Abstract
Background
The impact of blood pressure (BP) during adolescence on other cardiovascular risk factors in young adults is important for the primary prevention.
Objective
To evaluate BP, anthropometric indexes, metabolic and inflammatory profiles in young individuals stratified by their BP behavior recorded for 18 years.
Methods
A total of 116 individuals, of whom 63 were males, from the Rio de Janeiro study (follow-up of 17.76 ± 1.63 years), were assessed at two moments: A1 (12.40 ± 1.49 years) and A2 (30.09 ± 2.01 years). The 116 individuals were divided into two groups: GN (n = 71), of participants with normal BP at A1; and GH (n = 45), of those with abnormal BP at A1. BP, weight, height and body mass index (BMI) were measured at A1 and A2. At A2, abdominal circumference (AC) and laboratory, metabolic and inflammatory variables were included.
Results
1) No difference was observed between the groups as regards age and gender; 2) At A2, GH showed higher mean weight, BMI, BP, insulin, HOMA-IR (p < 0.001), leptin (p < 0.02), apolipoprotein B100 and A1 (p < 0.02), apolipoprotein B100 / apolipoprotein A1 ratio (p < 0.010); and higher prevalences of overweight/obesity (p < 0.001), of increased AC (p < 0.001) and of hypertension (p < 0.02); 3) No difference was observed between the groups as regards the inflammatory variables; 4) There was a positive correlation of BP at A1 with BP, BMI, insulin, leptin and HOMA-IR at A2 (p < 0.05).
Conclusion
BP in adolescence was associated with higher values of BP, and anthropometric and metabolic variables in young adulthood, but not with inflammatory variables.
Keywords: Blood Pressure, Young Adult, Atherosclerosis / physiopathology, Inflammation, Adipokines
Introduction
Despite all the knowledge accumulated on the pathophysiology of atherosclerosis and the therapeutic advances on cardiovascular diseases (CVD), mortality and morbidity rates related to these diseases remain high1. Early detection of individuals at a high risk and the implementation of prevention strategies are crucial to reduce them, and all efforts should be made to prevent the first cardiovascular event, which can be fatal and disabling, in addition to represent a high medical and social burden.
Understanding the behavior of the cardiovascular risk factors (RF) throughout the years is of the utmost importance, since they facilitate the development of changes in the cardiovascular system2. Several studies, such as that of the Bogalusa's group2 and PDAY3, have been of great value for the understanding of the risk factor, atherosclerosis, morbidity and mortality associated with these conditions in children, adolescents and young adults, demonstrating that atherosclerosis starts in childhood, that the progression of clinically significant lesions occurs from childhood until adulthood, and that the atherosclerotic lesions found correlate positively with the classic risk factors. Thus, the knowledge on blood pressure (BP) and other cardiovascular RF in young Brazilian populations is very important for the adoption of primary prevention measures.
On the other hand, great advance was achieved on the knowledge of the atherosclerotic disease with the understanding that atherosclerosis is an inflammatory disease of the vessel wall. To date, several molecular markers involved in the inflammatory process are being investigated as regards their role in the atherosclerotic process or their association with the pathogenesis of CVD. Among them, we can point out: oxidized LDL (ox-LDL); proinflammatory cytokines such as interleukin 1 (IL-1); tumor necrosis factor alpha (TNF-alpha); intercellular adhesion molecules (ICAM-1); hepatic stimulation proteins such as high-sensitivity C reactive protein (hs-CRP) and serum amyloid A protein (SAAP); and leukocyte count4.
Following the same line, studies on insulin, leptin and adiponectin have permitted a greater understanding of the impact of metabolic variables on the cardiovascular system and of the mechanisms involved in insulin resistance5-7.
In this context, the objective of the present study was to evaluate BP, anthropometric indexes and metabolic and inflammatory profiles of young individuals stratified by their BP behavior recorded for 18 years in their adolescence.
Methods
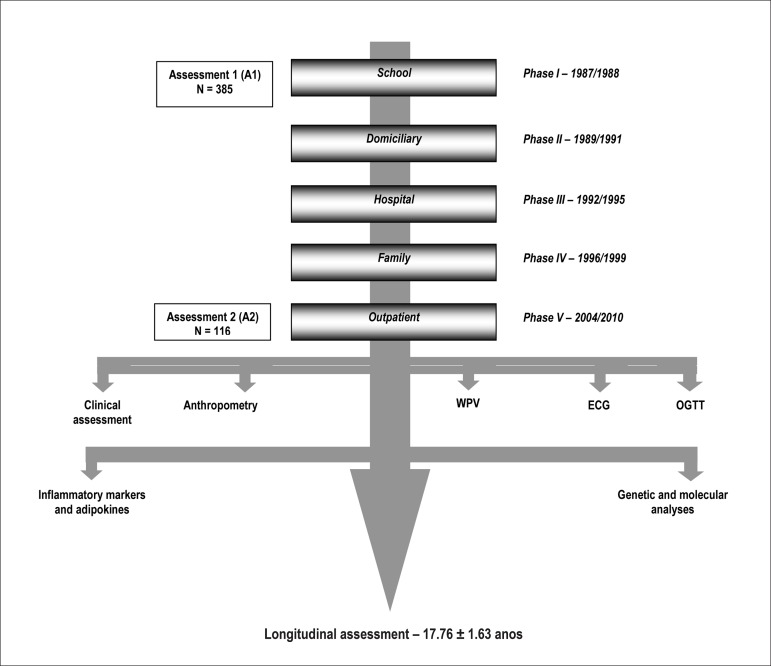
This study population is from the Rio de Janeiro Study (RJS)8-14, and is part of a line of research on BP and other cardiovascular RF in young individuals and their families, that has been developed in the University of the State of Rio de Janeiro since 1983. It was divided into two stages: in the first stage, children between 6 and 9 years of age, n = 3109 (1983-1986) were evaluated; in the second stage, adolescents between 10 and 15 years of age, n = 3906 (1987 - 1989), called target-students, were evaluated, and normality curves of systolic (SBP) and diastolic blood pressure (DBP) were obtained for each gender and age. In the second stage of the study, and equal number of target-students with BP ≥ 95th percentile (group with abnormal BP) and BP ≤ 50th percentile (group with normal BP) were randomly selected, and several cross-sectional assessments were made at different moments throughout the 18-year follow-up, called Domiciliary, Hospital, Family and Outpatient Phases (Figure 1).
Figure 1.
The Rio de Janeiro study design9-15. Second Stage. WPV: pulse wave velocity; ECG: electrocardiogram; OGTT: oral glucose tolerance test.
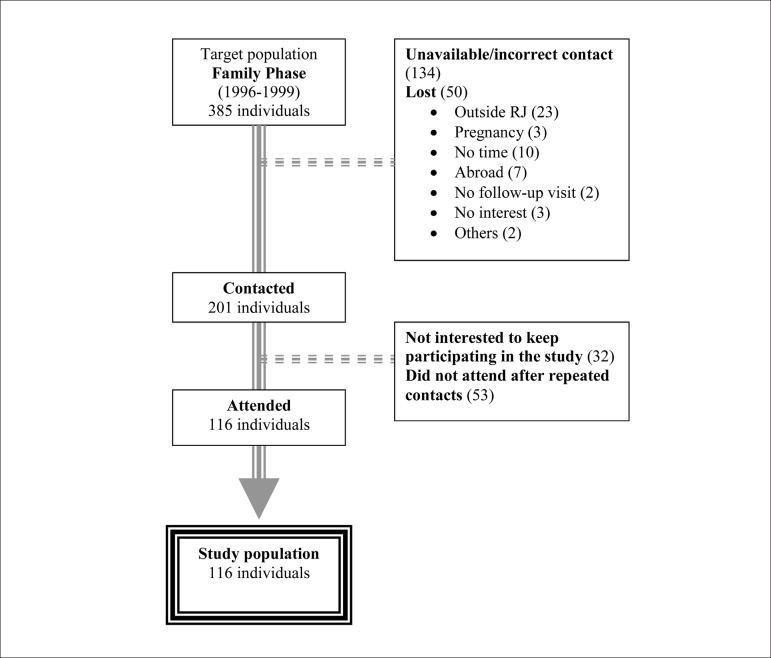
For the present study, inserted in the Outpatient Phase of the RJS, 385 individuals assessed in the Family Phase (1996-1999) as target-population were assessed. Figure 2 shows the final composition of the study sample and the reasons for the losses to follow-up of part of the population.
Figure 2.
Composition of the population sample of the present study
Data from 116 target-students (63 males and 53 females) were assessed at two moments with a mean follow-up of 17.76 ± 1.63 years, namely at: A1 (School Phase): at 12.40 ± 1.49 years, and A2 (Outpatient Phase): at 30.09 ± 2.01 years. Two groups were formed according to their BP behavior at A1: group of normal individuals (GN) with normal BP at A1 (n = 71), and group of hypertensive individuals (GH), of those with abnormal BP at A1 (n = 45).
In both assessments, a questionnaire was administered for the analysis of clinical, epidemiological and socio-cultural variables. BP, weight (W), and height (H) were measured, and the body mass index (BMI) was calculated.
At A2, the abdominal circumference (AC) was measured, and, after a 12-hour fasting, the following parameters were determined: blood glucose; total cholesterol (T Col); high-density lipoprotein (HDL-c); triglycerides (TGL), with calculation of low-density lipoprotein (LDL-c) when TGL < 400mg/dL; apolipoprotein B100 (Apo B100), and A1 (Apo A1); lipoprotein a [LP (a)]; insulin (ins); leptin; adiponectin; high-sensitivity C reactive protein (hs-CRP); E-selectin; vascular cell adhesion molecule 1 (VCAM-1); intercellular adhesion molecule receptor 1 (ICAM-1); and fibrinogen. The insulin resistance index was determined using the homeostasis model assessment for insulin resistance (HOMA-IR), considering insulin and fasting blood glucose.
High blood pressure (HBP) at A1 was defined when BP was ≥ 95th percentile for gender and age, and at A2, when BP ≥ 140 and/or 90 mmHg1,15. The presence of overweight/obesity (OW/O) was defined when BMI was ≥ 85th percentile for gender and age at A1; at A2, normal weight was defined when BMI values were < 25 kg/m2; overweight when BMI values were between 25 kg/m2and 29.99 kg/m2, and obesity when BMC values were ≥ 30 kg/m1,15. Increased values for abdominal circumference (AC) were defined as those > 102 cm for men and > 88 cm for women1,15. For metabolic variables, blood glucose levels < 100 mg/dL were considered normal; fasting blood glucose values between 100 mg/dL and 126 mg/dL were considered abnormal; and blood glucose ≥ 126 mg/dL were considered diabetes mellitus16. Normal values for lipid profile followed the recommendations of the IV Brazilian Guideline for Dyslipidemias and Atherosclerosis Prevention17. The presence of metabolic syndrome (MS) was defined according to the I Brazilian Guideline of MS18, adopting the cut-off point for fasting blood glucose ≥ 100 mg/dL, as proposed by Grundy et al19.
Quantitative analyses of insulin, leptin, E-selectin, VCAM-1, ICAM-1, and fibrinogen were made using the Luminex method, in a BioSystems A25 automated analyzer, with the Milliplex kit. Reference values (least detectable concentration) for each marker are: insulin 44.5 pg/mL; leptin 157.2 pg/mL; E-selectin 0.079 ng/mL; VCAM-1 0.016 ng/mL; ICAM-1 0.009 ng/mL, and fibrinogen 0.001 ng/mL.
Serum adiponectin was determined using the ELISA method. Its reference value (least detectable concentration) is 0.78 ng/mL. HOMA-IR was calculated using the equation proposed by Mathews in 1995: HOMA-IR = Fasting insulin (µU/mL) X fasting glucose (mmol/L) / 22.520.
Apo B100 and Apo A1 determinations were made using the turbidimetric and immunological method, and the reference values for Apo A1 were: for men: 110 - 170 mg/dL; for women: 120 - 190 mg/dL. For Apo B100: for men: 80 - 155 mg/dL; for women: 75 - 150 mg/dL. LP (a) was determined using the immunoturbidimetric method with labeled particles, considering the reference value < 30 mg/dL. These determinations were made in a BioSystems A25 automated analyzer, using the BioSys kit.
Determination of hs-CRP was made using the turbidimetric / high-sensitivity latex method, in a BioSystems A25 automated analyzer, with the BioSys kit. The reference values were those recommended in the literature (hs-CRP > 3mg/L)17.
This study was approved by the HUPE Research Ethics Committee (2130 CEP-HUPE). All participants gave written informed consent.
The information collected was stored in a single data basis using the Microsoft Access software program. Data were analyzed using the SPSS for Windows statistical program, version 8.0.0, Copyright SPSS Inc. 1989-1997.
The following statistical tests were used:
• Student's t test (t): for comparisons of two data series.
• Chi-square test (χ2): for comparison of frequency distributions of categorical variables of independent samples.
• Pearson's bivariate correlation (r): to analyze the correlation between continuous variables.
In all analyses, the significance level was set at 5%, admitting a probability "p" ≤ 0.05 to reject the null hypothesis.
Results
The overall characteristics of the 116 target-students analyzed and of the two groups formed based on their BP behavior obtained approximately 18 years earlier (GN = Normal; GH = Hypertensive) are shown in Table 1.
Table 1.
Overall characteristics, mean blood pressure and anthropometric variables of the total population and of the groups of normal (GN) and hypertensive (GH) individuals
| Parameter | Total (116) | GN (71) | GH (45) | p value |
|---|---|---|---|---|
| Age at A2 (years)* | 30.09 ± 2.01 | 30.17 ± 2.10 | 29.98 ± 1.87 | NS |
| Gender (M/F %)* | 54.31 / 45.69 | 52.1 / 47.9 | 57.8 / 42.2 | NS |
| Follow-up period (months)* | 213.12 ± 19.2 | 213.96 ± 19.08 | 211.68 ± 21.63 | NS |
| SBP A1 | 113.43 ± 14.47 | 105.80 ± 9.35 | 125.47 ± 12.89 | < 0.001 |
| SBP A2 | 121.84 ± 16.70 | 115.94 ± 14.38 | 130.89 ± 16.07 | < 0.001 |
| DBP A1 | 62.43 ± 12.46 | 55.94 ± 7.86 | 72.67 ± 11.50 | < 0.001 |
| DBP A2 | 80.51 ± 13.50 | 76.58 ± 11.55 | 86.53 ± 14.16 | < 0.001 |
| Weight A1 | 49.72 ± 11.88 | 45.99 ± 9.42 | 55.62 ± 13.02 | < 0.001 |
| Weight A2 | 75.38 ± 18.69 | 69.88 ± 15.29 | 84.05 ± 20.33 | < 0.001 |
| BMI A1 | 20.12 ± 2.27 | 19.03 ± 2.44 | 21.84 ± 3.68 | < 0.001 |
| BMI A2 | 26.04 ± 5.44 | 24.49 ± 4.47 | 28.51 ± 5.97 | < 0.001 |
| AC A2 | 90.34 ± 12.48 | 87.08 ± 10.84 | 95.61 ± 15.62 | < 0.001 |
p = not significant; GN: group normal; GH: group hypertensive; t: Student’s t test; BP: blood pressure; M: male; F: female; SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; AC: abdominal circumference.
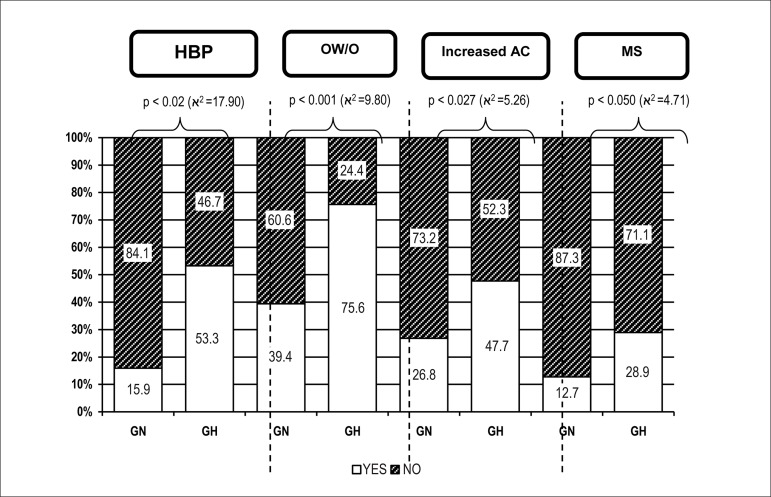
Table 1 shows the mean systolic (SBP) and diastolic blood pressures (DBP) at the two assessment moments A1 and A2 in the total population and in the groups normal and hypertensive. Comparing the BP behavior between GN and GH, we can observe that the mean SBP and DBP were higher in GH than in GN, with statistically significant differences at A1 and A2. GH showed a higher prevalence of HBP at A2 (p < 0.02) (Figure 3). The prevalence of HBP was higher in GH (Figure 3).
Figure 3.
Prevalence of hypertension, overweight/obesity, increased abdominal circumference, and metabolic syndrome at A2 in the groups of normal (GN) and hypertensive (GH) individuals. HBP: high blood pressure; OW/O: overweight/obesity; increased AC: increased abdominal circumference; MS: metabolic syndrome.
Anthropometric variables are shown in Table 1.
Higher mean weight and BMI were observed in GH, with significant differences at A1 and A2. The same behavior was observed for mean AC obtained at A2. The prevalences of OW/O and increased AC were higher in GH (Figure 3).
GH showed higher mean insulin, HOMA-IR (p < 0.001), leptin (p < 0.02), Apo B100 (p < 0.02) and Apo B100/Apo A1 ratio (p < 0.01) than GN at A2. No difference between the two groups was observed for the other lipid variables, adhesion molecules, and inflammatory variables (Tables 2 and 3).
Table 2.
Metabolic variables at A2 in the total population and in the groups normal (GN) and hypertensive (GH)
| Parameter | Total (n = 116) | GN (n = 71) | GH (n = 45) | p value |
|---|---|---|---|---|
| Blood glucose | 82.63 ± 12.53 | 81.28 ± 12.05 | 84.72 ± 13.11 | NS |
| Insulin (μUI/mL) | 12.64 ± 10.72 | 9.82 ± 7.48 | 17.09 ± 13.36 | < 0.001 |
| HOMA-IR | 2.73 ± 2.71 | 2.07 ± 1.83 | 3.77 ± 3.47 | < 0.001 |
| Apo B100 (ng/dl) | 72.79 ± 18.57 | 37.59 ± 16.24 | 49.03 ± 33.38 | < 0.010 |
| Apo B100/ Apo A1 | 42.03 ± 24.87 | 37.59 ± 16.24 | 49.04 ± 33.38 | < 0.004 |
| LP (A) (mg/dl) | 31.72 ± 41.24 | 30.01 ± 38.14 | 34.40 ± 46.04 | NS |
| Col T | 185.85 ± 37.09 | 183.38 ± 38.86 | 189.66 ± 34.27 | NS |
| LDL-c | 115.24 ± 31.46 | 112.34 ± 34.21 | 119.18 ± 27.20 | NS |
| HDL-c | 48.87 ± 15.53 | 48.00 ± 16.05 | 50.14 ± 14.85 | NS |
| TG | 107.88 ± 59.82 | 108.70 ± 63.25 | 106.67 ± 55.12 | NS |
| Leptin (pg/mL) | 16.69 ± 15.82 | 13.816.99 ± 13.478.87 | 21.245.98 ± 18.186.20 | < 0.02 |
| Adiponectin (ng/mL) | 8.638.00 ± 4.142.30 | 9.136.93 ± 4.322.83 | 7.849.93 ± 3.752.28 | NS |
GN: group normal; GH: group hypertensive; Apo B100: apolipoprotein B100; Apo A1: apolipoprotein A1; LP (a): lipoprotein a; Col-T: total cholesterol; HDL-c: highdensity lipoprotein; LDL-c: low-density lipoprotein; TG: triglycerides.
Table 3.
Inflammatory variables and adhesion molecules at A2 in the total population and in the groups of normal (GN) and hypertensive (GH) individuals
| Parameter | Total (n = 116) | GN (n = 71) | GH (n = 45) | p value |
|---|---|---|---|---|
| E-selectin | 42,31 ± 24,62 | 40,13 ± 23,64 | 45,77 ± 25,99 | NS |
| VCAM-1 | 1030,54 ± 316,81 | 1021,50 ± 323,38 | 1044,91 ± 309,21 | NS |
| ICAM-1 | 177,42 ± 101,08 | 177,30 ± 95,21 | 177,61 ± 110,90 | NS |
| hs-CRP | 0,2407 ± 0,33 | 0,2331 ± 0,33 | 0,2527 ± 0,33 | NS |
| Fibrinogen | 298,11 ± 69,41 | 296,24 ± 63,66 | 300,82 ± 77,78 | NS |
GN: group normal; GH: group hypertensive; VCAM-1: vascular cell adhesion molecule 1; ICAM-1: intercellular adhesion molecule receptor 1; hs-CRP: high-sensitivity C-reactive protein.
The aggregation of RFs demonstrated by the presence of MS at A2 was more prevalent in GH, albeit without statistical significance (28.9% vs. 12.7%; p = 0.05). Bivariate correlations of SBP and DBP with the risk variables at A2 are shown in Table 4. Positive and significant correlations were observed between SBP and DBP at A1 and the following variables at A2: SBP, DBP, BMI, leptin, insulin, and HOMA-IR.
Table 4.
Correlations of SBP and DBP at A1 with risk variables at A2
| Variable | Correlations with SBP | Correlations with DBP | ||
|---|---|---|---|---|
| r | p | r | p | |
| SBP at A2 | 0.511 | < 0.001 | 0.389 | < 0.001 |
| DBP at A2 | 0.416 | < 0.001 | 0.477 | < 0.001 |
| BMI at A2 | 0.339 | < 0.001 | 0.285 | 0.002 |
| Blood glucose | 0.079 | 0.410 | 0.076 | 0.430 |
| Cholesterol | 0.007 | 0.942 | 0.087 | 0.363 |
| HDL-c | -0.049 | 0.620 | 0.058 | 0.558 |
| LDL-c | -0.037 | 0.719 | 0.091 | 0.372 |
| TG | 0.038 | 0.695 | 0.032 | 0.747 |
| Apo A1 | -0.093 | 0.321 | -0.098 | 0.294 |
| Apo B100 | 0.146 | 0.117 | 0.183 | 0.049 |
| LPa | 0.004 | 0.967 | 0.028 | 0.763 |
| Leptin | 0.237 | 0.010 | 0.187 | 0.044 |
| Insulin | 0.233 | 0.012 | 0.229 | 0.014 |
| Homa IR | 0.204 | 0.032 | 0.192 | 0.045 |
| Adiponectin | -0.129 | 0.168 | -0.052 | 0.581 |
| E-selectin | 0.073 | 0.439 | 0.155 | 0.099 |
| VCAM-1 | 0.037 | 0.699 | 0.006 | 0.946 |
| ICAM-1 | 0.027 | 0.778 | -0.022 | 0.818 |
| hs-CRP | -0.005 | 0.956 | 0.018 | 0.848 |
| Fibrinogen | 0.080 | 0.448 | 0.032 | 0.763 |
SBP: systolic blood pressure; DBP: diastolic blood pressure; BMI: body mass index; HDL-c: high-density lipoprotein; LDL-c: low-density lipoprotein; TG: triglyceride; APO A1: apolipoprotein A1; APO B100: apolipoprotein B100; LPa: lipoprotein A; HOMA-IR: homeostasis model assessment for insulin resistance; VCAM-1: vascular cell adhesion molecule 1; ICAM-1: intercellular adhesion molecule receptor 1; hs-CRP: high-sensitivity C-reactive protein.
Discussion
There are increasingly more evidences that BP in childhood is an important RF for further development of HBP and CAD21. BP values observed in childhood tend to remain in the same percentile range throughout time, a phenomenon known as tracking, with BP repetition coefficients throughout childhood between 37% and 48%21.
In the present study, both the mean BP at A2 and the prevalence of HBP were higher in GH in comparison to GN. In addition, a positive and significant correlation of BP at A1 with BP at A2 was observed, thus suggesting that children and adolescents show a sustained BP behavior pattern throughout time.
We should point out that the original design of the RJS was characterized by the follow-up of two groups of young individuals with distinct BP behavior in the school phase: with BP ≥ 95th percentile and with BP ≤ 50th percentile. Thus, in subsequent study phases, prevalence rates of HBP higher than those observed in the general population in this young age range are expected.
Body composition is one of the main determinants of BP in children22. In the Muscatine study23, the authors demonstrated that more than half of the hypertensive children were also obese. In our country, several publications, especially those of the RJS cohort, had already suggested the importance of body composition in the determinism of BP in children and adolescents8-14,24,25.
In the present study, we observed higher mean weight and BMI in GH at the two assessment moments, higher prevalence of OW/O at A2, and positive and significant correlations of SBP and DBP at A1 with BMI at A2, thus also demonstrating an association between body composition and BP in this population.
An important aspect is to know the pattern of body fat distribution and its relation to RF26,27. Two classic international studies, the Bogalusa Heart Study26 and the CARDIA study27, reinforced the knowledge that central obesity as verified by an abnormal AC represents an important marker of coronary atherosclerosis in young populations. In Brazil, studies analyzing the relationship between AC and other RF, such as HBP14,22, have shown conflicting results, thus suggesting the need to improve the knowledge on the AC cut-off points in our population, as well as its relations to other RF and the determinism of further CVD. In the present study, a higher prevalence of increased AC was observed at A2 in hypertensive individuals (GH) in adolescence.
Adipocytes have the ability to secrete substances with important biological effects. Larger amounts of leptin are produced with the increase in fat tissue. Conversely, the production of adiponectin is reduced with the increase in fat mass, and its action it to increase insulin sensitivity28.
Among the adipokines, adiponectin and leptin are the two most important insulin-sensitizer hormones produced by the adipocytes. The role of each of them in the regulation of insulin sensitivity remains obscure28. Adiponectin is an important adipokine that modulates insulin resistance, with antiinflammatory and antiatherogenic properties. Low adiponectin concentrations are associated with the presence of cardiovascular RF6. At first, leptin was exclusively associated with obesity, but advances in the investigations have shown other possible associations with cardiovascular risk factors, such as inflammatory markers and even HBP6.
Langenberg et al29 suggested that the development of CVD and MS, in the presence of visceral obesity, could be a consequence of adipocytes dysfunction and subsequent change in the levels or expression of leptin and adiponectin receptors.
Corroborating the literature2,8-14,29, the present cohort showed higher prevalence of mean insulin and insulin resistance as assessed by HOMA-IR in early adulthood in GH individuals, thus reflecting a more unfavorable metabolic profile in this population. This group also had higher serum leptin levels in comparison to GN and lower mean adiponectin than GN, although the latter without a statistically significant difference. We point out the positive and significant correlations of SBP and DBP at A1 with insulin, HOMA-IR and leptin at A2, corroborating the findings described.
In Brazilian surveys with young populations, varying prevalences of the different lipid abnormalities were observed depending on the population studied and on the country region30,31. In the present study, the association of BP in childhood and adolescence with lipid variables in adulthood corroborated the literature. GH individuals showed a more unfavorable lipid profile than GN individuals, with higher mean Chol T, LDL-c, and TGL, although without statistically significant differences.
Apolipoproteins are protein molecules associated with lipids in the composition of lipoprotein particles and perform different and fundamental functions in the lipoprotein metabolism. The apo B/ apo A-1 ratio represents the balance between potentially atherogenic cholesterol particles (apo B) and anti-atherogenic cholesterol particles (apo A-1). It has been suggested that this ratio represents a better predictor of atherosclerotic disease than lipid and lipoprotein concentrations17,32. The physiological function of Lp (a) is not fully understood. However, in observational studies, it has been associated with the atherosclerotic plaque formation and progression. As demonstrated in a study in which Lp (a) levels of 504 patients were determined immediately prior to coronary angiography, these levels were strongly associated with the presence and extent of CAD (p < 0.001)33.
An important evidence correlating apolipoproteins with classic cardiovascular RF such as HBP in young individuals is documented in a Bogalusa cohort analysis, in which children of parents who suffered infarction had higher apo B levels, lower apo A-1 levels, and higher apo B / apo A-1 ratio, even though their LDL and HDL cholesterol levels were within normal limits34.
In the present study population, Apo B100 levels were higher in GH, as well as the Apo B100 / Apo A1 ratio, thus suggesting a positive atherogenic balance in early adulthood in the group with higher blood pressure in childhood and adolescence. LP (a) concentrations, in turn, were not significantly different between the groups.
Many coronary events are known to occur in patients in whom the presence of traditional RF is not observed. A great advance in the knowledge of the atherosclerotic disease was achieved with the understanding that atherosclerosis is an inflammatory disease of the vessel wall35.
To date, studies involving cell adhesion molecules (E-selectin, ICAM-1 and VCAM-1) and C-reactive protein (hs-CRP), fibrinogen and endothelial dysfunction have proven important in population analyses, but not in individual terms, whether for the diagnosis, prognosis or treatment of cardiovascular diseases36. There are evidences that increased hs-CPR levels could predict further HBP in normotensive individuals, although it is still early to use it broadly with this objective in the daily clinical practice36. Several prospective cross-sectional studies have related high fibrinogen levels to CAD, even after correction for covariables37,38. As demonstrated in a five-year follow-up of 2125 men, fibrinogen levels in the upper tercile of distribution were associated with a higher risk of CAD (adjusted RR of 2.5, 95% CI; p = 0.001)39.
Thus, currently, there has been growing interest in the study of the association between inflammation, traditional risk factors and atherosclerosis in children and adolescents, with the purpose of trying to identify the cardiovascular disease, at its early stages, as a higher potential for primary prevention40.
In the present cohort, although the concentrations of inflammatory markers and adhesion molecules studied had been higher in GH, these differences were not statistically different.
The major limitation of this study is the same experienced by several cohorts reported in the literature, and is related to the longitudinal character of the follow-up, which leads to progressive losses of part of the original sample.
We should point out that two groups were followed up separately: a group with normal, and a group with abnormal BP. Despite the reduction in the number of follow-up visits, the proportionality in each group was maintained in the several cross-sectional assessments made at different moments throughout 18 years. In addition, in the assessment made at adolescence (School phase of the second stage of the RJS), only data on weight, height, and blood pressure could be collected, given the epidemiological characteristic of this study phase, with the assessment of 3906 adolescents to obtain blood pressure curves by age and gender. Other clinical parameters were obtained only in later phases, when the assessments were made in an outpatient and hospital basis, and thus could not be observed throughout time.
Finally, we should point out that the population sample of the RJS is a non-hospital population, comprising the Brazilian cohort in this age range that has been followed up for the longest period to date.
Conclusion
The findings of the present study demonstrated a strong relationship between BP behavior in adolescence and BP, anthropometric and metabolic variables, but not inflammatory variables, in early adulthood, 18 years later, thus suggesting that primary prevention measures of cardiovascular RF should start early in life.
Footnotes
Author contributions
Conception and design of the research; Acquisition of data; Analysis and interpretation of the data; Statistical analysis; Critical revision of the manuscript for intellectual content: Campana EMG, Brandão AA, Pozzan R, Magalhães MEC, Fonseca FL, Pizzi OL, Freitas EV, Brandão AP. Writing of the manuscript: Campana EMG.
Study Association
This article is part of the thesis of doctoral submitted by Erika Maria Gonçalves Campana, from Universidade Estadual do Rio de Janeiro - UERJ.
Sources of Funding
This study was funded by FAPERJ.
References
- 1.Sociedade Brasileira de Cardiologia. Sociedade Brasileira de Hipertensão. Sociedade Brasileira de Nefrologia VI Diretrizes brasileiras de hipertensão. Arq Bras Cardiol. 2010;95(1) supl.1:1–51. [PubMed] [Google Scholar]
- 2.Berenson GS, Srnivasan SR, Bogalusa Heart Study Group Cardiovascular risk factors in young with implications for aging: the Bogalusa Heart Study. Neurobiol Aging. 2005;26(3):303–307. doi: 10.1016/j.neurobiolaging.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Zieske AW, Malcom GT, Strong JP. Natural history and risk factors of atherosclerosis in children and youth: the PDAY Study. Pediatr Pathol Mol Med. 2002;21(2):213–237. doi: 10.1080/15227950252852104. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Ridkler PM. Novel inflammatory markers of coronary risk: theory versus practice. Circulation. 1999;100(11):1148–1150. doi: 10.1161/01.cir.100.11.1148. [DOI] [PubMed] [Google Scholar]
- 5.Marcovia SM, Albero JJ, Dati F, Ledue TB, Ritchie RF. International Federation of Clinical Chemistry standardization project for measurements of apolipoproteins A-I and B. Pt 1Clin Chem. 1991;37(10):1676–1682. [PubMed] [Google Scholar]
- 6.Gil-Campos M, Canete RR, Gil A. Adiponectin, the missing link in insulin resistance and obesity. Clin Nutr. 2004;23(5):963–974. doi: 10.1016/j.clnu.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Gauthier A, Dubois S, Bertrais S, Gallois Y, Aube C, Gagnadoux F, et al. The leptin to adiponectin ratio is a marker of the number of metabolic syndrome criteria in French adults. J Metabol Syndr. 2011;1(1):2–6. [Google Scholar]
- 8.de Brandão AP. A importância do desenvolvimento físico no comportamento da curva de pressão arterial em crianças de 6 a 9 anos de idade. Arq Bras Cardiol. 1987;48(4):203–209. [PubMed] [Google Scholar]
- 9.Brandão AP, Brandão AA, Araujo EM. The significance of physical development on the blood pressure curve of children between 6 and 9 years of age and its relationship with familial aggregation. J Hypertens Suppl. 1989;7(1):S37–S39. doi: 10.1097/00004872-198902001-00011. [DOI] [PubMed] [Google Scholar]
- 10.Pozzan R, Brandão AA, da Silva SL, Brandão AP. Hyperglycemia, hyperinsulinemia, overweight, and high blood pressure in young adults: the Rio de Janeiro Study. Pt 2Hypertension. 1997;30(3):650–653. doi: 10.1161/01.hyp.30.3.650. [DOI] [PubMed] [Google Scholar]
- 11.Magalhães ME, Pozzan R, Brandão AA, Cerqueira RC, Roussoulières AL, Czwarcwald C, et al. Early blood pressure level as a mark of familial aggregation of metabolic cardiovascular risk factors - the Rio de Janeiro Study. Pt 2J Hypertens. 1998;16(12):1885–1889. doi: 10.1097/00004872-199816121-00006. [DOI] [PubMed] [Google Scholar]
- 12.Brandão AA, Pozzan R, Magalhães ME, Brandão AP. Aggregation of metabolic abnormalities, overweight and high blood pressure, in young subjects followed-up for a 10-year-period. The Rio de Janeiro Study. [Abstract] J Am Coll Cardiol. 2000;35(Suppl A):264A–264A. [Google Scholar]
- 13.Brandão AA, Pozzan R, Freitas EV, Magalhães ME, Brandão AP. Blood pressure and overweight in adolescents ant their association with insulin resistance and metabolic syndrome. J Hypertens. 2004;22(Suppl 1):111S–111S. [Google Scholar]
- 14.Campana EM, Brandão AA, Pozzan R, França Mde F, Fonseca FL, Pizzi OL, et al. Pressão arterial em jovens como marcador de risco cardiovascular. Estudo do Rio de Janeiro. Arq Bras Cardiol. 2009;93(6):608-15, 657-65. doi: 10.1590/s0066-782x2009001200016. [DOI] [PubMed] [Google Scholar]
- 15.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2) Suppl 4th Report:555–576. [PubMed] [Google Scholar]
- 16.Sociedade Brasileira de Diabetes Atualização brasileira sobre diabetes. [Acesso em 2008 nov 2]. Disponível em http://www.diabetes.org.br/politicas/abdonline.php.
- 17.Sposito AC, Caramelli B, Fonseca FA, Bertolami MC, Afiune A, Neto, Souza AD, et al. Sociedade Brasileira de Cardiologia IV Diretriz brasileira sobre dislipidemias e prevenção da aterosclerose. Arq Bras Cardiol. 2007;88(supl 1):1–18. [Google Scholar]
- 18.Brandão AP, Brandão AA, Nogueira AR, Suplicy H, Guimarães JI, Oliveira JEP, et al. Sociedade Brasileira de Cardiologia I Diretriz brasileira de diagnóstico e tratamento da síndrome metabólica. Arq Bras Cardiol. 2005;84(supl 1):1–28. [PubMed] [Google Scholar]
- 19.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. American Heart Association. National Heart, Lung, and Blood Institute Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. Erratum in: Circulation 2005;112(17):e297. Circulation. 2005;112(17):e298. [DOI] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1995;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.Brandão AA, Magalhães ME, Pozzan R, França MF, Pozzan R, Freitas EV, et al. Hipertensão arterial no jovem como marcador para a prevenção cardiovascular primária. Rev SOCERJ. 2002;15(4):247–255. [Google Scholar]
- 22.Guimarães IC, de Almeida AM, Santos AS, Barbosa DB, Guimarães AC. Pressão arterial: efeito do índice de massa corporal e da circunferência abdominal em adolescentes. Arq Bras Cardiol. 2008;90(6):393–399. doi: 10.1590/s0066-782x2008000600007. [DOI] [PubMed] [Google Scholar]
- 23.Rames LK, Clarke WR, Connor WE, Reiter MA, Lauer RM. Normal blood pressure and the evaluation of sustained blood pressure elevation in childhood: the Muscatine Study. Pediatrics. 1978;61(2):245–251. [PubMed] [Google Scholar]
- 24.Silva MA, Rivera IR, Ferraz MR, Pinheiro AJ, Alves SW, Moura AA, et al. [Prevalence of cardiovascular risk factors in child and adolescent students in the city of Maceió]. Arq Bras Cardiol. 2005;84(5):387–392. doi: 10.1590/s0066-782x2005000500007. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro RQ, Lotufo PA, Lamounier JA, Oliveira RG, Soares JF, Botter DA. [Additional cardiovascular risk factors associated with excess weight in children and adolescents: the Belo Horizonte heart study]. Arq Bras Cardiol. 2006;86(6):408–418. doi: 10.1590/s0066-782x2006000600002. [DOI] [PubMed] [Google Scholar]
- 26.Freedman DS, Kahn HS, Mei Z, Grummer-Strawn LM, Dietz WH, Srinivasan SR, et al. Relation of body mass index and waist-to-height ratio to cardiovascular disease risk factors in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr. 2007;86(1):33–40. doi: 10.1093/ajcn/86.1.33. [DOI] [PubMed] [Google Scholar]
- 27.Lee C, Jacobs DR, Jr, Schreiner PJ, Iribarren C, Hankinson A. Abdominal obesity and coronary artery calcification in young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Nutr. 2007;86(1):48–54. doi: 10.1093/ajcn/86.1.48. [DOI] [PubMed] [Google Scholar]
- 28.Barbalho SM, Mclellan KC, Lerario AC. Metabolic syndrome and its relationship with insulin resistance, endothelial dysfunction and atherogenesis. J Brazilian Soc Food Nutr. 2007;32(1):89–100. [Google Scholar]
- 29.Langenberg C, Bergstrom J, Scheidt-Nave C, Pfeilschifter J, Barrett-Connor E. Cardiovascular death and the metabolic syndrome: role of adiposity-signaling hormones and inflammatory markers. Diabetes Care. 2006;29(6):1363–1369. doi: 10.2337/dc05-2385. [DOI] [PubMed] [Google Scholar]
- 30.Mendes GA, Martinez TL, Izar MC, Amancio OM, Novo NF, Matheus SC, et al. Perfil lipídico e efeitos da orientação nutricional em adolescentes com história familiar de doença arterial coronariana prematura. Arq Bras Cardiol. 2006;86(5):361–365. doi: 10.1590/s0066-782x2006000500006. [DOI] [PubMed] [Google Scholar]
- 31.Scherr C, Magalhães CK, Malheiros W. Análise do perfil lipídico em escolares. Arq Bras Cardiol. 2007;89(2):73–78. doi: 10.1590/s0066-782x2007001400001. [DOI] [PubMed] [Google Scholar]
- 32.Lima LM, Carvalho Md, Sousa MO. Apo B/apo A-I ratio and cardiovascular risk prediction. Arq Bras Cardiol. 2007;88(6):e187–e190. doi: 10.1590/s0066-782x2007000600014. [DOI] [PubMed] [Google Scholar]
- 33.Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353(1):46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 34.Freedman DS, Scrinivasan SR, Shear CL, Franklin FA, Webber LS, Berenson GS. The relation of apolipoproteins A-I and B in children to parental myocardial infarction. N Engl J Med. 1986;315(12):721–726. doi: 10.1056/NEJM198609183151202. [DOI] [PubMed] [Google Scholar]
- 35.Willerson JT, Ridkler PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21) Suppl 1:II2–I10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 36.Melo SE, Yugar-Toledo JC, Coca AP, Moreno H., Junior Arterial hypertension, atherosclerosis and inflammation: the endothelium as target organ. Rev Bras Hipertens. 2007;14(4):234–238. [Google Scholar]
- 37.de la Serna G. Fibrinogen: a new major risk factor for cardiovascular disease: a review of the literature. J Fam Pract. 1994;39(5):468–477. [PubMed] [Google Scholar]
- 38.Izar MC, Fonseca FA, Ihara SS, Kasinski N, Sang WH, Lopes IE, et al. Risk factors, biochemical markers, and genetic polymorphisms in early coronary artery disease. Arq Bras Cardiol. 2003;80(4):379–395. doi: 10.1590/s0066-782x2003000400003. [DOI] [PubMed] [Google Scholar]
- 39.Cantin B, Després JP, Lamarche B, Moorjani S, Lupien PJ, Bogaty P, et al. Association of fibrinogen and lipoprotein(a) as a coronary heart disease risk factor in men (The Quebec Cardiovascular Study) Am J Cardiol. 2002;89(6):662–666. doi: 10.1016/s0002-9149(01)02336-0. [DOI] [PubMed] [Google Scholar]
- 40.Litwin M, Michalkiewicz J, Niemirska A, Gackowska L, Kubiszewska I, Wierzbicka A, et al. Inflammatory activation in children with primary hypertension. Pediatr Nephrol. 2010;25(9):1711–1718. doi: 10.1007/s00467-010-1548-4. Erratum in Pediatr Nephrol. 2010;25(12):2549. [DOI] [PubMed] [Google Scholar]