Abstract
Background
Some risk factors for atherosclerosis are followed by non-alcoholic fatty liver disease (NAFLD). We wanted to use Multislice computed tomography (MSCT) as technique for searching relationship between NAFLD and coronary artery disease (CAD).
Objective
The relationship between NAFLD and CAD was investigated using MSCT.
Methods
A total of 372 individuals with or without cardiac symptoms who had undergone MSCT angiography were included in the study. The patients were divided into two groups according to the presence of NAFLD. Coronary artery segments were visually evaluated via MSCT angiography. Based on the coronary artery stenosis degree, those with no or minimal plaques were considered normal, whereas those who had stenosis of less than 50% and at least one plaque were considered to have non-obstructive coronary artery disease (non-obsCAD). The patients who had at least one plaque and coronary artery stenosis of 50% or more were considered to have obstructive coronary artery disease (obsCAD). NAFLD was determined according to the MSCT protocol, using the liver density.
Results
According to the liver density, the number of patients with non-alcoholic fatty liver disease (group 1) was 204 (149 males, 54.8%) and with normal liver (group 2) was 168 (95 males, 45.2%). There were 50 (24.5%) non-obsCAD and 57 (27.9%) obsCAD cases in Group 1, and 39 (23.2%) non-obsCAD and 23 (13.7%) obsCAD cases in Group 2.
Conclusions
The present study using MSCT demonstrated that the frequency of coronary artery disease in patients with NAFDL was significantly higher than that of patients without NAFDL.
Keywords: Fatty Liver, Hepatitis
Introduction
Today, non-alcoholic fatty liver disease (NAFLD) is considered as the most common chronic liver disease in Western populations1,2. Since the cases are generally asymptomatic, the true prevalence of NAFLD is unknown. Hepatic enzymes are within normal ranges in 70% of the patients. Adult screening studies found the prevalence of NAFLD to be 10%-15% in normal-weight individuals, but 70%-80% in obese people3,4. NAFLD comprises a wide spectrum of hepatic damage ranging from simple steatosis and steatohepatitis to advanced fibrosis and cirrhosis3. Risk factors for atherosclerosis including hypertension, obesity, diabetes, metabolic syndrome, dyslipidemia and insulin resistance, accompany NAFLD5-8.
Computed tomography (CT) is the right modality for detecting fatty liver disease9. The attenuation value differences between liver and spleen are used for hepatosteatosis diagnosis. The mean liver attenuation value minus the mean spleen attenuation value presenting a difference of ≤ 10 Hounsfield Units indicates hepatosteatosis9,10.
Multislice computed tomography (MSCT) coronary angiography is considered a non-invasive modality for the detection and classification of coronary artery disease (CAD)11,12.
The present study investigated the relation between CAD and non-alcoholic fatty liver disease using MSCT angiography protocol.
Methods
Patients' Clinical Characteristics
The present study comprises 372 patients with or without cardiac symptoms, who underwent MSCT angiography in our clinic between January 2008 and September 2012. Data were collected retrospectively and the ethical committee approval was obtained.
Study groups included individuals who did not consume alcohol or had an alcohol consumption of less than 20 g/day ethanol. People with positive serology for hepatitis B or C or who had a history of chronic liver disease were excluded from the study.
Dyslipidemia was defined as a fasting serum triglyceride level ≥ 150 mg/dl, low-density lipoprotein (LDL) cholesterol level ≥ 140 mg/dl, and/or high-density lipoprotein (HDL) cholesterol level < 40 mg/dl, and those receiving or not active medical treatment for this13.
Before CT scan, the height (Human weighing machine, NAN TARTI AŞ, Turkey) and body weight (TANITA Body Composition Analyzer, TANITA Corporation, Japan) of the participants were measured, and their body mass indexes (BMI) were calculated. Those with a BMI lower than 25 kilogram (kg)/square meter (m2) (BMI<25 kg/m2) were considered normal-weight, those with a BMI between 25 and 30 kg/m2 (25 kg/m2 ≤ BMI < 30 kg/m2) were considered over-weight, and those with a BMI ≥ 30 kg/m2 (30 kg/m2 ≤ BMI) were considered obese.
MSCT Image Reconstruction and CAD evaluation
MSCT angiography was performed via tomography device Somatom Sensation 64 (Siemens, Forchheim, Germany). The acquisition parameters were a gantry rotation time of 330 milliseconds, tube voltage of 120 kilowatt, 250 milliamps (mAs), and detector collimation of 0.6 millimeter. Scans were obtained within a breath-hold in approximately 8.4-13.1 seconds in the craniocaudal direction from the level of the carina to the subcostal level. During MSCT angiography scanning, 80-to-110 milliliters (ml) of non-ionic contrast agent (Iomeron 400, Bracco s.p.a., Milan, Italy), depending on the patient's body weight, was given through an antecubital vein at a rate of 5.0 milliliter (ml)/second (s) followed by a bolus administration of 40 ml of normal saline. Automatic peak contrast intensity in the ascending aorta was determined to be +140 Hounsfield units. The images were reconstructed using a retrospective electrocardiographic gating technique (with a slice thickness of 0.6 mm and a reconstruction index of 0.6 mm). Multiplanar and three-dimensional volume rendering images were created from thin axial sections, and coronary artery anatomy was studied.
All coronary artery segments were visually examined. Among the study participants, those with no or minimal plaques were considered normal (Figure 1), those with stenosis less than 50% and which had at least one plaque were considered to have non-obstructive coronary artery disease (non-obsCAD) (Figures 2a and 2b), and those with at least one plaque and coronary artery stenosis ≥50% were considered to have obstructive coronary artery disease (Figures 3a and 3b). MSCT coronary angiography examinations were performed by radiologists, cardiovascular surgeons and cardiologists.
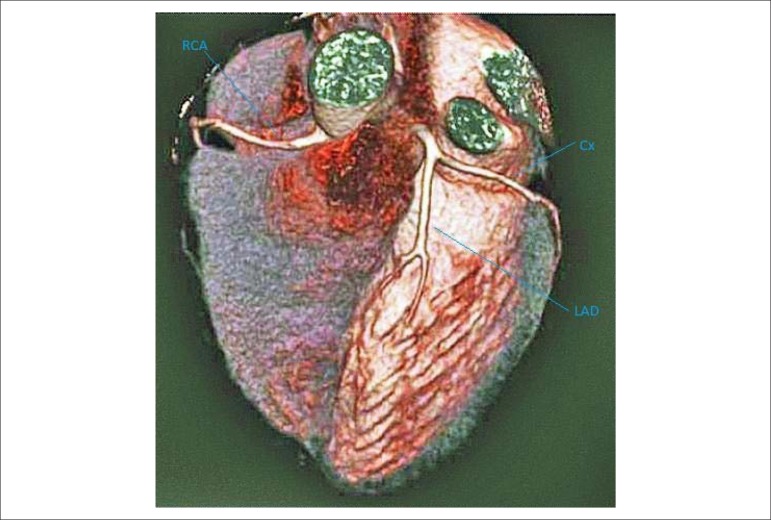
Figure 1.
3-D reconstruction image are showing normal coronary arteries (RCA: right coronary artery; Cx: circumflex coronary artery; LAD: left anterior descending artery).
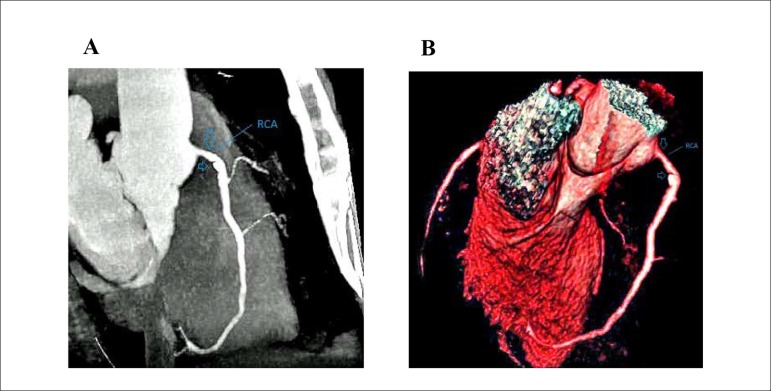
Figure 2.
A) Multiplanar reconstruction image is showing mild stenotic calcified coronary plaques at proximal area of RCA(arrows) (RCA: right coronary artery). B) 3-D reconstruction image are showing mild stenotic calcified coronary plaques at proximal area of RCA(arrows) (RCA: right coronary artery)
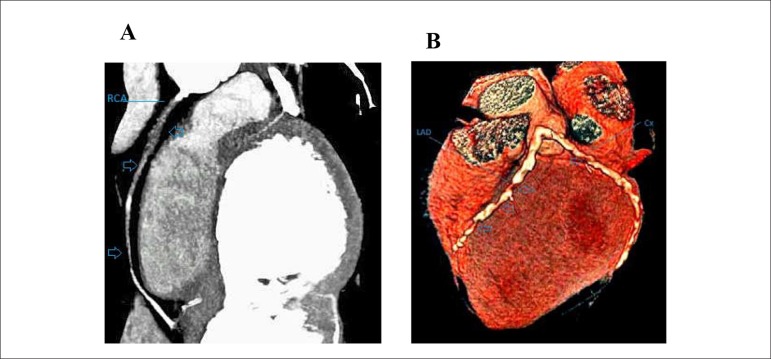
Figure 3.
A) Multiplanar reconstruction image is showing total vessel occlusion of the right coronary artery due to diffuse soft plaque (arrows). B) 3-D reconstruction image is showing considerable atherosclerosis with diffuse calcifications of LAD and Cx (arrows)(LAD: the left anterior descending artery; Cx: circumflex coronary artery)
Assessment of NAFLD
Most individuals with NAFLD have no symptoms and signs of liver disease. Hepatomegaly may be the unique physical finding. The most common laboratory abnormality is mild-to-moderate elevation in serum hepatic enzyme levels. The diagnosis of NAFLD is made based on proven fatty infiltration in the liver of the individuals without chronic liver disease (primary or secondary) and without alcohol consumption. Although abdominal ultrasonography is the most commonly used modality, the present study used the non-contrasted images of the liver obtained by MSCT angiography scanning protocol.
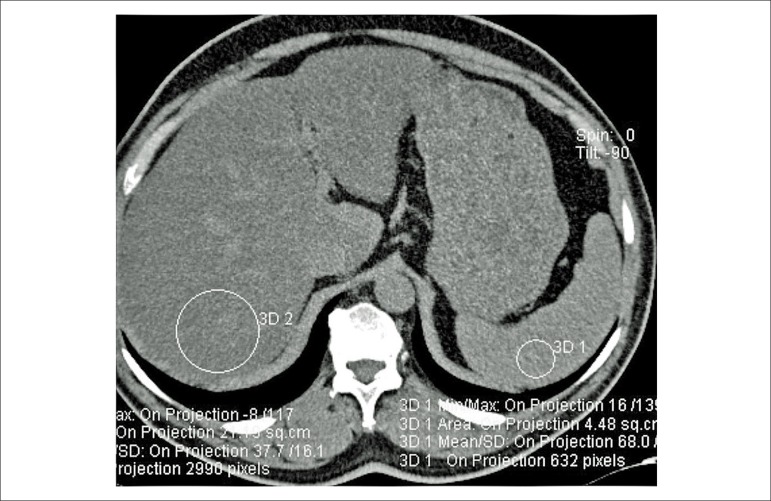
The individuals, whose non-contrast CT scans in the MSCT angiography protocol involved the level between the carina and subcostal plane, were included in the study. Densities of the liver and spleen were measured (Figure 4). Individuals with hepatic density lesser than spleen density by 10 HU or higher were named as Group 1. The other study participants without hepatosteatosis were considered to have normal livers and they were named as Group 2. Hepatic and splenic density measurement was done by drawing circular region of interests (ROIs) on three axial slices, and the mean values were recorded. Vascular and biliary structures were avoided while drawing the ROIs (Figure 4).
Figure 4.
Diffuse fat deposition in the liver (non-contrast CT section). Liver density is 37 HU and spleen density is 68 HU.
Statistical Analysis
Statistical analysis was done using the SPSS software version (SPSS Inc., Chicago, IL, USA). The comparison of nonparametric data between the groups was performed with the Pearson's Chi-square analysis. Parametric data were presented as minimum, maximum, and mean ± standard deviation. The comparison of parametric data between the groups was performed with the independent student t-test. Results were considered statistically significant if the two-tailed p value was lower than 0.01 (p < 0.01) (Table 1). Different characteristics between the groups including age, gender, dyslipidemia, smoking were subjected to Binary Logistic Regression Analysis. Results were considered statistically significant if the two-tailed p value was lower than 0.01 (p < 0.01) (Table 2).
Table 1.
Data and statistical results about groups
| Group 1 (n = 204) | Group 2 (n = 168) | p value | |
|---|---|---|---|
| Age (±SD) | 50,8 ± 10,9 | 48,09 ± 11,5 | 0,018¥ |
| Gender (male) | 149 (% 73) | 95 (% 56,5) | 0,001§ |
| Hepatic density (HU) | 43,7 ± 9 | 62,2 ± 5,2 | 0¥ |
| Diabetes | |||
| Nondiabetic | 130 (% 63,7) | 124 (% 73,8) | 0,112§ |
| Oral a/d | 54 (% 26,5) | 33 (% 19,8) | |
| Parenteral a/d | 20 (% 9,4) | 11 (% 6,5) | |
| Dyslipidemia | 161 (% 78,9) | 108 (% 64,3) | 0,002§ |
| Hypertension | 125 (% 61,3) | 94 (% 56) | 0,323§ |
| Smoking | 96 (% 47,1) | 56 (% 33,3) | 0,007§ |
| Alcohol consumption ≥ 20 g/day (± SD) | - | - | - |
| Body weight | |||
| Excessive weight | 61 (% 29,9) | 39 (% 23,2) | 0§ |
| Obesity | 104 (% 51) | 34 (% 20,2) | |
| CAD disease | |||
| Non-obs CAD | 50 (% 24,5) | 39 (% 23,2) | 0,002§ |
| Obs CAD | 57 (% 27,9) | 23 (% 13,7) | |
P value was presented as a result of Student t-test.
P value was presented as a result of Pearson Chi-square test. SD: Standart deviation; HU: Haunsfiled Unit; a/d: Antidiabetic agent; CAD: Coronary artery disease; non-obs: Non-obstructive; obs: Obstructive.
Table 2.
Statistical effect of different characteristics in groups
| Unadjusted OR | %95 CI | p value | Adjusted OR | %95 CI | p value | |
|---|---|---|---|---|---|---|
| Age (± SD) | 1,082 | 1,039-1,127 | 0,000 | 1,065 | 1,029-1,101 | 0,000 |
| Gender | 2,498 | 0,972-6,425 | 0,057 | - | - | - |
| Dyslipidemia | 0,111 | 0,035-0,355 | 0,000 | 0,121 | 0,039-0,377 | 0,000 |
| Smoking | 1,883 | 0,840-4,223 | 0,125 | - | - | - |
OR: Odds Ratio; SD: Standart deviation.
Results
According to hepatic density measurements of the 372 individuals participated in the study, 204 (149 males, 54.8%) had non-alcoholic fatty liver disease (Group 1) whereas 168 (95 males, 45.2%) were normal (Group 2). The present study found that the difference between the prevalence of coronary artery disease found in the group with NAFLD and in the group with normal liver tissue was statistically significant.
All subjects were examined for coronary artery disease including smoking, hypertension, diabetes mellitus, dyslipidemia, and familial coronary artery disease. The age distribution of the participants ranged between 24 and 74 years (y) (mean ± standard deviation 49.6 ± 11.2 y). Of these, 244 (65.6%) were male and 128 (34.4%) were female. The mean attenuation value of the liver parenchyma was measured to be 52 ± 11.9 HU (range, 14-75). While the number of patients with hypertension (HT) was 102 (27.4%), the number of patients with dyslipidemia was 131 (35.2%). Among the participants, 239 (64.2%) had not diabetes mellitus (DM), the number of those receiving an antidiabetic agent was 133 (35.8%); 96 (25.8%) of them were receiving an oral antidiabetic agent and 37 (9.9%) of them were receiving a parenteral antidiabetic agent. There were 152 (40.9%) active smokers and 220 (59.1%) nonsmokers. Based on BMI, 96 (25%) patients were normal-weight, 149 (40.1%) were over-weight and 127 (34.1%) were obese. Fatty liver disease was detected in 168 (45.2%) of study participants. Number of patients with normal liver was 204 (54.8%). (Table 3)
Table 3.
Data about study participants
| All participants ( n = 372) | ||
|---|---|---|
| Age (± SD) | 49.6 ± 11,2 years( range 24-74 years) | |
| Gender (male) | 244 (65,6%) | |
| Hepatic density (HU) | 52 ± 11.9 HU (range, 14-75 HU) | |
| Diabetes | ||
| Nondiabetic | 239 (64.2%) | |
| Oral a/d | 96 (25.8%) | |
| parenteral a/d | 37 (9.9%) | |
| Dyslipidemia | 131 (35,2%) | |
| Hypertension | 102 (27,4%) | |
| Smoking ( active smokers) | 152 (40,9%) | |
| Alcohol consumption ≥ 20 g/day (± SD) | - | |
| Body weight | ||
| Excessive weight | 149 (40.1%) | |
| Obesity | 127 (34.1%) | |
¥: P value was presented as a result of Student t-test. §: P value was presented as a result of Pearson Chi-square test. SD: Standart deviation; HU: Haunsfiled Unit; a/d: Antidiabetic agent.
The mean liver density was 43 ± 9.1 HU (range 14-56) in males, and 45.5 ± 8.4 HU (range 31-58) in females of Group 1. The corresponding figures were 61.8 ± 4.7 HU (range, 56-75), and 62.6 ± 5.7 HU (range, 54-74) in the males and females of Group 2, respectively. Mean liver densities of the groups according to their ages are demonstrated in Figure 5.
Figure 5.
Mean hepatic density according to ages
Evaluation of the coronary arteries of the study participants revealed that 203 of them (107 males, 52.7%) had normal coronary arteries, 89 (69 males, 77.5%) had non-obsCAD, and 80 (68 males, 21.5%) had obsCAD.
The number of males without coronary artery disease was 62 (41.6%), those with non-obsCAD was 42 (28.2%), and those with obsCAD was 45 (30.2%) in Group 1. The number of females without coronary artery disease was 35 (63.6%), with non-obsCAD was 8 (14.5%), and with obsCAD was 12 (21.8%).
In Group 2, there were 45 (47.4%) males without coronary artery disease, 27 (28.4%) males with non-obsCAD, and 23 (24.2%) males with obsCAD. The number of females without coronary artery disease was 61 (83.6%), with non-obsCAD was 12 (16.4%), and with obsCAD was 0 in Group 2.
Individuals in Group 1 were older and dyslipidemic more than Group 2. In additionaly, Group 1 has more males and smokers than the Group 2. These characteristics of persons affected on obsCAD were evaluated with Binary Logistic Regresion Analysis in Table 3. Age and dyslipidemia affected on obsCAD were considered statistical significant (p < 0.01).
Discussion
MSCT coronary angiography is an important method for detecting CAD in the early stage. A study which compared MSCT angiography and invasive coronary angiography for the evaluation of coronary arteries and coronary artery segments larger than 1.5 mm found the sensitivity of MSCT angiography to be 94% and specificity to be 97%14. Besides, CT is also used for the diagnosis of hepatic steatosis. Sensitivity and specificity of CT for the diagnosis of hepatic steatosis is 82% and 100%, respectively9. The present study used the hepatic CT images used in the MSCT angiography scanning protocol.
Based on MSCT, the present study found that coronary artery disease prevalence in patients with NAFLD was significantly higher than that of those with normal liver tissue (p < 0.01). Statistical comparison between the two groups is presented in Table 2.
Studies from other countries reported that NAFLD was more common among females15,16. However, a study from Turkey found the frequency of non-alcoholic hepatic steatosis to be lower in females (32.7%)17. Some prevalence studies verified the diagnosis of NAFLD in 76% of 146 liver biopsy samples obtained from obese patients that underwent bariatric surgery; a smaller-scale study in Turkey, however, reported the prevalence of NAFLD to be 72% among obese patients18,19.
A gradually increasing number of studies indicate NAFLD as the hepatic manifestation of metabolic syndrome20,21. Although metabolic syndrome is a well-known precursor of CAD22-24, the association between NAFLD and CAD remains unclear.
There are studies demonstrating that proinflammatory cytokines including tumor necrosis factor alpha (TNF-α), C reactive protein (CRP) and plasminogen activator inhibitor I (PAI-I) have been increased in patients with both NAFLD and CAD25. It has been emphasized that the increase in proinflammatory markers enhances future CAD events25. It has been also highlighted that this might independ from metabolic syndrome and related risk factors. Some studies conducted in insulin users demonstrated that insulin resistance is a predictor for CAD events and plays an important role in the development of unfavorable clinical outcomes for NAFLD patients26,27.
Association between NAFLD, from simple steatosis to advanced form of NAFLD, and high risk of CAD has been attributed to increased oxidative stress and subclinical inflammation26,28,29.
A study conducted by Perseghin stated that NAFLD was characterized by the appearance of early metabolic and vascular pathological changes of atherosclerosis. However, despite all these findings, it has been emphasized that the evidences indicating the association between NAFLD and CAD are weak30.
Although the close association between NAFLD and CAD has not been clarified yet, fat deposition in NAFLD is considered to increase free fatty acids that lead to CAD by causing low-grade inflammation31. The presence of NAFLD in patients with type 2 diabetes has suggested NAFLD as a strong predictor for CAD32.
Brea et al33 found an association between NAFLD and carotid atherosclerosis. Targher et al34 suggested a relation between NAFLD and carotid artery wall thickness in type 2 diabetes mellitus patients controlled with diet. Lin et al35 stated that NAFLD was an independent risk factor for ischemic CAD.
Study Limitations
While measuring the liver density of some cases, the optimal selection of appropriate hepatic regions not including vascular and biliary structures has not been possible due to inadequate spatial resolution. During MSCT coronary angiography, we had occasional difficulties in detecting the stenosis degree in massive calcified plaques. Moreover, there have been difficulties in differentiating probable coronary artery soft plaques from the respiratory artifacts on the images of the cases with respiratory distress.
Conclusion
Based on MSCT, the present study found that the difference between the prevalence of coronary artery disease found in the group with NAFLD and in the group with normal liver tissue was statistically significant.
We can say that the likelihood of CAD in individuals with hepatosteatosis not consuming or consuming less than 20 g/day of alcohol is higher than in the individuals without hepatosteatosis.
We think that this hypothesis should be verified with larger studies.
Acknowledgments
We thank Assoc. Prof. Ismail Keskin*, PhD for his contributions to the evaluation of results and statistical analysis.
*Selçuk University, Zootechnics Division, Department of Biometry and Genetics, Konya, TURKEY.
Footnotes
Author contributions
Conception and design of the research, Acquisition of data, Analysis and interpretation of the data: Efe D. Statistical analysis, Writing of the manuscript, Critical revision of the manuscript for intellectual content: Aygün F.
Study Association
This study is not associated with any post-graduation program.
Sources of Funding
There were no external funding sources for this study.
References
- 1.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 2.Mulhall BP, Ong JP, Younossi ZM. Non-alcoholic fatty liver disease: an overview. J Gastroenterol Hepatol. 2002;17(11):1136–1143. doi: 10.1046/j.1440-1746.2002.02881.x. [DOI] [PubMed] [Google Scholar]
- 3.Clark JM. Weight loss as a treatment for nonalcoholic fatty liver disease. J Clin Gastroenterol. 2006;40(Suppl 1):S39–S43. doi: 10.1097/01.mcg.0000168641.31321.fa. [DOI] [PubMed] [Google Scholar]
- 4.Ruhl CE, Everhart JE. Epidemiology of nonalcoholic fatty liver. Clin Liver Dis. 2004;8(3):501–519. doi: 10.1016/j.cld.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Akahoshi M, Amasaki Y, Soda M, Tominaga T, Ichimaru S, Nakashima E, et al. Correlation between fatty liver and coronary risk factors: a population study of elderly men and women in Nagasaki, Japan. Hypertens Res. 2001;24(4):337–343. doi: 10.1291/hypres.24.337. [DOI] [PubMed] [Google Scholar]
- 6.Akbar DH, Kawther AH. Nonalcoholic fatty liver disease in Saudi type 2 diabetic subjects attending a medical outpatient clinic: prevalence and general characteristics. Diabetes Care. 2003;26(12):3351–3352. doi: 10.2337/diacare.26.12.3351-a. [DOI] [PubMed] [Google Scholar]
- 7.Gupte P, Amarapurkar D, Agal S, Baijal R, Kulshrestha P, Pramanik S, et al. Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol. 2004;19(8):854–858. doi: 10.1111/j.1440-1746.2004.03312.x. [DOI] [PubMed] [Google Scholar]
- 8.Assy N, Kaita K, Mymin D, Levy C, Rosser B, Minuk G. Fatty infiltration of liver in hyperlipidemic patients. Dig Dis Sci. 2000;45(10):1929–1934. doi: 10.1023/a:1005661516165. [DOI] [PubMed] [Google Scholar]
- 9.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124(1):71–79. doi: 10.1053/gast.2003.50004. [DOI] [PubMed] [Google Scholar]
- 10.Piekarski J, Goldberg HI, Royal SA, Axel L, Moss AA. Difference between liverand spleen CT numbers in the normal adult: its usefulness in predicting the presence of diffuse liver disease. Radiology. 1980;137(3):727–729. doi: 10.1148/radiology.137.3.6934563. [DOI] [PubMed] [Google Scholar]
- 11.Motoyama S, Kondo T, Anno H, Suqiura A, Ito Y, Mori K, et al. Atherosclerotic plaque characterization by 0.5-mm-slice multislice computed tomo graphic imaging. Circ J. 2007;71(3):363–366. doi: 10.1253/circj.71.363. [DOI] [PubMed] [Google Scholar]
- 12.Jinzaki M, Sato K, Tanami Y, Yamada M, Kuribayashi S, Anzai T, et al. Novel method of displaying coronary CT angiography: angiographic view. Circ J. 2006;70(12):1661–1662. doi: 10.1253/circj.70.1661. [DOI] [PubMed] [Google Scholar]
- 13.Teramoto T, Sasaki J, Ueshima H, Egusa G, Kinoshita M, Shimamoto K, et al. Risk factors of atherosclerotic disease. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerosis cardiovascular diseases for Japanese. J Atheroscler Thromb. 2007;14(6):267–277. doi: 10.5551/jat.e578. [DOI] [PubMed] [Google Scholar]
- 14.Leschka S, Alkadhi H, Plass A, Desbiolles L, Grünenfelder J, Marincek B, et al. Accuracy of MSCT coronary angiography with 64-slice technology: first experience. Eur Heart J. 2005;26(15):1482–1487. doi: 10.1093/eurheartj/ehi261. [DOI] [PubMed] [Google Scholar]
- 15.Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis. Med Clin North Am. 1996;80(6):1147–1166. doi: 10.1016/s0025-7125(05)70483-1. [DOI] [PubMed] [Google Scholar]
- 16.Lee RG. Nonalcoholic steatohepatitis: a study of 49 patients. Hum Pathol. 1989;20(6):594–598. doi: 10.1016/0046-8177(89)90249-9. [DOI] [PubMed] [Google Scholar]
- 17.Sonsuz A, Özdemir MB, Basaranoglu M, Sentürk A, Akin P. Metabolic disorders associated with nonalcoholic steatohepatitis: an analysis of risk factors. European Association for the Study of the Liver and Turkish Association for the Study of the Liver; Istanbul: 1998. 1998. pp. 49–49. Easl Postgraduate Course-First Hepatology Day, 1998 June 26-27. Proceedings. Syllabus and abstract book. [Google Scholar]
- 18.de Oliveira CP, de Mello ES, Alves VA, Saviero SM, Strauss E. Changes in histological criteria lead to different prevalences of nonalcoholic steatohepatitis in severe obesity. Ann Hepatol. 2007;6(4):255–261. [PubMed] [Google Scholar]
- 19.Bahçecioglu IH, Sentürk H, Mert A, Basaran G, Akçakaya M, Keles I, et al. Karaciger Yaglanmasi: 100 olgu. Turk J Gastroenterol. 1996;7(2):205–210. [Google Scholar]
- 20.Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28(7):1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 21.Targher G. Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: the plot thickens. Diabet Med. 2007;24(1):1–6. doi: 10.1111/j.1464-5491.2007.02025.x. [DOI] [PubMed] [Google Scholar]
- 22.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143(10):722–778. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 23.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 24.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 25.Targher G, Chonchol M, Miele L, Zoppini G, Pichiri I, Muggeo M. Nonalcoholic fatty liver disease as a contributor to hypercoagulation and thrombophilia in the metabolic syndrome. Semin Thromb Hemost. 2009;35(3):277–287. doi: 10.1055/s-0029-1222606. [DOI] [PubMed] [Google Scholar]
- 26.Diamant M, Nieuwland R, Pablo RF, Sturk A, Smit JW, Radder JK. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation. 2002;106(19):2442–2447. doi: 10.1161/01.cir.0000036596.59665.c6. [DOI] [PubMed] [Google Scholar]
- 27.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franchini M, Targher G, Montagnana M, Lippi G. The metabolic syndrome and the risk of arterial and venous thrombosis. Thromb Res. 2008;122(6):727–735. doi: 10.1016/j.thromres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Morange PE, Renucci JF, Charles MA, Aillaud MF, Giraud F, Grimaux M, et al. Plasma levels of free and total TFPI, relationship with cardiovascular risk actors and endothelial cell markers. Thromb Haemost. 2001;85(6):999–1003. [PubMed] [Google Scholar]
- 30.Perseghin G. The role of non-alcoholic fatty liver disease in cardiovascular disease. Dig Dis. 2010;28(1):210–213. doi: 10.1159/000282088. [DOI] [PubMed] [Google Scholar]
- 31.Targher G, Bertolini L, Scala L, Zoppini G, Zenari L, Falezza G. Non-alcoholic hepatic steatosis and its relation to increased plasma biomarkers of inflammation and endothelial dysfunction in non-diabetic men. Role of visceral adipose tissue. Diabet Med. 2005;22(10):1354–1358. doi: 10.1111/j.1464-5491.2005.01646.x. [DOI] [PubMed] [Google Scholar]
- 32.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54(12):3541–3546. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 33.Brea A, Mosquera D, Martin E, Arizti A, Cordero JL, Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case-control study. Arterioscler Thromb Vasc Biol. 2005;25(5):1045–1050. doi: 10.1161/01.ATV.0000160613.57985.18. [DOI] [PubMed] [Google Scholar]
- 34.Targher G, Bertolini L, Padovani R, Poli F, Scala L, Zenari L, et al. Non-alcoholic fatty liver disease is associated with carotid artery wall thickness in diet-controlled type 2 diabetic patients. J Endocrinol Invest. 2006;29(1):55–60. doi: 10.1007/BF03349177. [DOI] [PubMed] [Google Scholar]
- 35.Lin YC, Lo HM, Chen JD. Sonographic fatty liver, overweight and ischemic heart disease. World J Gastroenterol. 2005;11(31):4838–4842. doi: 10.3748/wjg.v11.i31.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]