Abstract
Objectives: The aim of this study was to identify (prognostic) factors that may predict the development of recurrent endometrial cancer and may improve the choice of adjuvant therapy subsequently.
Methods: Data of all patients, diagnosed with primary endometrial cancer in Orbis Medical Center Sittard between 2002 and 2010, were analyzed retrospectively. Cox regression analysis was performed for identification of independent prognostic factors; survival was calculated by using the Kaplan-Meier method.
Study design: Data of all patients, diagnosed with primary endometrial cancer in Orbis Medical Center Sittard between 2002 and 2010, were analyzed retrospectively. Cox regression analysis was performed for identification of independent prognostic factors; survival was calculated by using the Kaplan-Meier method.
Multiple factors were associated with recurrence. Age, histological type and progesteron receptor expression (PR) were identified as independent prognostic factors. Risk profile (according to the PORTEC-1 study) and PR were also independent prognostic factors. Furthermore, PR (p < 0.001) and histological type (p = 0.013) were associated with disease specific survival after recurrence.
Conclusion: Although the survival of endometrial cancer is good, the prognosis of recurrent disease is poor. Recurrence of endometrial cancer and disease free survival rates are associated with several (independent) factors. The effect of adjuvant treatment may improve through more sufficient selection of patients by using the new prognostic factors and through better selection of the type of adjuvant therapy.
Key words: Endometrial cancer, recurrence, survival, prognostic factors, progesterone receptor expression.
Keywords: Endometrial cancer, recurrence, survival, prognostic factors, progesterone receptor expression
Introduction
Endometrial cancer is the most common malignancy of the female genital tract in developed countries (ACOG, 2005; Amant et al., 2005; Fung-Kee-Fung et al., 2006; Ferlay et al., 2008; Otsuka et al., 2010; Odagiri et al., 2011). In the Netherlands, endometrial cancer accounted for 1930 new cancer diagnoses in 2010. Moreover, the incidence of endometrial cancer in the Netherlands is rising, mainly due to the proportional rise in the ageing population as well as the rising prevalence of obesity (Benedet et al., 2000; ACOG, 2005; Bray et al., 2005). Although the prognosis for endometrial cancer is good (due to early diagnosis), approximately 13% of all endometrial cancers recur (Fung-Kee-Fung et al., 2006). The prognosis for recurrent disease is poor; the median survival hardly exceeds 12 months. Currently, the absolute and proportional number of patients with recurrent endometrial cancer increases (Odagiri et al., 2011).
It is important to identify (prognostic) factors that may predict the development of recurrent disease and improve the choice of adjuvant therapy subsequently. This analysis of recurrent endometrial cancer identifies clinical and histopathological variables that are associated with recurrence of endometrial cancer.
Methods
All patients treated between 2002 and 2010 for primary endometrial cancer in Orbis Medical Center Sittard, an independent teaching hospital in the South of the Netherlands, were included. All data were analyzed retrospectively. Patients with non-endometrial cancer, like sarcomas, were excluded. The follow-up ended on 30 November 2011.
For tumour staging, the FIGO 1988 classification system was used for the years 2002-2009 (Odicino et al., 2008). In 2010, the revised FIGO 2009 classification system was used (Pecorelli, 2009). Surgery was performed by laparotomy. The choice for lymphadenectomy and adjuvant therapy was made based on national guidelines; lymphadectomy was performed when tumour positive lymph nodes were suspected or in the context of staging; adjuvant radiotherapy was used in patients with low stage disease with intermediate high or high risk profile (IKNL, 2011). Estrogen and progesterone receptor expression were assessed by consulting the medical record, where it was defined as either positive or negative.
The recurrence free interval (RFI) was defined as the time between date of surgical staging and date of (histological or radiological confirmed) recurrence. The recurrence free survival (RFS) was defined as the time between date of surgical staging and either date of (histological or radiological confirmed) recurrence, date of death – irrespective of cause – or date of the end of the study. The cause of death was extracted from the patients file. Death was defined as disease specific when death was a consequence of (complications from) the disease or therapy.
Statistics were performed using IBM SPSS statistics 19. A p-value < 0.05 was considered to be statistically significant. The 2 sided t-test and nonparametric tests including χ2 test and Fisher’s exact test were used to determine differences between groups and proportions. Cox regression analysis was performed for identification of independent prognostic factors, with recurrence free survival (RFS) and development of recurrence as the outcome measures. Variables found to be significant on univariate analysis were entered into a multiple variate Cox proportional hazards model. The number of variables entered together in the multiple variate Cox proportional hazards model did depend on the number of events, approved by an independent statistician. The proportional hazards assumption was checked using the log-minus-log plot. Overall and disease specific survival and survival after recurrence was calculated by using the Kaplan-Meier method.
Results
Between 2002 and 2010, 219 patients were diagnosed having endometrial cancer. The median follow up time for the total cohort was 40 months. Ten patients (4.6%) did not undergo any kind of surgery. So, 209 patients (95.4%) were operated, all with curative intent; in 26 patients (12.4%) of them, endometrial cancer recurred.
Characteristics
The characteristics of the total cohort are presented in Table I. The mean age of the patients with endometrial cancer at time of diagnosis was 65.3 ± 10.3 years, median age was 65 years. Patients with recurrence where significantly older than patients without recurrence (69.1 ± 10.5 years versus 64.7 ± 10.2 years) (p = 0.042).
Table I. Clinicopathological characteristics.
| Total cohort N = 209 |
Recurrent disease N = 26 |
No recurrent disease N = 183 |
p-value | |
|---|---|---|---|---|
| Mean age (SD) | 65.3 (10.3) | 69.1 (10.5) | 64.7 (10.2) | 0.042 |
| Mean BMI (SD) (n = 183) | 30.0 (7.3) | 31.5 (8.7) | 29.8 (7.1) |
NS |
| DET (n = 180) Increased |
97.2% |
95.2% |
97.5% |
NS |
| CA125 (n = 188) Increased |
31.4% |
41.7% |
29.9% |
NS |
| Histology Endometrioid Non-endometrioid |
85.2% 14.8% |
65.4% 34.6% |
88.0% 12.0% |
0.006 |
| Grade (n = 178) Low grade High grade |
84.3% 15.7% |
64.7% 35.3% |
86.3% 13.7% |
0.032 |
| Histological type Type I Type II |
71.3% 28.7% |
42.3% 57.7% |
75.4% 24.6% |
< 0.001 |
| Estrogen receptor expression (n = 181) positive negative |
86.7% 13.3% |
69.2% 30.8% |
89.7% 10.3% |
0.010 |
| Progesterone receptor expression (n = 181) positive negative |
87.3% 12.7% |
69.2% 30.8% |
90.3% 9.7% |
0.007 |
Clinicopathological characteristics of all patients operated for endometrial cancer (n = 209), followed by a comparison between patients with and without recurrent disease.
Mean age: Mean age at time of diagnosis in years.
BMI: Body mass index in kg/m2.
DET: Double endometrial thickness.
Grade: Low = grade 1 and 2, high = grade 3.
Histological type: Type I = endometrioid grade 1 and 2, type II = endometrioid grade 3 and non-endometrioid carcinomas.
The mean body mass index (BMI) at time of diagnosis was 30.0 ± 7.3 kg/m2. Patients with recurrence did not have a significantly different BMI than patients without recurrence (31.5 ± 8.7 kg/m2 versus 29.8 ± 7.1 kg/m2).
The cut-off value of the double endometrial thickness (DET), measured by transvaginal ultrasonography as the sum of the two adjacent layers of endometrium, was 4 millimeters. DET was increased in 175 patients (97.2%). The prevalence of increased DET was not significantly different between patients who recurred (95.2%) versus patients who did not (97.5%).
The cut-off level of CA-125 measured by blood analysis was 20 kU/L. CA-125 was increased in 59 patients (31.4%). The prevalence of increased CA-125 was not significantly different between patients who recurred (41.7%) versus patients who did not (29.9%).
Of all endometrial carcinomas, 178 (85.2%) were endometrioid, 24 (11.5%) were papillary serous and 7 (3.3%) were clear cell tumours. The prevalence of endometrioid carcinomas was significantly lower in patients who recurred (65.4%) versus patients who did not (88.0%) (p = 0.006).
Of all endometrioid carcinomas, 74 (41.6%) were grade 1, 76 (42.7%) were grade 2 and 28 (15.7%) were grade 3. When divided in low grade (grade 1 and 2) and high grade (grade 3), 150 carcinomas (84.3%) were low grade. The prevalence of low grade endometrioid carcinomas was significantly lower in patients who recurred (64.7%) versus patients who did not (86.3%) (p = 0.032).
Endometrioid grade 1 and 2 tumours were defined as histological type I endometrial cancer; endometrioid grade 3 and non-endometrioid tumours were defined as histological type II endometrial cancer. Of all 209 patients, 149 patients (71.3%) had type I. The prevalence of type I was significantly lower in patients who recurred (42.3%) versus patients who did not (75.4%) (p < 0.001).
Estrogen receptor expression (ER) was positive in 157 tumours (86.7%). The percentage of ER was significantly lower in tumours of patients who recurred (69.2%) versus patients who did not (89.7%) (p = 0.010).
Progesterone receptor expression (PR) was positive in 158 tumours (87.3%). The percentage of PR was significantly lower in tumours of patients who recurred (69.2%) versus patients who did not (90.3%) (p = 0.007).
Of all 209 patients, 169 patients (80.9%) were diagnosed having FIGO-stage I, 10 (4.8%) having FIGO-stage II, 24 (11.5%) having FIGO stage III and 6 (2.9%) having FIGO-stage IV. The recurrence rate was significantly different between patients within different FIGO-stages; 6.5%, 20.0%, 37.5% and 66.7% in FIGO-stage I, II, III and IV respectively (p < 0.001) (Table II).
Table II. FIGO-stage and recurrence rate.
| FIGO-stage | Patients (%) | Recurrence rate |
|---|---|---|
| I | 169 (80.9) | 6.5% |
| II | 10 (4.8) | 20.0% |
| III | 24 (11.5) | 37.5% |
| IV | 6 (2.9) | 66.7% |
The number of patients with endometrial cancer (n = 209) and the recurrence rate within the different FIGO-stages.
Additionally, there has been made a distinction between low stage (FIGO-stage I-IIA) and high stage (FIGO-stage IIB-VI) disease (IKNL, 2011). Overall, 172 patients (82.3%) had low stage and 37 patients (17.7%) had high stage disease. The recurrence rate was significantly lower in patients with low FIGO-stage (6.4%) versus patients with high FIGO-stage (40.5%) (p < 0.001).
Treatment
Of all 209 patients that were operated for endometrial cancer, 202 patients (96.7%) underwent at least a total abdominal hysterectomy (TAH) with bilateral salpigooöphorectomy (BSO). The remaining 7 patients (3.3%) underwent other surgical procedures, as in these patients endometrial cancer was accidentally found after vaginal hysterectomy due to prolapse or menstrual disorders.
Besides the TAH with BSO, 16 patients (7.7%) underwent lymph node sampling or lymph node dissection (LND). Of these patients, 6 underwent full pelvic and paraaortic lymphadenectomy and 7 underwent lymphadenectomy of suspicious lymph nodes only. In 3 patients the extensiveness of lymphadenectomy remains unclear. The prevalence of LND was not significantly different between patients who recurred (11.5%) versus patients who did not (7.1%).
Of all 16 patients who underwent LND, 5 patients (31.3%) had positive lymph nodes.
The prevalence of positive lymph nodes was not significantly different between patients who recurred (33.3%) versus patients who did not (30.8%).
Recurrence
The recurrence site was vaginal in 8 patients (30.8%), pelvic in 4 patients (15.4%), distant in 10 patients (38.5%), vaginal and pelvic in 2 patients (7.7%), vaginal and distant in 1 patient (3.8%) and pelvic and distant in 1 patient (3.8%). Altogether, 12 patients (46.2%) had recurrence involving distant disease: 8 patients (66.7%) had only abdominal metastasis, 1 had abdominal and lung metastasis, 1 had abdominal, lung and bone metastasis and 2 had abdominal and pleural metastasis (Table III).
Table III. Site of recurrences.
| Site of recurrence | Patients (%) |
|---|---|
| Vaginal | 11 (42.3) |
| Pelvic | 7 (26.9) |
| Distant | 12 (46.2) |
| Abdominal | 12 |
| Lung | 2 |
| Pleural | 2 |
| Bone | 1 |
The number of patients with recurrent endometrial cancer (n = 26) by site of recurrence; 4 patients had recurrence in 2 sites; 4 patients had distant recurrence in 2 or more sites.
The median RFI for all 26 patients with recurrence was 17 months. Nine patients (37.5%) recurred within the first year, 13 (54.2%) within the second year, 2 (8.3%) within the third year and 2 (7.7%) after 5 years. Altogether, 24 patients (92.3%) recurred within 3 years.
The median RFI was not significantly different between patients with vaginal recurrence (4 months) and patients with pelvic or distant recurrence (16 months). The median RFI was also not significantly different between patients with different sites of distant recurrent disease.
Survival
Of all 209 patients, 40 patients (19.1%) were dead by the end of follow up, 167 patients (79.9%) were alive. Survival was unknown in 2 patients (1.0%). Of the 40 patients who died, 20 patients (50%) had no evidence of recurrent disease.
The disease specific survival (DSS) for all 209 patients was 94% at 2 years and 86% at 5 years. The overall survival (OS) was 92% at 2 years and 78% at 5 years. The DSS for patients with recurrence (n = 26) was 71% at 2 years and 32% at 5 years, median DSS was 34 months. The OS for patients with recurrence was 67% at 2 years and 20% at 5 years, median OS was 32 months.
The 5-year DSS was significantly lower in patients with recurrence (31%) versus patients without (95%) (p < 0.001). The 5-year OS was significantly lower in patients with recurrence (20%) versus patients without (89%) (p < 0.001) (Fig. 1).
Fig. 1.
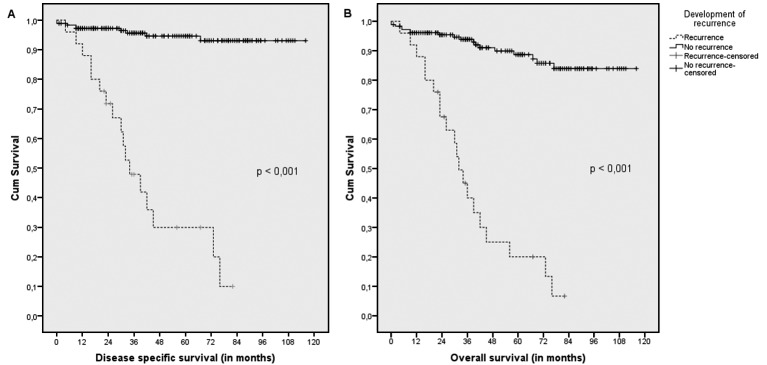
Disease specific and overall survival in patients with endometrial cancer
Kaplan-Meier estimates of disease specific survival (A) and overall survival (B), regarding development of recurrence; demonstrating a significantly worse outcome in patients with recurrence (p < 0.001).
The DSS after recurrence was 27% at 2 years and 20% at 5 years, median DSS was 11 months. The OS after recurrence was 22% at 2 years and 8% at 5 years, median OS was 9 months.
Uni-and multiple variate analysis
On univariate analysis, the following prognostic factors were related to RFS: histology, grade, histological type (type II vs. I), ER, PR, cervical invasion, myometrial invasion (no or = 50% invasion vs. > 50%), lymph vascular space invasion, peritoneal cytology, ovarian metastasis, FIGO-stage and both presence and type of adjuvant therapy (Table IV). Age was also considered as an unfavorable factor for the development of recurrence (p = 0.019). The hazard ratio for recurrence by annual increase in age was 1,048 (95% CI: 1.008-1.089).
Table IV. Uni- en multiple variate Cox regression analysis.
| Factors | hazard ratio | 95% confidence interval | p-value |
|---|---|---|---|
| Univariate | |||
| Age | 1.048 | 1.008-1.089 | 0.019 |
| Body mass index | 1.026 | 0.972-1.083 | NS |
| Increased CA-125 | 1.554 | 0.688-3.510 | NS |
| Histology (non-endometrioid vs. endometrioid) | 3.742 | 1.665-8.411 | 0.001 |
| Grade (high vs. low grade) | 2.890 | 1.067-7.827 | 0.037 |
| Histological type (type II vs. type I) | 3.756 | 1.725-8.180 | 0.001 |
| Estrogen receptor expression | 0.260 | 0.113-0.599 | 0.002 |
| Progesterone receptor expression | 0.238 | 0.103-0.550 | 0.001 |
| Cervical invasion | 5.692 | 2.580-12.556 | < 0.001 |
| Myometrial invasion (serosa/> 50% vs. < 50%/no invasion) | 2.895 | 1.338-6.264 | 0.007 |
| Lymph-vascular space invasion | 3.418 | 1.507-7.753 | 0.003 |
| Peritoneal cytology (positive vs. negative cytology) | 5.145 | 2.232-11.861 | < 0.001 |
| Ovarian metastasis | 6.772 | 2.516-18.231 | < 0.001 |
| FIGO-stage (high vs. low stage) | 8.531 | 3.911-18.607 | < 0.001 |
| Lymph node dissection | 1.861 | 0.555-6.248 | NS |
| Adjuvant therapy | 3.916 | 1.702-9.008 | 0.001 |
| Type of adjuvant therapy • radiotherapy vs. no adjuvant therapy • chemotherapy vs. no adjuvant therapy • radiotherapy + chemotherapy vs. no adjuvant therapy • radiotherapy + chemotherapy vs. radiotherapy |
2.290 31.803 5.334 2.334 |
0.883-5.934 8.826-114.594 1.130-25.186 0.500-10.886 |
NS < 0.001 0.035 NS |
| Type of radiotherapy • internal vs. external • internal + external vs. external • internal + external vs. internal |
0.581 1.802 2.803 |
0.073-4.601 0.487-6.664 0.289-27.135 |
NS NS NS |
| Multiple variate 1 | |||
| Age Histological type (type II vs. type I) Progesterone receptor expression |
1.049 2.668 0.325 |
1.006-1.093 1.177-6047 0.133-0.797 |
0.024 0.019 0.014 |
| Multiple variate 2 | |||
| Risk profile (high vs. low risk profile) Progesterone receptor expression |
3.170 0.320 |
1.306-7.695 0.136-0.755 |
0.011 0.009 |
Cox proportional hazards model for the prediction of recurrence, demonstrating multiple factors associated with the development of recurrence.
Grade: Low = grade 1 and 2, high = grade 3.
Histologic type: Type I = endometrioid grade 1 and 2, type II = endometrioid grade 3 and non-endometrioid carcinomas.
FIGO-stage: Low stage = FIGO-stage I-IIA, high stage = FIGO-stage IIB-IV.
Risk profile: Low and high risk profile according to PORTEC-1 study.
Multiple variate Cox regression analysis revealed that age (HR 1.049, 95% CI 1.006-1.093, p = 0.024), histological type (HR 2.668, 95% CI 1.177-6.047, p = 0.019) and PR (HR 0.325, 95% CI 0.133-0.797, p = 0,014) were independent prognostic factors.
FIGO stage (HR 5.930, 95% CI 2.687-13.089, p < 0.001) and PR (HR 0.319, 95% CI 0.135-0.756, p = 0.009) were also independent prognostic factors in multiple variate Cox regression analysis.
Subsequently, patients were divided in two groups (ie low and high risk profile) according to FIGO-stage and the trias of age, grade and myometrial invasion, based on the classification reported in the PORTEC-1 study (Creutzberg, 2000; IKNL, 2011) which is used widely in considering the indication for adjuvant therapy. Multiple variate Cox regression analysis revealed that risk profile (HR 3.170, 95% CI 1.306-7.695, p = 0.011) and PR (HR 0.320, 95% CI 0.136-0.755, p = 0.009) were independent prognostic factors. (Table IV).
Survival after recurrence
In a post-hoc analysis, all factors mentioned above were analyzed into having any associations with disease specific and overall survival after recurrence. Histological type and PR were revealed as independent factors associated with DSS after recurrence (p = 0.013 and p < 0.001 respectively) (Fig. 2) and OS after recurrence (p = 0.046 and p < 0.001 respectively).
Fig. 2.
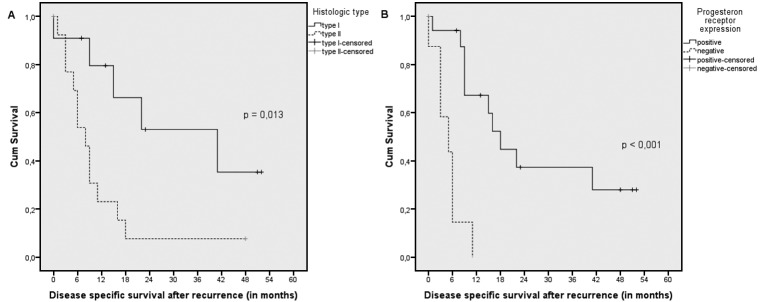
Disease specific survival after recurrence
Kaplan-Meier estimates of disease specific survival after recurrence regarding (A) histological type, demonstrating a significantly better outcome in patients with type I endometrial cancer (p = 0.013) and (B) progesterone receptor expression, demonstrating a significantly better outcome in patients with positive receptor expression (p < 0.001).
Discussion
Since 2002, the recurrence rate in patients treated for endometrial cancer in the South of the Netherlands is 12.4%. The median RFI is 17 months, irrespective of the site of recurrence. The recurrence rate and median RFI are equal to recurrence rates (10-15%) and RFI’s (13-22 months) in world wide reported analyses (Fung-Kee-Fung et al., 2006; Zusterzeel et al., 2008; Esselen et al., 2011; Odagiri et al., 2011). However, in conflict to publication of the American College of Obstetricians and Gynecologists (ACOG, 2005) and Van Wijk et al. (2007), RFI for vaginal recurrence is not shorter than for pelvic or distant recurrence. This may be explained by the fact that in our study there was only a small number of events with short follow-up. However, the most recent study of Fujimoto et al. (2009) supports our findings.
The (prognostic) factors that are associated with the development of recurrent disease include age, histology, grade, histological type, ER, PR, cervical invasion, myometrial invasion, LVSI, peritoneal cytology, ovarian metastasis, FIGO-stage and both presence and type of adjuvant therapy. Subsequently, multiple variate Cox regression analysis revealed that age, histological type and PR are independent factors associated with recurrent endometrial cancer. One report supports our findings regarding age (Zusterzeel et al., 2008) while another paper supports our findings regarding histological type (Fung-Kee-Fung et al., 2006). The role of PR in developing recurrent endometrial cancer is also supported by other worldwide reported analyses, which conclude that low PR is associated with recurrent endometrial cancer (Ingram et al., 1989; Pijnenborg et al., 2005; Suthipintawong et al., 2008).
Of all recurrences, half was seen in high stage disease. This may influence the analysis, because high stage disease will very likely recur. To prevent this, stage is included in the variable ‘risk profile’. Multiple variate Cox regression analysis revealed that PR and risk profile (according to the widely used classification of the PORTEC-1 study (Creutzberg et al., 2000)) are both identified as independent prognostic factors.
The PORTEC-1 study (Creutzberg et al., 2000) pointed out that the trias of age, myometrial invasion and tumour grade has to play a role in considering the indication for adjuvant therapy. Although the PORTEC-1 trial pointed out that there was a decrease of local recurrences, there was no survival benefit.
However, recurrent endometrial cancer is associated with poor prognosis and poor disease specific and overall survival. Progesterone receptor expression and histological type were associated with survival after recurrence. Other studies have indicated histological grading of the tumour, recurrence free interval and site of recurrence as the most important prognostic factors in recurrent endometrial cancer (Sartori et al., 2003; Karagol et al., 2006; Sohaib et al., 2007; Otsuka et al., 2010; Odagiri et al., 2011). Although the number of patients are small and the follow up is short, this study may be the first report demonstrating an association between PR and survival after recurrence. This finding may be associated with the fact that positive PR contributes to the effect of hormone-based therapy, thereby prolonging overall survival (Yang et al., 2011). However, hormone therapy was only used in half of the patients with positive PR.
The choice of adjuvant therapy is based on international guidelines regarding FIGO-stage and the trias of age, grade and myometrial invasion. We suggest that possibly new factors, such as progesterone receptor expression, may be useful in identifying those patients with a high risk for recurrent endometrial cancer.
Further studies are needed to investigate their predictive value in clinical outcome. For example, recently L1CAM has shown to be an important prognostic factor in low stage endometrial cancers and may be used for the indication for adjuvant therapy (Zeimat et al., 2013). Also other factors like MSI, PI3K-AKT, Wnt/β-catenin and P53 should be added to improve identification of those patients who are at risk for recurrence (Nout et al., 2012). This will improve the selection of the patients that may benefit from adjuvant treatment subsequently, intending to decline the recurrence rate and to improve survival.
References
- ACOG. practice bulletin, clinical management guidelines for obstetriciangynecologists, number 65, August 2005: management of endometrial cancer. Obstet Gynecol. 2005;106(2):413–425. doi: 10.1097/00006250-200508000-00050. [DOI] [PubMed] [Google Scholar]
- Amant F, Moerman P, Neven P, et al. Endometrial cancer. Lancet. 2005;366(9484):491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- Benedet JL, Bender H, Jones H, et al. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000;70(2):209–262. [PubMed] [Google Scholar]
- Bray F, Dos Santos Silva I, Moller H, et al. Endometrial cancer incidence trends in Europe: underlying determinants and prospects for prevention. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1132–1142. doi: 10.1158/1055-9965.EPI-04-0871. [DOI] [PubMed] [Google Scholar]
- Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355(9213):1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- Esselen KM, Boruta DM, del Carmen M, et al. Defining prognostic variables in recurrent endometrioid endometrial cancer: a 15-year singleinstitution review. Int J Gynecol Cancer. 2011;21(6):1078–1083. doi: 10.1097/IGC.0b013e31821872f4. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Nanjyo H, Fukuda J, et al. Endometrioid uterine cancer: histopathological risk factors of local and distant recurrence. Gynecol Oncol. 2009;112(2):342–347. doi: 10.1016/j.ygyno.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Fung M, Dodge J, Elit L, et al. Follow-up after primary therapy for endometrial cancer: a systematic review. Gynecol Oncol. 2006;101(3):520–529. doi: 10.1016/j.ygyno.2006.02.011. [DOI] [PubMed] [Google Scholar]
- IKNL Werkgroep Oncologische. Landelijke richtlijn Endometriumcarcinoom, Versie: 3.0. 2011 [Google Scholar]
- Ingram SS, Rosenman J, Heath R, et al. The predictive value of progesterone receptor levels in endometrial cancer. Int J Radiat Oncol Biol Phys. 1989;17(1):21–27. doi: 10.1016/0360-3016(89)90365-9. [DOI] [PubMed] [Google Scholar]
- Karagol H, Saip P, Uygun K, et al. Evaluation of prognostic factors and comparison of systemic treatment modalities in patients with recurrent or metastatic endometrial carcinoma. Med Oncol. 2006;23(4):543–548. doi: 10.1385/MO:23:4:543. [DOI] [PubMed] [Google Scholar]
- Nout RA, Bosse T, Creutzberg CL, et al. Improved risk assessment of endometrial cancer by combined analysis of MSI, PI3K-AKT, Wnt/β-catenin and P53 pathway activation. Gynecol Oncol. 2012;126(3):466–473. doi: 10.1016/j.ygyno.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Odagiri T, Watari H, Hosaka M, et al. Multivariate survival analysis of the patients with recurrent endometrial cancer. J Gynecol Oncol. 2011;22(1):3–8. doi: 10.3802/jgo.2011.22.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odicino F, Pecorelli S, Zigliani L, et al. History of the FIGO cancer staging system. Int J Gynaecol Obstet. 2008;101:205–210. doi: 10.1016/j.ijgo.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Otsuka I, Uno M, Wakabayashi A. Predictive factors for prolonged survival in recurrent endometrial carcinoma: Implications for follow-up protocol. Gynecol Oncol. 2010;119(3):506–510. doi: 10.1016/j.ygyno.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Pijnenborg JM, Romano A, de Veen GC. Aberrations in the progesterone receptor gene and the risk of recurrent endometrial carcinoma. J Pathol. 2005;205(5):597–605. doi: 10.1002/path.1738. [DOI] [PubMed] [Google Scholar]
- Sartori E, Laface B, Gadducci A, et al. Factors influencing survival in endometrial cancer relapsing patients: a Cooperation Task Force (CTF) study. Int J Gynecol Cancer. 2003;13(4):458–465. doi: 10.1046/j.1525-1438.2003.13328.x. [DOI] [PubMed] [Google Scholar]
- Sohaib SA, Houghton SL, Meroni R, et al. Recurrent endometrial cancer: patterns of recurrent disease and assessment of prognosis. Clin Radiol. 2007;62(1):28–34. doi: 10.1016/j.crad.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Suthipintawong C, Wejaranayang C, Vipupinyo C. Prognostic significance of ER, PR, Ki67, c-erbB-2, and p53 in endometrial carcinoma. J Med Assoc Thai. 2008;91(12):1779–1784. [PubMed] [Google Scholar]
- Van Wijk FH, Huikeshoven FJ, Abdulkadir L, et al. Recurrent endometrial cancer: a retrospective study. Eur J Obstet Gynecol Reprod Biol. 2007;130(1):114–120. doi: 10.1016/j.ejogrb.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metab. 2011;22(4):145–152. doi: 10.1016/j.tem.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeimet AG, Reimer D, Huszar M, et al. L1CAM in Early-Stage Type I Endometrial Cancer: Results of a Large Multicenter Evaluation. J Natl Cancer Inst. 2013;Epub ahead of print doi: 10.1093/jnci/djt144. [DOI] [PubMed] [Google Scholar]
- Zusterzeel PL, Bekkers RL, Hendriks JC, et al. Prognostic factors for recurrence in patients with FIGO stage I and II, intermediate or high risk endometrial cancer. Acta Obstet Gynecol Scand. 2008;87(2):240–246. doi: 10.1080/00016340701876163. [DOI] [PubMed] [Google Scholar]


