Abstract
Background
The role of angiotensin-converting enzyme genetic polymorphisms as a predictor of echocardiographic outcomes on heart failure is yet to be established. The local profile should be identified so that the impact of those genotypes on the Brazilian population could be identified. This is the first study on exclusively non-ischemic heart failure over a follow-up longer than 5 years.
Objective
To determine the distribution of angiotensin-converting enzyme genetic polymorphism variants and their relation with echocardiographic outcome of patients with non-ischemic heart failure.
Methods
Secondary analysis of the medical records of 111 patients and identification of the angiotensin-converting enzyme genetic polymorphism variants, classified as DD (Deletion/Deletion), DI (Deletion/Insertion) or II (Insertion/Insertion).
Results
The cohort means were as follows: follow-up, 64.9 months; age, 59.5 years; male sex, 60.4%; white skin color, 51.4%; use of beta-blockers, 98.2%; and use of angiotensin-converting-enzyme inhibitors or angiotensin receptor blocker, 89.2%. The angiotensin-converting enzyme genetic polymorphism distribution was as follows: DD, 51.4%; DI, 44.1%; and II, 4.5%. No difference regarding the clinical characteristics or treatment was observed between the groups. The final left ventricular systolic diameter was the only isolated echocardiographic variable that significantly differed between the angiotensin-converting enzyme genetic polymorphisms: 59.2 ± 1.8 for DD versus 52.3 ± 1.9 for DI versus 59.2 ± 5.2 for II (p = 0.029). Considering the evolutionary behavior, all echocardiographic variables (difference between the left ventricular ejection fraction at the last and first consultation; difference between the left ventricular systolic diameter at the last and first consultation; and difference between the left ventricular diastolic diameter at the last and first consultation) differed between the genotypes (p = 0.024; p = 0.002; and p = 0.021, respectively).
Conclusion
The distribution of the angiotensin-converting enzyme genetic polymorphisms differed from that of other studies with a very small number of II. The DD genotype was independently associated with worse echocardiographic outcome, while the DI genotype, with the best echocardiographic profile (increased left ventricular ejection fraction and decreased left ventricular diameters).
Keywords: Heart failure, Genetic polymorphism, Angiotensin-converting enzyme
Introduction
Heart failure (HF) is the second major cause of hospitalization in Brazil1. In the United States, 32 billion dollars will be spent during 20132 with that syndrome. In addition, those patients' quality of life is severely impaired. Despite the reduction in morbidity and mortality due to new drugs, that gain has not been uniform, and clinical outcome can be unfavorable. One of the mechanisms that can justify such differences is genetics.
The genetic influence, comprising all stages of the syndrome3, has been studied in the following phases: pre-installation4; development5; and clinical phase (disease natural history6 and therapeutic response7). Those results are controversial8 and the studies have been carried out in foreign populations; thus, their impact on the Brazilian population remains unclear.
The major mechanism of that genetic influence is via modulation of the activity of the sympathetic nervous (SNS) and renin-angiotensin-aldosterone (RAAS) systems, which promote cardiac remodeling and sodium and water retention, characteristics of HF. Variations in the activity of those systems would determine different pathophysiological responses, and, thus, varied clinical outcome.
Some genetic markers, the genetic polymorphisms (GP), have been identified and associated with the molecular processes of that neuro-humoral response, such as beta-adrenergic receptors7, angiotensin synthesis9, nitric oxide metabolism10, and angiotensin converting enzyme (ACE)4,11-19. The later, object of this study, is the major agent of the RAAS.
Regarding RAAS, the major GP was the ACE Deletion/Insertion (DI) of 287 base pairs of the intron 16 (GPACE)20. The GPACE, especially the Deletion/Deletion (DD) genotype, was associated with the risk for HF21, mortality22, rejection to heart transplants23, and echocardiographic variations24. However, that relationship has not been observed in some publications11,19,25.
Published studies have controversial results and small sample sizes, and have been carried out in populations different from the Brazilian one, regarding geographical, epidemiological and ethnical aspects. In addition, patients with non-ischemic HF are usually underrepresented in studies on the topic, involving different pathophysiological mechanisms26 and variable therapeutic responses27.
Thus, the present study aimed at determining the frequency of the GPACE variants and their relation with the clinical and echocardiographic outcomes of patients with non-ischemic HF.
Methods
Study design
This is a longitudinal study of a cohort of patients. Medical data were retrospectively and prospectively collected from their medical records, beginning at the patient's arrival at the HF Clinics of a university-affiliated hospital, from December 2009 to January 2012.
Patients
This study consecutively selected 111 patients (67 men and 44 women) diagnosed with systolic non-ischemic HF, on a minimum 12-months follow-up. The major characteristics of the sample were as follows: mean age, 59.5 ± 1.3 years (range: 26 - 89 years); male prevalence (60.4%); and ethnical composition (white, 51.4%; black, 36.0%; others, 12.6%). The mean follow-up time was 64.9 ± 3.9 months.
Inclusion criteria
Patients with symptomatic non-ischemic HF, according to the Framingham criteria, and systolic ventricular dysfunction with ejection fraction ≤ 50% on two-dimensional echocardiography (Simpson's method) were considered eligible to the study.
Exclusion criteria
The presence of significant coronary arterial disease defined as coronary lesion ≥ 75% in two or more epicardial arteries or ≥ 75% in left main coronary artery28 led to exclusion from this study.
Heart failure etiology
The HF etiologies were classified into four groups: idiopathic (36.0%); hypertensive (20.7%); alcoholic (18.9%); and others (24.3%). The diagnosis was established by the physician at the HF Clinics, according to previously described criteria29.
Clinical, laboratory and electrocardiographic parameters
Clinical data were extracted from medical records. Skin color was defined by the attending physician and classified as white, black or others. The functional class was determined according to the New York Heart Association functional classification, at the beginning and end of follow-up.
Laboratory tests were periodically performed at the discretion of the attending physician. The most recently available exams were considered for analysis to express the patient's current clinical status.
All patients underwent electrocardiography (ECG), and were assessed regarding QRS duration, presence of left bundle-branch block (LBBB) and atrial fibrillation (AF).
Echocardiographic variables
The following parameters were assessed: left ventricular systolic diameter (LVSD); left ventricular diastolic diameter (LVDD); and left ventricular ejection fraction (LVEF). Echocardiography was performed by a medical team blinded to the patients' genotypes. Two echocardiographies were performed, one at the beginning and another at the end of follow-up, with a mean interval between exams of 65.5 ± 4.3 months (range: 12 - 232 months), so that the evolution of those parameters could also be observed.
Genotyping
The GPACE variants were analyzed from blood samples collected. After storage under a temperature of 5-15ºC, the samples were processed and the DNA extracted according to the salting out procedure30. After extraction, the polymorphism was genotyped by use of polymerase chain reaction (PCR) and classified as DD, DI or Insertion/Insertion (II).
Statistical Analysis
All data obtained were analyzed by use of the statistical program Statistical Package for the Social Science for Mac (SPSS), version 21. In all tests, the rejection level of the null hypothesis was fixed as 0.05 or 5% (p < 0.05) and the 95% confidence interval (CI) was used. The central trend measurements were expressed as mean ± standard deviation.
The following tests were used: chi-square, Student t test and analysis of variance (ANOVA).
The genotype and haplotype frequencies were tested for Hardy-Weinberg equilibrium31, by using the ARLEQUIN software, 2000 version.
The project was approved by the Committee on Ethics and Research of the Pedro Ernesto university-affiliated hospital (December 16th 2009). All patients provided written informed consent.
The present study was partially financed by the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) after approval of the Inovacor project.
Results
Genetic profile of the population studied
In the population studied, the D allele occurred 163 times (73%), while the I allele, 59 times (27%). Regarding genotypes, 57 (51.4%) were classified as DD, 49 (44.1%) as DI, and only 5 (4.5%) as II. The population studied was in Hardy-Weinberg equilibrium.
Characteristics of the population sample
There was a predominance of the male sex and white individuals, and a high incidence of systemic arterial hypertension (SAH) and smoking. However, the prevalences of diabetes mellitus and dyslipidemia were relatively low. No significant difference in the genotypes was observed for any of the clinical or laboratory characteristics assessed (Table 1).
Table 1.
Clinical characteristics of the population studied according to the genetic polymorphisms of the angiotensin-converting enzyme
| Clinical variable* | Mean | DD (n = 57) | DI (n = 49) | II (n = 5) | Statistical test | p Value |
|---|---|---|---|---|---|---|
| Follow-up (months) | 64.9 ± 3.9 | 65.2 ± 6.1 | 64.7 ± 5.1 | 63.6 ± 13.6 | F = 0.004 | 0.996 |
| HF duration (months) | 97.0 ±6.9 | 89.9 ± 7.6 | 107.6 ± 12.7 | 73.4 ± 15.0 | F = 1.067 | 0.348 |
| Age (years) | 59.5 ±1.3 | 61.1 ± 12.6 | 57.8 ± 14.6 | 57.2 ± 10.7 | F = 0.852 | 0.429 |
| Male gender | 67 (60.4) | 35 (61.4) | 27 (56.3) | 4 (80.0) | X2 = 1.61 | 0.560 |
| Ethnicity | ||||||
| White | 57 (51.4) | 27 (47.4) | 25 (52.1) | 4 (80.0) | X2 = 2.158 | 0.707 |
| Black | 40 (36) | 22 (38.6) | 17 (35.4) | 1 (20.0) | ||
| Others | 14 (12.6) | 8 (14.0) | 6 (12.5) | 0 (0) | ||
| BMI (kg/m2) | 26.1±0.6 | 26.0 ± 0.9 | 26.1 ± 0.8 | 28.0 ± 2.2 | 0.231 | 0.794 |
| Arterial hypertension | 78 (70.3) | 41 (71.9) | 33 (68.8) | 4 (80.0) | X2 = 0.338 | 0.845 |
| Diabetes mellitus | 24 (21.6) | 13 (22.8) | 9 (18.8) | 2 (40.0) | X2 = 1.267 | 0.531 |
| Anemia | 17 (15.3) | 11 (19.3) | 6 (12.5) | 0 | X2 = 1.879 | 0.391 |
| Dyslipidemia | 43 (38.7) | 23 (40.4) | 17 (35.4) | 3 (60.0) | X2 = 1.228 | 0.541 |
| Atrial fibrillation | 22 (19.8) | 12 (21.1) | 8 (16.7) | 2 (40.0) | X2 = 1.751 | 0.781 |
| Current smoker | 7 (6.3) | 8 (14.3) | 3 (6.3) | 2 (40.0) | X2 = 7.350 | 0.775 |
| Former smoker | 45 (40.5) | 24 (42.1) | 19 (39.6) | 1 (20.0) | ||
| Former smoker | 21 (19.1) | 8 (14.3) | 10 (20.8) | 3 (60.0) | X2 = 7.350 | 0.118 |
| Former alcoholic | 42 (38.2) | 20 (35.7) | 20 (41.7) | 1 (20.0) | ||
| initial NYHA** I | 25 (22.5) | 13 (22.8) | 12 (25.0) | 0 (0) | X2 = 5.400 | 0.714 |
| initial NYHA** II | 51 (45.9) | 25 (43.9) | 22 (45.8) | 4 (80.0) | ||
| initial NYHA** III | 23 (20.7) | 14 (24.6) | 8 (16.7) | 1 (20.0) | ||
| initial NYHA** IV | 3 (9.9) | 5 (8.8) | 6 (12.5) | 0 (0) | ||
| mean initial NYHA | 2.18 ± 0.09 | 2.19 ± 0.12 | 2.17 ± 0.14 | 2.20 ± 0.20 | F = 0.012 | 0.988 |
| final NYHA I | 41 (36.9) | 19 (33.3) | 19 (39.6) | 3 (60.0) | X2 = 7.664 | 0.264 |
| final NYHA II | 53 (47.7) | 26 (45.6) | 25 (52.1) | 2 (40.0) | ||
| final NYHA III | 14 (12.6) | 11 (19.3) | 3 (6.1) | 0 (0) | ||
| final NYHA IV | 3 (2.7) | 1 (1.8) | 2 (4.2) | 0 (0) | ||
| mean final NYHA | 1.81 ± 0.07 | 1.89 ± 0.10 | 1.76 ± 0.11 | 1.40 ± 0.25 | F = 1.224 | 0.298 |
| Hemoglobin (g/dL) | 14.2 ± 1.3 | 12.57 ± 1.94 | 16.02 ± 20.28 | 14.2 ± 1.30 | F = 0.834 | 0.437 |
| Creatinine (mg/dL) | 1.03 ± 0.18 | 1.03 ± 0.31 | 1.12 ± 0.18 | 0.40 ± 0.24 | F = 0.336 | 0.715 |
| Uric acid (mg/dL) | 6.5 ± 0.2 | 6.51 ± 2.20 | 6.52 ± 2.01 | 5.2 ± 1.48 | F = 0.92 | 0.402 |
| Sodium (mEq/L) | 138.9 ± 0.3 | 138.43 ± 3.60 | 139.40 ± 2.83 | 139.40 ± 2.41 | F = 1.213 | 0.302 |
| Potassium (mEq/L) | 4.1 ± 0.1 | 4.18 ± 0.66 | 4.02 ± 0.64 | 4.00 ± 0.71 | F = 0.817 | 0.445 |
| Total cholesterol (mg/dL) | 184.4 ± 4.6 | 187.4 ± 5.8 | 182.8 ± 7.7 | 165.8 ± 12.2 | F = 0.511 | 0.602 |
| EGFR (mL/min) | 74.6 ± 3.8 | 74.9 ± 5.5 | 72.9 ± 5.3 | 101.5 ± 34.1 | F = 0.707 | 0.497 |
Numerical variables expressed as mean ± standard deviation; categorical variables, expressed as n (%);
there was no data on initial NYHA class for one Group DI patient.
DD: deletion/deletion genotype; DI: deletion/insertion genotype; II: insertion/insertion genotype; Follow-up: follow-up duration (months); F: frequency; HF duration: disease evolution since disease diagnosis; BMI: body mass index; NYHA: New York Heart Association; EGFR: estimated glomerular filtration rate.
The idiopathic etiology prevailed (36.0%), followed by the hypertensive (20.7%); however, there was no statistically significant difference in the distribution of the etiologies regarding the GPACE (p = 0.248).
A high percentage of use of major beta-blockers (BB), ACE inhibitors (ACEI) and/or angiotensin-receptor blockers (ARB) was observed, with mean doses close to those recommended by the current Brazilian Guidelines for Heart Failure32. There was no difference concerning the distribution of the type of drugs used according to the GPACE (Table 2).
Table 2.
Medicamentous treatment of the Brazilian population studied according to the genetic polymorphisms of the angiotensin-converting enzyme*
| Drug | Mean | DD (n = 57) | DI (n = 49) | II (n = 5) | Statistical test | p Value |
|---|---|---|---|---|---|---|
| Beta-blocker | 108 (98.2) | 55 (98.2) | 47 (97.9) | 5 (100.0) | X2 = 0.111 | 0.946 |
| Carvedilol | 76 (71.0) | 34 (61.8) | 36 (78.3) | 5 (100.0) | X2 = 7.471 | 0.279 |
| Metoprolol | 16 (15.0) | 10 (18.2) | 4 (8.7) | 0 (0) | ||
| Bisoprolol | 14 (13.1) | 11 (20.0) | 5 (10.9) | 0 (0) | ||
| Target dose | 84.9 ± 3.7 | 84.3 ± 4.3 | 84.6 ± 5.8 | 91.2 ± 32.3 | F = 0.78 | 0.925 |
| ACEI | 60 (54.1) | 30 (52.6) | 26 (54.2) | 4 (80.0) | X2 = 1.394 | 0.498 |
| Captopril | 6 (10.0) | 3 (10.0) | 3 (11.5) | 0 (0) | X2 = 0.513 | 0.774 |
| Enalapril | 54 (90.0) | 27 (90.0) | 23 (88.5) | 4 (100.0) | ||
| Target dose | 66.7 ± 3.3 | 60.4 ± 5.9 | 71.6 ± 6.4 | 81.3 ± 1.9 | F = 1.233 | 0.299 |
| ARB: Losartan | 39 (35.1) | 22 (38.6) | 14 (29.2) | 2 (40.0) | X2 = 2.158 | 0.707 |
| Target dose | 73.1 ± 4.3 | 80.7 ± 10.3 | 63.3 ± 8.4 | 62.5 ± 3.8 | F = 0.574 | 0.569 |
| Espironolactona | 74 (66.7) | 39 (68.4) | 33 (68.8) | 2 (40.0) | X2 = 1.771 | 0.413 |
| Furosemida | 79 (71.2) | 43 (75.4) | 32 (66.7) | 3 (60.0) | X2 = 1.274 | 0.529 |
| Mean dose (mg) | 75.4 ± 5.7 | 80.5 ± 8.0 | 71.5 ± 8.7 | 46.7 ± 17.6 | F = 0.791 | 0.457 |
| Hydrochlorothiazide | 26 (23.4) | 14 (24.6) | 12 (25.0) | - | X2 = 1.624 | 0.444 |
| Digitalis | 40 (36.0) | 25 (43.9) | 13 (27.1) | 1 (20.0) | X2= 3.751 | 0.153 |
| Amiodarone | 13 (11.7) | 6 (10.5) | 6 (12.5) | 0 | X2 = 0.746 | 0.689 |
| Statins | 50 (45.0) | 29 (50.9) | 17 (35.4) | 4 (80.0) | X2 = 5.033 | 0.081 |
| Allopurinol | 18 (16.2) | 9 (15.8) | 7 (14.6) | 1 (20.0) | X2 = 0.112 | 0.946 |
Numerical variables expressed as mean ± standard deviation; categorical variables, expressed as n (%). DD: deletion/deletion genotype; DI: deletion/insertion genotype; II: insertion/insertion genotype; F: frequency; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin-receptor blocker.
Of the patients studied, 34 (30.6%) had QRS ≥ 120 ms, 38 (34.2%) had LBBB, and 22 (19.8%) had AF on ECG. The distribution of those variables according to the GPACE types was not statistically different.
Eight (7.2%) patients had implantable devices as follows: three (2.7%) had pacemakers; two (1.8%) had implantable cardioverter-defibrillators (ICD); two (1.8%) had undergone cardiac resynchronization therapy (CRT); and one (0.9%) had a combined device (ICD + CRT).
Echocardiographic outcomes
Approximately half of the cohort (49.5%) had severe LV systolic dysfunction when beginning follow-up, with LVEF ≤ 35%. That percentage increased to 58.5% by the end of the study.
Table 3 shows the echocardiographic data at the beginning and at the end of the study, and the evolution of such measurements. The means of the initial echocardiographic parameters (LVEF, LVSD and LVDD) did not significantly differ between the ACE genotypes. On final echocardiography, only LVSD was significantly different, with a lower mean for the DI GPACE.
Table 3.
Echocardiographic parameters of the population studied according to the genetic polymorphisms of the angiotensin-converting enzyme
| Variable* | Total mean | DD (n = 57) | DI (n = 49) | II (n = 5) | Statistical test | p Value |
|---|---|---|---|---|---|---|
| initial LVEF (%) | 34.0 ± 1.0 | 35.6 ± 1.5 | 32.1 ± 1.5 | 34.6 ± 3.4 | F = 1.469 | 0.235 |
| initial LVSD (mm) | 54.9 ± 1.0 | 54.1 ± 1.4 | 55.7 ± 1.4 | 55.4 ± 3.0 | F = 0.472 | 0.625 |
| initial LVDD (mm) | 65.9 ± 0.9 | 65.6 ± 1.2 | 66.1 ± 1.3 | 66.6 ± 3.1 | F = 0.112 | 0.894 |
| final LVEF (%) | 34.3 ± 1.2 | 32.8 ± 1.6 | 36.4 ± 1.8 | 29.4 ± 4.2 | F = 1.634 | 0.200 |
| final LVSD (mm) | 56.1 ± 1.3 | 59.2 ± 1.8 | 52.3 ± 1.9 | 59.2 ± 5.2 | F = 3.677 | 0.029 |
| final LVDD (mm) | 67.0 ± 1.2 | 69.4 ± 1.8 | 64.0 ± 1.8 | 69.0 ± 4.6 | F = 2.197 | 0.116 |
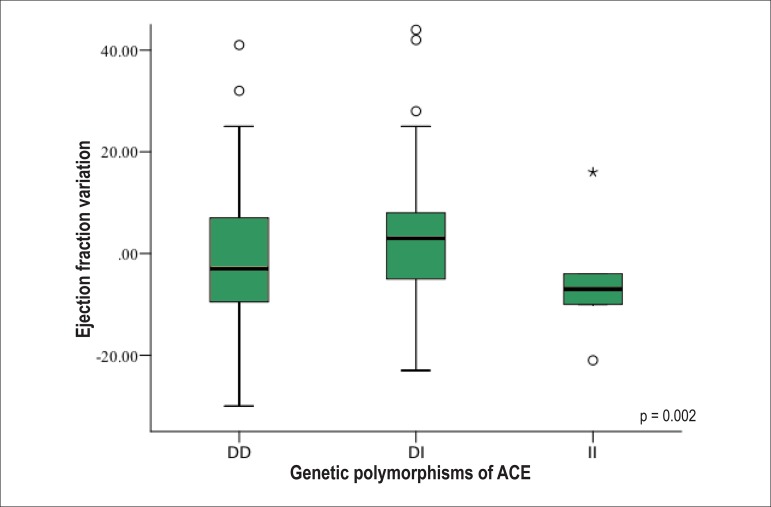
| ∆LVEF (%) | 0.36 ± 1.37 | -2.57 ± 14.86 | 4.62 ± 12.92 | -5.20 ± 13.48 | F = 3.857 | 0.024 |
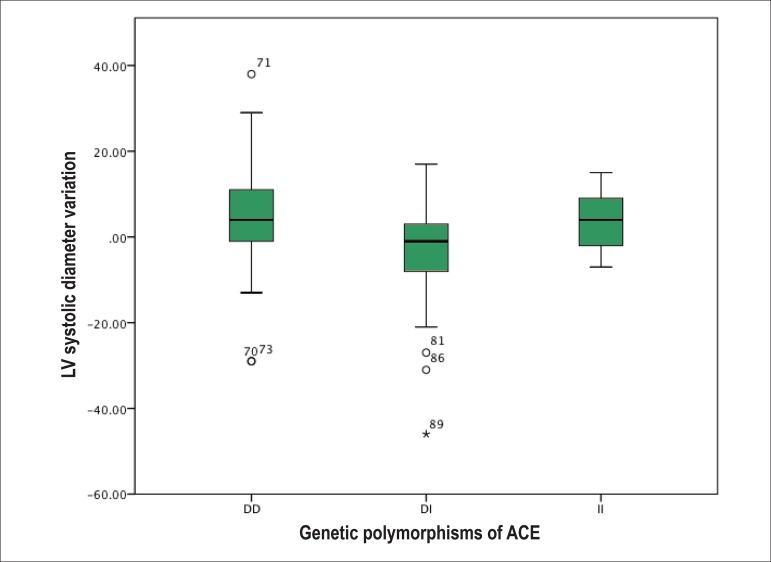
| ∆LVSD (mm) | 0.94 ± 1.17 | 4.60 ± 12.04 | -3.73 ± 11.28 | 3.80 ± 8.70 | F = 6.783 | 0.002 |
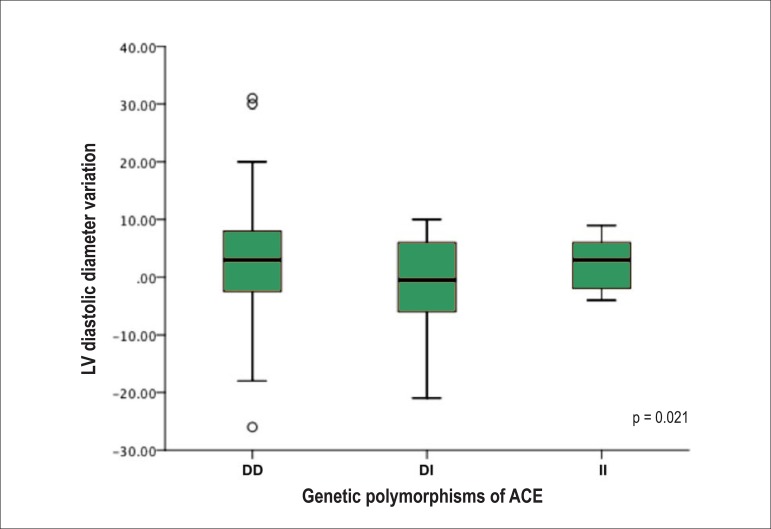
| ∆LVDD (mm) | 0.82 ± 1.04 | 3.38 ± 9.90 | -2.49 ± 11.47 | 2.40 ± 5.41 | F = 4.026 | 0.021 |
| Interval between exams (months) | 65.5 ± 4.3 | 63.2 ± 6.3 | 68.0 ± 6.4 | 65.4 ± 12.4 | F = 0.142 | 0.868 |
Variáveis numéricas estão expressas em média ± desvio padrão. DD: genótipo deleção/deleção; DI: genótipo deleção/inserção; II: genótipo inserção/inserção; F: frequência; FEVE: fração de ejeção do ventrículo esquerdo; DSVE: diâmetro sistólico do ventrículo esquerdo; DDVE: diâmetro diastólico do ventrículo esquerdo. ∆FEVE: diferença entre a fração de ejeção do ventrículo esquerdo da última e primeira consulta; ∆DSVE: diferença entre o diâmetro sistólico do ventrículo esquerdo da última e primeira consulta; ∆DDVE: diferença entre o diâmetro diastólico do ventrículo esquerdo da última e primeira consulta.
Analyzing the evolutionary behavior of each echocardiographic variable [difference between the LVEF at the last and first consultation (∆LVEF); difference between the LVSD at the last and first consultation (∆LVSD); and difference between the LVDD at the last and first consultation (∆LVDD)], the following distinct and significant patterns are observed: on average, DI showed an increase in LVEF as compared to the initial, while DD and II showed a decrease (Figure 1). Regarding the LV size, the DI genotype showed a reduction in LVSD and LVDD at the end of follow-up, while the DD and II genotypes showed an increase in the cavitary diameters (Figures 2 and 3, respectively).
Figure 1.
Ejection fraction variation between the end and the beginning of follow-up of the population studied according to the genetic polymorphisms of the angiotensinconverting enzyme (ACE). DD: deletion/deletion genotype; DI: deletion/insertion genotype; II: insertion/insertion genotype.
Figure 2.
Left ventricular (LV) systolic diameter variation between the end and the beginning of follow-up of the population studied according to the genetic polymorphisms of the angiotensin-converting enzyme (ACE). DD: deletion/deletion genotype; DI: deletion/insertion genotype; II: insertion/insertion genotype.
Figure 3.
Left ventricular (LV) diastolic diameter variation between the end and the beginning of follow-up of the population studied according to the genetic polymorphisms of the angiotensin-converting enzyme (ACE). DD: deletion/deletion genotype; DI: deletion/insertion genotype; II: insertion/insertion genotype.
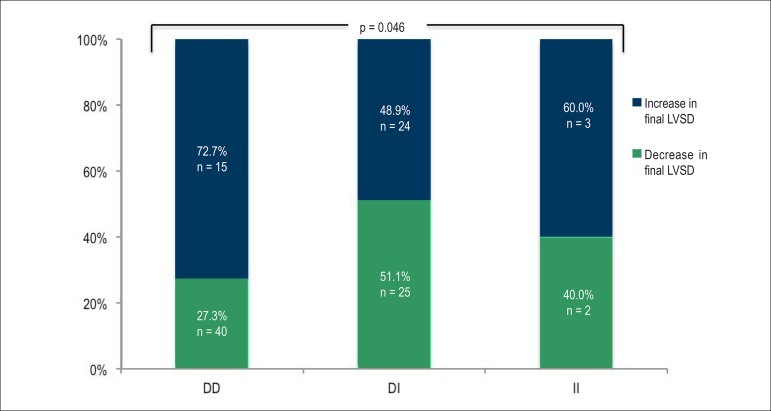
The qualitative analysis (increase versus decrease) of the ∆LVSD (Figure 4) and of the ∆ LVDD showed a difference between the GPACE with statistical significance for LVSD (p = 0.046), but not for LVDD (p = 0.095): the DD genotype had a greater number of patients with increased LVSD while the DI variant had a greater number of patients with decreased LVSD by the end of the study.
Figure 4.
Evolutionary behavior of the left ventricular systolic diameter of the population studied according to the genetic polymorphisms of the angiotensin-converting enzyme (ACE). DD: deletion/deletion genotype; DI: deletion/insertion genotype; II: insertion/insertion genotype; LVSD: left ventricular systolic diameter.
Discussion
This study describes the relationship between the GPACE variants and the clinical and echocardiographic outcomes in 111 patients with non-ischemic HF, with mean follow-up of 5.4 years (range, 12.0 - 249.7 months). Other international11,13 and national14,15 studies have carried out that analysis; however, this study is the first to assess exclusively non-ischemic HF in a Brazilian population with a mean follow-up time longer than five years.
Two findings of this study are worthy of note. First, the ACE genotypic profile of the population studied differed from that of most of previous publications, with an extremely low proportion of type II GPACE (only 4.5% of the patients). In addition, the echocardiographic evolutionary behavior represented by the variables ∆LVEF, ∆LVSD and ∆LVDD differed between the GPACEs, with worsening of those parameters in the DD genotype.
The low prevalence of the II genotype observed in this study can be related to the characteristics of the population studied, especially their ethnicity. The meta-analysis by Bai et al4, with 2,453 cases of HF of multiple etiologies, included only 6.4% of black individuals and 23.4% of those of Asian origin, while the population of this study consisted of 51% of white individuals, 36% of black, 13% of individuals with mixed heritage and none of Asian ethnicity. The differences in the prevalences of the ACE genotypes found in this study and in the study by Bai et als. were, respectively: 51.4% versus 31% for DD; 44.1% versus 46% for DI; and 4.5% versus 23% for II.
Tiago et al33, studying 157 black individuals with idiopathic HF in South Africa, have reported a GPACE distribution more similar to ours: 45.2% of DD; 38.2% of DI; and 6.5% of II. That might have resulted from the exclusive presence of Afro-descendants in that study. Velloso et al10 have described a similar association of other GPs and the skin color of individuals with HF: the GP prevalences of nitric oxide synthase differed between white and Afro-American individuals.
The different etiologies of non-ischemic HF did not relate to the genetic profile, and the absence of patients with ischemic HF might not have determined higher or lower incidence of any of the genotypes. Amir et al34, studying 195 patients with HF (124 ischemic and 71 non-ischemic), have already demonstrated no significant variation in genotypes regarding etiology.
The analysis of the echocardiographic variables showed a significant difference between the means of final LVSD according to the ACE genotypes. The DD GPACE showed higher means than the DI GPACE: 59.2 mm versus 52.3 mm, respectively. The small number of patients with the II genotype limited the analysis for that group. The evolutionary parameters ∆LVEF, ∆LVSD and ∆LVDD differed significantly between the GPACEs, with improvement in the EF and LV diameters (reverse remodeling) in the DI genotype. The DD and II genotypes showed an inverse behavior, with worsening of the EF and of the ventricular diameters (cardiac dilation).
That more severe evolutionary pattern related to the DD GPACE is in accordance with the findings of other authors16,24. The more marked cardiac dilation in those patients relates to the higher neuro-humoral activation, mainly of the RAAS. The GPACEs are responsible for approximately 50% of the variation in ACE levels, the DD genotype being associated with higher levels of that enzyme35. Elevated ACE levels are accompanied by increased synthesis of angiotensin and greater activation of that system36.
However, those results are not uniform. De Groote et al11 have found no difference in the echocardiographic parameters of 199 patients with HF, who had not initiated the BB use. The short interval between exams (only three months after optimization of the BB dose as compared to 65.5 months in this study) might have been insufficient to observe cardiac reverse remodeling in that study. Mahjoub et al17 have not detected echocardiographic differences between the GPACEs, but those authors have chosen a categorical analysis, dividing the sample into two groups according to the LVDD (≥ 69 mm versus < 69 mm), corresponding to higher or lower severity, respectively. The statistical analysis of the present study used the numerical values of the echocardiographic parameters as continuous variables, having, thus, higher discriminatory power.
The clinical profile of each cohort varies between studies. In addition to the already discussed relationship of ethnicity and GPACE prevalence, other factors seem to influence the participation of the ACE gene on HF natural history and pathophysiology. One of the major factors is drug treatment.
The percentage of BB use was 98.2%, with a target dose of 84.3% of that recommended, higher than that of most clinical trials14,18,20. The use of ACEI and/or ARB was 91.2%, and that of spironolactone, 68.4%. However, the excellence of that treatment can interfere with the patients' clinical outcomes, hindering the observation of differences according to GPACE.
McNamara et al12,13 have assessed the pharmacogenetic interaction, observing the use of BB12 and ACEI13 and the GPACEs. The DD genotype was associated with worse clinical and echocardiographic outcome, but the impact of that GP was attenuated by the treatment with BB and ACEI. In other words, for that group of patients, the neuro-humoral block might have neutralized the excessive RAAS activity secondary to the DD GPACE. Thus, under optimized therapy, the three genotypes, DD, DI and II, began to behave in a similar manner regarding clinical outcome.
In another study, the combination of two GPACE genetic variants with the GP in the angiotensin II receptor has shown an independent association with clinical outcomes37.
Thus, the polygenic character described for other physical characteristics, such as height38 or lipid profile39, might also seem to play a role in the HF pathophysiology and in RAAS action. The simultaneous study of multiple GPs in the same population has identified that only combinations of genotypes have been associated with clinical and/or echocardiographic outcomes19,20. A panel of genetic markers might be more efficient in detecting more severely ill individuals than isolated GPs.
The present study has some limitations. First, the relatively small number of individuals studied, especially the reduced number of individuals with the II genotype, hindered a more conclusive data analysis. In addition, data collection from medical records represents, by definition, a limitation. However, it is worth noting that such limitation might have been attenuated by the high quality of the service provided at a well-structured HF clinic, with defined protocols, professional training and regular auditing. Last, because this is also a retrospective study, a selection bias might have occurred with the inclusion of a smaller number of more severely ill patients. However, the II genotype, theoretically more prevalent in less critical patients, had the lowest prevalence, which counteracts that selection bias.
The application of genetics to the HF context has become a potentially interesting and attractive tool for risk and severity stratification, as well as a marker of therapeutic response. The complex genetic architecture, represented by the already known polygenic heritage of other characteristics, illustrates the study difficulty on the subject. However, better understanding that area might have a great impact on medical practice, especially cardiology. Thus, the difficulties observed should not be seen as negative results, but as an incentive for further studies that would fill gaps and develop the knowledge in that important area.
Conclusion
The frequency of alleles and variants of GPACE has differed in most international and also national studies on HF, emphasis given to the small number of individuals with the II variant.
The echocardiographic parameters differed significantly between the GPACE variants. The DD genotype related to a worse echocardiographic outcome over a 5.4-year follow-up.
Footnotes
Author contributions
Conception and design of the research: Albuquerque FN, Brandão AA, Silva DA, Mourilhe-Rocha RM, Duque GS, Albuquerque DC; Acquisition of data: Albuquerque FN, Silva DA, Mourilhe-Rocha RM, Duque GS, Gondar AFP, Neves LMA, Bittencourt MI; Analysis and interpretation of the data: Albuquerque FN, Brandão AA, Silva DA, Pozzan R, Albuquerque DC; Statistical analysis: Albuquerque FN, Pozzan R; Obtaining funding: de Albuquerque FN, Albuquerque DC; Writing of the manuscript: Albuquerque FN, Brandão AA, Pozzan R, Albuquerque DC; Critical revision of the manuscript for intellectual content: Albuquerque FN, Brandão AA, Albuquerque DC.
Study Association
This article is part of the thesis of master submitted by Felipe Neves de Albuquerque during the Post-Graduation in Medical Science of the Faculdade de Ciências Médicas of Universidade do Estado do Rio de Janeiro.
Sources of Funding
This study was partially funded by FAPERJ.
References
- 1.Godoy HL, Silveira JA, Segalla E, Almeida DR. Hospitalização e mortalidade por insuficiência cardíaca em hospitais públicos no município de São Paulo. Arq Bras Cardiol. 2011;97(5):402–407. doi: 10.1590/s0066-782x2011005000096. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. Erratum in: Circulation. 2013;127(1):e841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopes LR, Elliott PM. Genetics of heart failure. Biochim Biophys Acta. 2013 Jan 03; doi: 10.1016/j.bbadis.2012.12.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Bai Y, Wang L, Hu S, Wei Y. Association of angiotensin-converting enzyme I/D polymorphism with heart failure: a meta-analysis. Mol Cell Biochem. 2012;361(1-2):297–304. doi: 10.1007/s11010-011-1115-8. [DOI] [PubMed] [Google Scholar]
- 5.McNamara DM. Genomic variation and neurohormonal intervention in heart failure. Heart Fail Clin. 2010;6(1):35–43. doi: 10.1016/j.hfc.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira SB, Velloso MW, Chermont S, Quintão MM, Nunes Abdhala R, Giro C, et al. ß-adrenergic receptor polymorphisms in susceptibility, response to treatment and prognosis in heart failure: Implication of ethnicity. Mol Med Rep. 2012 Oct 09; doi: 10.3892/mmr.2012.1120. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Liu WN, Fu KL, Gao HY, Shang YY, Wang ZH, Jiang GH, et al. ß1 adrenergic receptor polymorphisms and heart failure: a meta-analysis on susceptibility, response to ß-blocker therapy and prognosis. PLoS One. 2012;7(7): doi: 10.1371/journal.pone.0037659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talameh JA, McLeod HL, Adams KF, Jr, Patterson JH. Genetic tailoring of pharmacotherapy in heart failure: optimize the old, while we wait for something new. J Card Fail. 2012;18(4):338–349. doi: 10.1016/j.cardfail.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Chang SN, Lin JW, Juang JM, Tsai CT, Hwang JJ, Chiang FT. Association between genetic polymorphisms in the renin-angiotensin system and systolic heart failure revised by a propensity score-based analysis. Cardiology. 2010;116(4):279–285. doi: 10.1159/000321123. [DOI] [PubMed] [Google Scholar]
- 10.Velloso MW, Pereira SB, Gouveia L, Chermont S, Tardin OM, Gonçalves R, et al. Endothelial nitric oxide synthase Glu298Asp gene polymorphism in a multi-ethnical population with heart failure and controls. Nitric Oxide. 2010;22(3):220–225. doi: 10.1016/j.niox.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 11.de Groote P, Helbecque N, Lamblin N, Hermant X, Amouyel P, Bauters C, et al. Beta-adrenergic receptor blockade and the angiotensin-converting enzyme deletion polymorphism in patients with chronic heart failure. Eur J Heart Fail. 2004;6(1):17–21. doi: 10.1016/j.ejheart.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 12.McNamara DM, Holubkov R, Janosko K, Palmer A, Wang JJ, MacGowan GA, et al. Pharmacogenetic interactions between-blocker therapy and the angiotensin-converting enzyme deletion polymorphism in patients with congestive heart failure. Circulation. 2001;103(12):1644–1648. doi: 10.1161/01.cir.103.12.1644. [DOI] [PubMed] [Google Scholar]
- 13.McNamara DM, Holubkov R, Postava L, Janosko K, MacGowan GA, Mathier M, et al. Pharmacogenetic interactions between angiotensin-converting enzyme inhibitor therapy and the angiotensin-converting enzyme deletion polymorphism in patients with congestive heart failure. J Am Coll Cardiol. 2004;44(10):2019–2026. doi: 10.1016/j.jacc.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 14.Cuoco MA, Pereira AC, Mota Gde F, Krieger JE, Mansur AJ. Polimorfismo genético, terapia farmacológica e função cardíaca seqüencial em pacientes com insuficiência cardíaca. Arq Bras Cardiol. 2008;90(4):252–256. [PubMed] [Google Scholar]
- 15.Cuoco MA, Pereira AC, de Freitas HF, de Fátima Alves da Mota G, Fukushima JT, Krieger JE, et al. Angiotensin-converting enzyme gene deletion polymorphism modulation of onset of symptoms and survival rate of patients with heart failure. Int J Cardiol. 2005;99(1):97–103. doi: 10.1016/j.ijcard.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 16.Huang W, Xie C, Zhou H, Yang T, Sun M. Association of the angiotensin-converting enzyme gene polymorphism with chronic heart failure in Chinese Han patients. Eur J Heart Fail. 2004;6(1):23–27. doi: 10.1016/j.ejheart.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Mahjoub S, Mehri S, Bousaada R, Ouarda F, Zaroui A, Zouari B, et al. Association of ACE I/D polymorphism in Tunisian patients with dilated cardiomyopathy. J Renin Angiotensin Aldosterone Syst. 2010;11(3):187–191. doi: 10.1177/1470320310368874. [DOI] [PubMed] [Google Scholar]
- 18.Sanderson JE, Yu CM, Young RP, Shum IO, Wei S, Arumanayagam M, et al. Influence of gene polymorphisms of the renin-angiotensin system on clinical outcome in heart failure among the Chinese. Pt 1Am Heart J. 1999;137(4):653–657. doi: 10.1016/s0002-8703(99)70218-8. [DOI] [PubMed] [Google Scholar]
- 19.Montgomery HE, Keeling PJ, Goldman JH, Humphries SE, Talmud PJ, McKenna WJ. Lack of association between the insertion/deletion polymorphism of the angiotensin-converting enzyme gene and idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1995;25(7):1627–1631. doi: 10.1016/0735-1097(95)00109-h. [DOI] [PubMed] [Google Scholar]
- 20.Zakrzewski-Jakubiak M, de Denus S, Dubé MP, Bélanger F, White M, Turgeon J. Ten renin-angiotensin system-related gene polymorphisms in maximally treated Canadian Caucasian patients with heart failure. Br J Clin Pharmacol. 2008;65(5):742–751. doi: 10.1111/j.1365-2125.2007.03091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernández-Solà J, Nicolás JM, Oriola J, Sacanella E, Estruch R, Rubin E, et al. Angiotensin-converting enzyme gene polymorphism is associated with vulnerability to alcoholic cardiomyopathy. Part 1Ann Intern Med. 2002;137(5):321–326. doi: 10.7326/0003-4819-137-5_part_1-200209030-00007. [DOI] [PubMed] [Google Scholar]
- 22.Andersson B, Sylvén C. The DD genotype of the angiotensin-converting enzyme gene is associated with increased mortality in idiopathic heart failure. J Am Coll Cardiol. 1996;28(1):162–167. doi: 10.1016/0735-1097(96)00098-8. [DOI] [PubMed] [Google Scholar]
- 23.Girnita DM, Ohmann EL, Brooks MM, Webber SA, Burckart GJ, Ferrell RE, et al. Gene polymorphisms impact the risk of rejection with hemodynamic compromise: a multicenter study. Transplantation. 2011;91(12):1326–1332. doi: 10.1097/TP.0b013e31821c1e10. [DOI] [PubMed] [Google Scholar]
- 24.Candy GP, Skudicky D, Mueller UK, Woodiwiss AJ, Sliwa K, Luker F, et al. Association of left ventricular systolic performance and cavity size with angiotensin-converting enzyme genotype in idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;83(5):740–744. doi: 10.1016/s0002-9149(98)00981-3. [DOI] [PubMed] [Google Scholar]
- 25.Sanderson JE, Young RP, Yu CM, Chan S, Critchley JA, Woo KS. Lack of association between insertion/deletion polymorphism of the angiotensin-converting enzyme gene and end-stage heart failure due to ischemic or idiopathic dilated cardiomyopathy in the Chinese. Am J Cardiol. 1996;77(11):1008–1010. doi: 10.1016/s0002-9149(97)89160-6. [DOI] [PubMed] [Google Scholar]
- 26.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342(15):1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 27.Follath F, Cleland JG, Klein W, Murphy R. Etiology and response to drug treatment in heart failure. J Am Coll Cardiol. 1998;32(5):1167–1172. doi: 10.1016/s0735-1097(98)00400-8. [DOI] [PubMed] [Google Scholar]
- 28.Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39(2):210–218. doi: 10.1016/s0735-1097(01)01738-7. [DOI] [PubMed] [Google Scholar]
- 29.Mangini S, Silveira FS, Silva CP, Grativvol PS, Seguro LF, Ferreira SM, et al. Decompensated heart failure in the emergency department of a cardiology hospital. Arq Bras Cardiol. 2008;90(6):400–406. doi: 10.1590/s0066-782x2008000600008. [DOI] [PubMed] [Google Scholar]
- 30.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salanti G, Amountza G, Ntzani EE, Ioannidis JP. Hardy-Weinberg equilibrium in genetic association studies: an empirical evaluation of reporting, deviations, and power. Eur J Hum Genet. 2005;13(7):840–848. doi: 10.1038/sj.ejhg.5201410. [DOI] [PubMed] [Google Scholar]
- 32.Bocchi EA, Marcondes-Braga FG, Bacal F, Ferraz AS, Albuquerque D, Rodrigues D, et al. Sociedade Brasileira de Cardiologia Atualização da Diretriz brasileira de insuficiência cardíaca crônica - 2012. Arq Bras Cardiol. 2012;98(1) supl. 1:1–33. doi: 10.1590/s0066-782x2012001000001. [DOI] [PubMed] [Google Scholar]
- 33.Tiago AD, Badenhorst D, Skudicky D, Woodiwiss AJ, Candy GP, Brooksbank R, et al. An aldosterone synthase gene variant is associated with improvement in left ventricular ejection fraction in dilated cardiomyopathy. Cardiovasc Res. 2002;54(3):584–589. doi: 10.1016/s0008-6363(02)00281-x. [DOI] [PubMed] [Google Scholar]
- 34.Amir RE, Amir O, Paz H, Sagiv M, Mor R, Sagiv M, et al. Genotype-phenotype associations between chymase and angiotensin-converting enzyme gene polymorphisms in chronic systolic heart failure patients. Genet Med. 2008;10(8):593–598. doi: 10.1097/gim.0b013e3181804b9c. [DOI] [PubMed] [Google Scholar]
- 35.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86(4):1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348(20):2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 37.Andersson B, Blange I, Sylvén C. Angiotensin-II type 1 receptor gene polymorphism and long-term survival in patients with idiopathic congestive heart failure. Eur J Heart Fail. 1999;1(4):363–369. doi: 10.1016/s1388-9842(99)00045-8. [DOI] [PubMed] [Google Scholar]
- 38.Lango Allen H, Estrada K, Lettre G, Berndt SI, Weedon MN, Rivadeneira F, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467(7317):832–838. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shuldiner AR, Pollin TI. Genomics: variations in blood lipids. Nature. 2010;466(7307):703–704. doi: 10.1038/466703a. [DOI] [PubMed] [Google Scholar]