicing contracts should be negotiated with the manufacturers. In addition, simplification of the consumables should be taken into consideration and laboratory reagents and culture media should have a long shelf life. Special prices for medication should be negotiated with the drug manufacturers and simple treatment protocols should be put into action in order to reach the best cost-effective therapies.
5. Documentation and registration: We believe that within each pilot-centre on-line data registration of all ART activities is mandatory. Administrative staff and (para) medicals have to be aware of the importance of correct and trustable data registration. The ultimate goal is to offer all pilot-centres a similar registration programme, which should be customer-friendly with a limited but sufficient number of items (increased personnel compliancy) (Ombelet et al., 2012). Continuous monitoring of service activities will be centralized, and provide feed-back to clinics for clinical and laboratory policy adjustments, information to couples on clinic performance, and information to society. Confidence can then be built and maintained.
6. Psychological and socio-cultural follow-up: When implementing low-cost (accessible) infertility services in DC it is extremely important to study social, psychological, sexual, legal and ethical aspects of infertility and infertility treatment and take study findings into account when setting up gender and cultural sensitive infertility services. Considering psychological and sociocultural follow-up the most important aims can be summarized as follows:
• Informing the design of culture and gender sensitive treatment and counseling procedures, ethical guidelines and informed consent forms for the selected pilot-centres.
• Describing the psychological well-being of the infertile women and men along the infertility treatment trajectory (before, during, immediately and one year after treatment); their expectations, experiences and suggestions regarding treatment procedures and aspects of quality of care; and the social repercussions and other social implications infertility treatments may have.
• Enhancing the level of knowledge and understanding with regard to socio-cultural, psychical, quality of care and ethical aspects of infertility care in DC.
Selection of countries / pilot-centres
Decision making on infertility treatment in developing countries assumes answers to quite a few questions: How should the infertility problem be defined? How often does infertility occur? What is the income in that specific country and what can be spend on health care? How cheap should IVF be in order to be accessible to a considerable part of the population? With what alternative health interventions should infertility treatment be compared? How cost-effective should IVF be in order to compete with those other interventions?
In this respect we believe that measurements of the (utility-measure oriented) Quality of Life over the infertile life-course in developing countries are urgently needed.
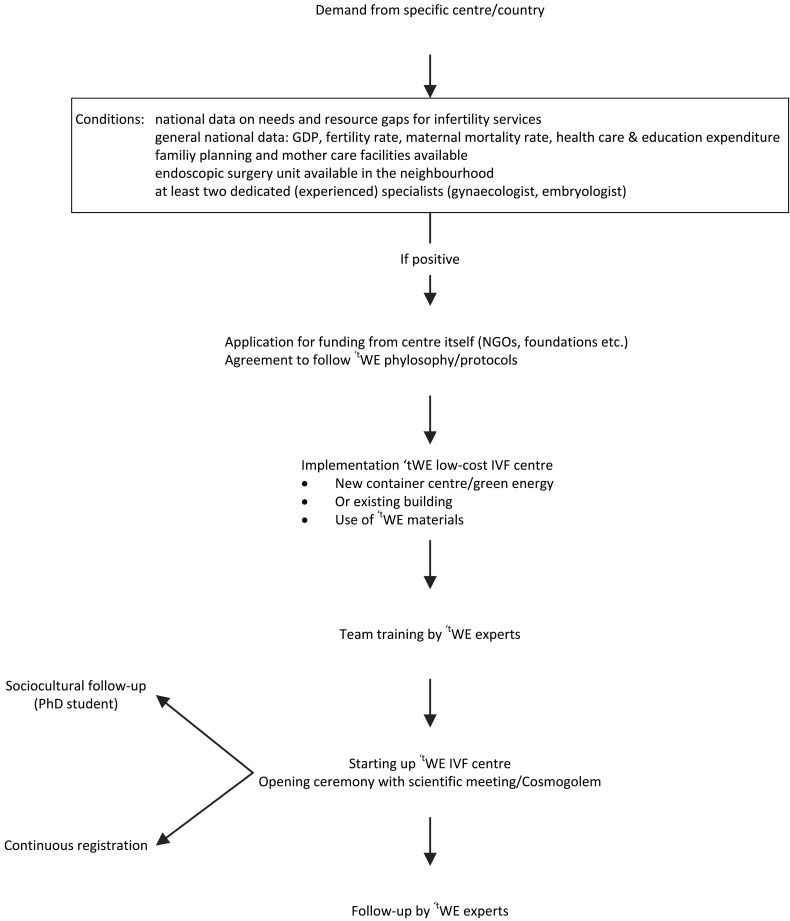
The selection of countries where the first pilot centres are implemented will be based on (1) available data on the resources, needs and resource gaps for infertility services on a national level, (2) percentage of GDP spent on education and health care, (3) the availability of endoscopic surgery facilities in the neighbourhood, (4) a good quality family planning unit, (5) good quality mother care facilities and (6) the availability of at least one experienced and dedicated gynaecologist and biologist (Fig. 3).
Fig. 3. The tWE strategy from application to implementation (GDP = gross domestic product).
The community/region including the local health care authorities should be empowered to support the program from the beginning. Table II gives an overview of the most important recommendations to consider when starting tWE pilot-centres in developing countries.
Table II. Implementing accessible infertility care pilot-centres in selected developing countries: recommendations (C Huyser, personal communication).
| 1. Risk analysis of the country including GPD, health care expenditures, budget for education, total fertility rate, maternal and infant mortality rate, etc |
| 2. The community/region should be empowered to support the program (communication channels, …) |
| 3. Selection of patients: one-step diagnostic clinic |
| 4. Some couples have to be referred to a level 3 centre of excellence (if ICSI needed) or to an endoscopic surgery unit |
| 5. Be aware of infectious conditions and STDs • Aseptic conditions to perform procedures • Screening of patients • Prevention of STD transmission • Unique profiles and risks in different countries • Semen decontamination methods for sperm processing |
| 6. ART should be designed to be robust, repeatable and efficient |
| 7. Equipment should be basic, sturdy and strong |
| 8. Products should be robust, ready to use and with a long half-life • Sperm processing materials are best aseptically packaged (set or kit) and stored at room temperature • Embryo culture media should be robust, short term, pre-packaged in small quantities • Disposables (pipette tips, screening dishes,….) can be pre-packaged as “a set per patient” |
| 9. Information to the community should be discrete and applicable, taking into account sociocultural and religious differences |
| 10. A training program with regular follow-up / audits should be available for the medical and paramedical staff of the pilot-centres. |
Selection of patients and IVF protocol
Figure 4 shows the inclusion and exclusion criteria for the selection of patients, the result of the expert meeting in Arusha.
Fig. 4. The Walking Egg project at a glance: from dream to reality.
In the initial phase we will only treat childless couples with specific age-limits.
Whether or not HIV-positive women and men should be treated remains a debatable subject. It was decided that HIV positive women can only be treated in those countries where antiretroviral therapy is provided, either by the government or by non-profit organizations.
Since ICSI will not be offered in the initial phase, severe male factor infertility cases are excluded and referred to a level 3 centre of excellence. We have to consider that in more than 70 % of ART cases our simplified tWE lab method can be successfully used due to the low number of spermatozoa needed per oocyte (see above).
Advocacy and networking
Global access to infertility care can only be implemented and sustained if it is supported by local policy makers and the international community. Many international organizations have already expressed their desire to collaborate including the WHO (World Health Organisation), ESHRE (European Society for Human Reproduction and Embryology) and ISMAAR (International Society for Mild Approaches to Assisted Reproduction). We will also need the media, patient organizations and interested politicians to change the existing moral and socio-cultural beliefs which are isolating and ostracizing infertile couples (Fig. 4).
Conclusion
The magnitude of childlessness in developing countries has dimensions beyond its prevalence and aetiology. Differences between the developed and developing world are emerging because of the different availability in infertility care and different sociocultural value surrounding procreation and childlessness. There is a growing belief that individual health needs of impoverished people have a place next to their public health needs. Although reproductive health education and prevention of infertility are number one priorities, the need for accessible diagnostic procedures and new simplified reproductive technologies is very high. The success and sustainability of ART in resource-poor settings will depend to a large extend on our ability to optimise these techniques in terms of availability, affordability and effectiveness. The Walking Egg npo aims to raise awareness surrounding childlessness in resource-poor countries and to make infertility care in all its aspects, including assisted reproductive technologies, available and accessible for a much larger part of the population. By simplifying the diagnostic and IVF laboratory procedures and by modifying the ovarian stimulation protocols for IVF assisted reproductive techniques can be offered at affordable prices. The implementation of low-cost infertility centres in resource poor countries, if possible integrated in existing Reproductive Health Care Centres, will be a crucial step to reach the ultimate goal of “universal access to infertility care”.
The selection of pilot-centers will depend on different factors such as budget for education and health care in that specific country, the availability of effective family planning and mother care facilities, a dedicated person who can coordinate the study and shows interest for sociological support, before, during and after treatment,
Infertility will likely become one of the more predominant components of future reproductive health care practice. Taking advantage of information and communication technologies will increase the effectiveness and accessibility of health care services, as well as change patient behaviors to seek timely treatment. As evidence-based affordable solutions begin to drive global guidance within both public and private health care system solutions, access to care for the infertile couple will become one of the largest emerging fields in global medicine.
Fig. 5.
Acknowledgments
I gratefully acknowledge all the experts who were involved in the Walking Egg Project since many years (Rudi Campo, Nathalie Dhont, Danie Franken, Trudie Gerrits, Carin Huyser, Geeta Nargund, Guido Pennings, Hassan Sallam, Frank Van Balen, Jonathan Van Blerkom, Sheryl Vanderpoel, Annie Vereecken, Koen Vanmechelen, and many others).
I also like to thank Nathalie Dhont and Jan Goossens for their critical editorial review and Annelies Thijssen and Liesbeth Grondelaers for their technical support in preparing this manuscript.
References
- Boivin J, Bunting L, Collins JA, et al. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–1512. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome. Br J Obstet Gynaecol. 1998;105:836–848. doi: 10.1111/j.1471-0528.1998.tb10227.x. [DOI] [PubMed] [Google Scholar]
- Campo R, Molinas CR, Rombauts L, et al. Prospective multicentre randomized controlled trial to evaluate factors influencing the success rate of office diagnostic hysteroscopy. Hum Reprod. 2005;20:258–263. doi: 10.1093/humrep/deh559. [DOI] [PubMed] [Google Scholar]
- Daar AS, Merali Z. In Vayena E, Rowe PJ and Griffin PD (Eds) Current Practices and Controversies in Assisted Reproduction. Geneva, Switzerland: World Health Organization; 2002. Infertility and social suffering: the case of ART in developing countries. pp. 15–21. [Google Scholar]
- Dhont N. Clinical, epidemiological and socio-cultural aspects of infertility in resource-poor settings. www.fvvo.eu. FV&V in ObGyn. 2011;3:77–88. [PMC free article] [PubMed] [Google Scholar]
- Dhont N. The Walking Egg non-profit organisation. www.fvvo.eu. FV&V in ObGyn. 2011;3:253–255. [PMC free article] [PubMed] [Google Scholar]
- Dhont N, Muvunyi C, Luchters S, et al. HIV infection and sexual behaviour in primary and secondary infertile relationships: a case--control study in Kigali, Rwanda. Sex Transm Infect. 87:28–34. doi: 10.1136/sti.2010.042879. [DOI] [PubMed] [Google Scholar]
- Dyer SJ, Abrahams N, Hoffman M, van der Spuy ZM. ‘Men leave me as I cannot have children’: women’s experiences with involuntary childlessness. Hum Reprod. 2002;17:1663–1668. doi: 10.1093/humrep/17.6.1663. [DOI] [PubMed] [Google Scholar]
- Dyer SJ, Abrahams N, Hoffman M, et al. Infertility in South Africa: women’s reproductive health knowledge and treatment-seeking behaviour for involuntary childlessness. Hum Reprod. 2002;17:1657–1662. doi: 10.1093/humrep/17.6.1657. [DOI] [PubMed] [Google Scholar]
- Dyer SJ, Abrahams N, Mokoena NE, et al. “you are a man because you have children”: experiences, reproductive health knowledge and treatment-seeking behaviour among men suffering from couple infertility in South Africa. Hum Reprod. 2004:960–977. doi: 10.1093/humrep/deh195. [DOI] [PubMed] [Google Scholar]
- Dyer SJ, Abrahams N, Mokoena NE, et al. Psychological distress among women suffering from couple infertility in South Africa: a quantitative assessment. Hum Reprod. 2005;20:1938–1943. doi: 10.1093/humrep/deh845. [DOI] [PubMed] [Google Scholar]
- Fathalla MF, Sinding SW, Rosenfield A, et al. Sexual and reproductive health for all: a call for action. Lancet. 2006;368:2095–2100. doi: 10.1016/S0140-6736(06)69483-X. [DOI] [PubMed] [Google Scholar]
- Frydman R, Ranoux C. INVO: a simple, low cost effective assisted reproductive technology. Hum Reprod. 2008;(ESHRE Monographs):85–89. [Google Scholar]
- Gerrits T, Shaw M. Biomedical infertility care in sub-Saharan Africa: a social science review of current practices, experiences and viewpoints. FV&V in ObGyn. 2010;2:194–207. [PMC free article] [PubMed] [Google Scholar]
- Gleicher N, Oleske DM, Kaspa I, et al. Reducing the risk of high-order multiple pregnancy after ovarian stimulation with gonadotropins. N Engl J Med. 2000;343:2–7. doi: 10.1056/NEJM200007063430101. [DOI] [PubMed] [Google Scholar]
- Glynn JR, Buvé A, Caraël M, et al. Decreased fertility among HIV-1-infected women attending antenatal clinics in three African cities. J Acquir Immune Defic Syndr. 2000;25:345–352. doi: 10.1097/00042560-200012010-00008. [DOI] [PubMed] [Google Scholar]
- Gnoth C, Herrmann P, Freundl G. Opinion: natural family planning and the management of infertility. Arch Gynecol Obstet. 2002;267 doi: 10.1007/s00404-002-0293-8. [DOI] [PubMed] [Google Scholar]
- Gnoth C, Godehardt D, Godehardt E, et al. Time to pregnancy: results of the German prospective study and impact on the management of infertility. Hum Reprod. 2003;18:1959–1966. doi: 10.1093/humrep/deg366. [DOI] [PubMed] [Google Scholar]
- Greil AL. Infertility and psychological distress: a critical review of the literature. Soc Sci Med. 1997;45:1679–1704. doi: 10.1016/s0277-9536(97)00102-0. [DOI] [PubMed] [Google Scholar]
- Guilhem D. New reproductive technologies, ethics and legislation in Brazil: a delayed debate. Bioethics. 2011;15:218–230. doi: 10.1111/1467-8519.00233. [DOI] [PubMed] [Google Scholar]
- Huyser C, Fourie J. SPERM ONLY PLEASE: Prevention of infections in an assisted reproduction laboratory in a developing country. FV&V in ObGyn. 2010;(Monograph “Artificial Insemination: An Update”):97–106. [Google Scholar]
- Ingerslev HJ, Hojgaard A, Hindkjaer J, et al. A randomized study comparing IVF in the unstimulated cycle with IVF following clomiphene citrate. Hum Reprod. 2001;16:696–702. doi: 10.1093/humrep/16.4.696. [DOI] [PubMed] [Google Scholar]
- Inhorn M. Global infertility and the globalization of new reproductive technologies: illustrations from Egypt. 2003;56:1837–1851. doi: 10.1016/s0277-9536(02)00208-3. [DOI] [PubMed] [Google Scholar]
- Kato K, Takehara Y, Segawa T, et al. Minimal ovarian stimulation combined with elective single embryo transfer policy: age-specific results of a large, single-centre, Japanese cohort. Reprod Biol Endocrinol. 2012;10:35. doi: 10.1186/1477-7827-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabaitu J, Bachengana C, Seeley J. Marital instability in a rural population in south-west Uganda: implications for the spread of HIV-1 infection. Africa (Lond) 1994;64:243–251. [PubMed] [Google Scholar]
- Nargund G, Waterstone J, Bland JM JM, et al. Cumulated conception and live birth rates in natural (unstimulated) IVF cycles. Hum Reprod. 2001;16:259–262. doi: 10.1093/humrep/16.2.259. [DOI] [PubMed] [Google Scholar]
- Nargund G, Fauser BC, Macklon NS, et al. Rotterdam ISMAAR Consensus Group on Terminology for Ovarian Stimulation for IVF. The ISMAAR proposal on terminology for ovarian stimulation for IVF. Hum Reprod. 2007;22:2801–2804. doi: 10.1093/humrep/dem285. [DOI] [PubMed] [Google Scholar]
- Ombelet W, Vandeput H, Van de Putte G, et al. Intrauterine insemination after ovarian stimulation with clomiphene citrate: predictive potential of inseminating motile count and sperm morphology? Hum Reprod. 1997;12:1458–1463. doi: 10.1093/humrep/12.7.1458. [DOI] [PubMed] [Google Scholar]
- Ombelet W, Deblaere K, Bosmans E, et al. Semen quality and intrauterine insemination. Reprod Biomed Online. 2003;7:485–492. doi: 10.1016/s1472-6483(10)61894-9. [DOI] [PubMed] [Google Scholar]
- Ombelet W, Campo R. Affordable IVF for developing countries. Reprod Biomed Online. 2007;15:257–265. doi: 10.1016/s1472-6483(10)60337-9. [DOI] [PubMed] [Google Scholar]
- Ombelet W, Cooke I, Dyer S, et al. Infertility and the provision of infertility medical services in developing countries. Hum Reprod Update. 2008;14:605–621. doi: 10.1093/humupd/dmn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ombelet W, Campo R, Frydman R, et al. The Arusha project: Accessible infertility care in developing countries – a reasonable option. FV&V in ObGyn. 2010;(Monograph):107–115. [Google Scholar]
- Ombelet W. Global access to infertility care in developing countries: a case of human rights, equity and social justice. FV&V in ObGyn. 2011;3:257–266. [PMC free article] [PubMed] [Google Scholar]
- Ombelet W, Campo R, Franken D, et al. The Walking Egg Project: an example of medical eduction and training. FV&V in ObGyn. 2012;(Monograph):66–75. [Google Scholar]
- Papreen N, Sharma A, Sabin K, et al. Living with infertility: experiences among Urban slum populations in Bangladesh. Reprod Health Matters. 2000;8:33–44. doi: 10.1016/s0968-8080(00)90004-1. [DOI] [PubMed] [Google Scholar]
- Pennings G. Ethical issues of infertility treatment in developing countries. Hum Reprod ESHRE Monographs. 2008:15–20. [Google Scholar]
- Pennings G, de Wert G, Shenfield F, et al. ESHRE Task Force on Ethics and Law. Providing infertility treatment in resource-poor countries. Hum Reprod. 2009;24:1008–1011. doi: 10.1093/humrep/den503. [DOI] [PubMed] [Google Scholar]
- Rojanasakul A, Choktanasiri W, Suchartwatanachai C. Simplified IVF ‘ : program for developing countries. J Med Assoc Thai. 1994;77:12–18. [PubMed] [Google Scholar]
- Rutstein SO, Iqbal HS. Infecundity, Infertility, and Childlessness in Developing Countries. DHS Comparative Reports, WHO. 2004 [Google Scholar]
- Sallam HN. Infertility in developing countries: funding the project. Hum Reprod ESHRE Monographs. 2008:97–101. [Google Scholar]
- Swain JE. A self-Contained Culture Platform Using Carbon Dioxide Produced from a Chemical Reaction Supports Mouse Blastocyst Development. In Vitro J Reprod Dev. 2011;57:551–555. doi: 10.1262/jrd.11-022m. [DOI] [PubMed] [Google Scholar]
- Van Balen F, Gerrits T. Quality of infertility care in poor-resource areas and the introduction of new reproductive technologies. Hum Reprod. 2001;16:215–219. doi: 10.1093/humrep/16.2.215. [DOI] [PubMed] [Google Scholar]
- Van Balen F, Bos HMW. The social and cultural consequences of being childless in poor-resource areas. FV&V in ObGyn. 2009;1:106–121. [PMC free article] [PubMed] [Google Scholar]
- Van Blerkom J, Manes C. Development of preimplantation rabbit embryos in vivo and in vitro. II. A comparison of qualitative aspects of protein synthesis. Devel Biol. 1974;40:40–51. doi: 10.1016/0012-1606(74)90105-5. [DOI] [PubMed] [Google Scholar]
- Verberg MF, Macklon NS, Nargund G, et al. Mild ovarian stimulation for IVF. Hum Reprod Update. 2009;15:13–29. doi: 10.1093/humupd/dmn056. [DOI] [PubMed] [Google Scholar]
- Verhulst SM, Cohlen BJ, Hughes E, et al. Intra-uterine insemination for unexplained subfertility. Cochrane Database Syst Rev. 2006;4(CD001838) doi: 10.1002/14651858.CD001838.pub3. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Geneva: World Health Organization; 2010. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed. [Google Scholar]