Abstract
Background:
Exercise Training (ET) and Grape Seed Extract (GSE) as an antioxidant have many positive effects on controlling diabetes mellitus and its complications.
Objectives:
This study aimed to determine the effects of GSE alone or combined with ET on body weight, plasma lipid profile, blood pressure, and heart rate in STZ-induced diabetic rats.
Methods:
In this study, male Wistar rats were randomly assigned to five groups: sedentary control, sedentary diabetic, trained diabetic, GSE treated sedentary diabetic, and GSE treated trained diabetic. ET was conducted on the treadmill daily for 8 weeks. One way ANOVA followed by LSD test was used for statistical analysis.
Results:
Reduction of body weight, high density lipoproteins, heart rate, and systolic blood pressure and increment of total cholesterol, triglyceride, low density lipoprotein, and very low density lipoproteins were observed after STZ injection. Co-administration of GSE and ET had more positive effects on lipid profile compared to each method alone. In addition, GSE and ET modified heart rate partially, while their combination was more effective in improvement of heart rat in conscious rats. On the other hand, administration of ET or GSE alone did not affect systolic blood pressure and body weight, while their combination restored systolic blood pressure completely and improved body weight partially.
Conclusions:
The study findings indicated that ET combined with GSE had more beneficial effects compared to each one alone on the complications of STZ induced diabetes. This may constitute a convenient and inexpensive therapeutic approach to diabetic complications.
Keywords: Exercise, Grape Seed Extract, Streptozotocin, Bradycardia, Hypotension
1. Background
Injection of Streptozotocin (STZ), the most commonly used agents in experimental diabetes (1), to rats produces a diabetic state characterized by hyperglycemia, loss of weight, hypotension, bradycardia (2, 3), increase in plasma Total Cholesterol (TC), Triglycerides (TG), Low-Density Lipoprotein cholesterol (LDL-c), Very Low-Density Lipoprotein cholesterol (VLDL-c), and decrease in High Density Lipoprotein (HDL) (4).
In management of diabetes and its complications, Exercise Training (ET) has many positive effects, such as increase in insulin sensitivity, decrease in glycosylated hemoglobin (HbA1c), improvement of blood lipid profiles and blood pressure (5), and improvement of systemic vascular resistance and heart rate (6). It has also been shown that the incidence of cardiovascular morbidity and mortality during diabetes was reduced by ET (7). In addition, ten weeks ET reversed hypotension and bradycardia induced by STZ in rats (8).
Since oxidative stress contributes to complications of Diabetes Mellitus (DM) (9-12), pharmacological agents that ameliorate oxidative stress may improve diabetes and its complications. It has been reported that treatment of diabetic hypertensive rats with vitamin E decreased blood pressure (13).
Grape Seed Extract (GSE) has many favorable effects on human health, such as lowering of LDL-c, reduction of Cardiovascular Diseases (CVD), and scavenging of free radicals (14). The antioxidant power of proanthocyanidins of the grape seeds is twenty times greater than vitamin E and fifty times greater than vitamin C (14). Badavi et al. showed that GSE improved hypertension and heart rate induced by lead exposure (15).
However, to our knowledge, no studies have been conducted on the effects of GSE alone or combined with exercise on the lipid profile, body weight, heart rate, and blood pressure of diabetic models. Therefore, the current study aims to determine the effects of GSE alone or combined with ET on the lipid profile, body weight, heart rate, and blood pressure of STZ-induced diabetic rats.
2. Materials and Methods
2.1. Animals and Treatment
In this study, 45 male Wistar rats weighing 200 - 240 g were obtained from the animal house of physiology research center at Ahwaz Jundishapour University of Medical Sciences, Ahwaz, Iran. The animals were randomly assigned to five groups each containing 9 rats: Sedentary Control (SC), Sedentary Diabetic (SD), Trained Diabetic (TrD), sedentary diabetic treated with GSE (ExD), and trained diabetic treated with GSE (TrExD). GSE was dissolved in 1 mL distilled water and administered orally via gavage needle once a day. The duration of the protocol was 8 weeks. Diabetes was induced by a single intraperitoneal injection of STZ (60 mg / kg body weight) dissolved in 0.3 mL normal saline (16). The experimental protocol and procedures were submitted and approved by the Institutional Animal Care and Use Committee of the University.
2.2. Drugs
STZ was obtained from Sigma (St. Louis, Mo). Besides, Ketamine and Xylazine were prepared by Alfasan Co (Woderen-Holland).
2.3. Exercise Training Protocol
The rats performed ET on treadmill daily for 8 weeks, 1 day after the diabetic state was verified as shown in Table 1.
Table 1. Exercise Training Protocol for the Rats on the Treadmill .
| Week | Belt Speed (m / min) | Inclination (˚) | Total Time (min) |
|---|---|---|---|
| 1 | 16 | 0 | 30 |
| 2 | 16 | 5 | 30 |
| 3 | 16 | 10 | 45 |
| 4 | 16 | 12 | 45 |
| 5 | 16 | 12 | 60 |
| 6 | 16 | 12 | 60 |
| 7 | 16 | 12 | 60 |
| 8 | 16 | 12 | 60 |
2.4. Preparation of Grape Seed Extract
VitisVinifera grape seeds were confirmed by Qazvin Agricultural Research Center, Qazvin, Iran. Voucher specimen was available in the herbariumat the Department of Phamacognosy, Faculty of Pharmacy, Ahvaz Jundishapur University of Medical Sciences, Ahwaz, Iran. Grape seeds were separated from the grapes manually, air-dried (in the shade, 25 - 30°C) for one week, and milled to fine powder. The grape seed powder was macerated in 70% ethanol (25% w/v) for three days at room temperature and was stirred three times a day. Then, the mixture was filtered with cheese cloth, the filtrate was dried at room temperature (about 25°C) to evaporate ethanol, and GSE was obtained (25 - 30 %) as a powder (17).
2.5. Plasma Lipid Profile Determination
Immediately after cardiac puncture under anesthesia with ketamine and xylasine, blood samples were obtained from the heart and transferred into EDTA containing tubes. The samples were then centrifuged at 4000 g for 10 min to obtain plasma. Afterwards, the plasma was stored at -70°C for biochemical analysis. Enzymatic colorimetric methods (Pars Azmune, Tehran, Iran) were used for measurement of TC and TG levels. In addition, HDL-c was determined by enzymatic colorimetric method (Pars Azmune) after precipitation of non- HDL-c lipoproteins by phosphotungstic acid and magnesium chloride in the plasma. Besides, VLDL was calculated as follows (18):
VLDL = Total serum triglycerides / 5
Moreover, LDL-c was calculated based on Friedwald’s equation (19) for less than 400 mg / dL TG-containing samples:
LDL-c = Total serum cholesterol–(VLDL–Total serum HDL).
2.6. Heart Rate and Blood Pressure Recording
Heart rate and blood pressure were recorded once a week. Prior to blood pressure and heart rate measurement, conscious rats were placed in a restrainer, pre-warmed, and allowed to rest for about 25 min. Then, these variables were determined by tail plethysmography coupled to a computer system (Powerlab, AD Instrument, Australia). Overall, three consecutive recordings (at least 5 min apart) were performed and the average of the recordings was calculated for each rat (15).
2.7. Statistical Analysis
The results were expressed as mean ± SEM. The data were first analyzed for normal distribution using kolmogrov-smirnof test. Then, comparisons were made between the study groups using one way and repeated measures ANOVA followed by LSD tests. Besides, P values < 0.05 were considered as statistically significant.
3. Results
3.1. Blood Lipid Profile in Different Groups
Eight weeks after STZ injection, significant changes were observed in the plasma lipid profile, such as lowered HDL-c, and elevated TC, TG, LDL-c, and VLDL (P < 0.001) (Table 2). However, ET improved HDL-c (P = 0.006), TC, TG, LDL-c, and VLDL (P < 0.001). In addition, GSE had improving effects on TC (P < 0.001), HDL-c (P = 0.001), and LDL-c (P = 0.001), but did not change TG and VLDL. On the other hand, in comparison to ET or GSE alone, co-administration of GSE and ET had more improving effects on TC, TG, HDL-c, LDL-c, and VLDL (P < 0.001). Moreover, plasma lipid profile values in the GSE + ET treated diabetic animals were the same as the corresponding values of the sedentary control group.
Table 2. Plasma Lipid Profile in Different Groups after 8 Weeks (Mean ± SEM, n = 6 - 7).
| Parameter Groups | TC (mg / dL) | TG (mg / dL) | HDLc (mg / dL) | LDLc (mg / dL) | VLDL (mg / dL) |
|---|---|---|---|---|---|
| SC | 79.33 ± 3.77 | 64.5 ± 5.55 | 47.83 ± 1.97 | 18.6 ± 1.97 | 12.9 ± 1.11 |
| SD | 163.86 ± 7.18 a | 157.14 ± 7.68 a | 33.29 ± 1.51 a | 99.14 ± 5.45 a | 31.43 ± 1.54 a |
| TrD | 112 ± 6.93 a, b | 117.5 ± 7.74 a, b | 41.5 ± 2.26 a, b | 47 ± 4.83 a, b | 23.5 ± 1.55 a, b |
| ExD | 134.43 ± 6.86 a, b | 140.29 ± 5.98 a | 43.14 ± 1.77 b | 63.23 ± 6.49 a, b | 28.057 ± 1.2 a |
| TrExD | 87.43 ± 6.72 b | 79.71 ± 4.47 b | 51.57 ± 2.21 b | 19.91 ± 7.28 b | 15.94 ± 0.89 b |
Abbreviations: TC, total cholesterol; TG, triglycerides; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol; VLDL, very low-density lipoprotein cholesterol; SC, sedentary control; SD, sedentary diabetic;TrD, trained diabetic;ExD, sedentary diabetic that received grape seed extract; TrExD, trained diabetic that received grape seed extract
aP < 0.05 significantly different from SC,
bP < 0.05 significantly different from SD (One-Way ANOVA followed by LSD multiple comparison tests)
3.2. Body Weight Changes
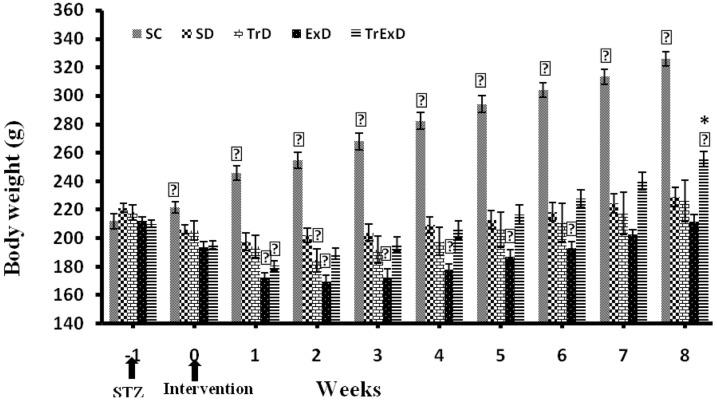
At the beginning of the experiment, no significant difference was found among the study groups regarding their body weight (Figure 1). However, the control groups gained weight throughout 9 weeks. On the other hand, the body weight of the sedentary diabetic group reduced during the first 2 weeks after STZ injection. In trained diabetic and GSE treated sedentary diabetic groups also, this reduction continued for 3 weeks and then increased slightly to reach the pre-STZ values at week 8. Nevertheless, the body weight of the GSE + ET treated diabetic group reduced initially during the first 2 weeks after STZ injection, then began to increase throughout the next 7 weeks, and reached a value that was significantly different from the pre-STZ value (P < 0.001) as well as from the control group (Figure 1).
Figure 1. Weekly Body Weight Changes (Mean  ± SEM, n = 7 - 8) in Different Groups During 8 Weeks.
Abbreviations: SC, sedentary control; SD, sedentary diabetic; TrD, trained diabetic; ExD, GSE treated sedentary diabetic; TrExD, GSE treated trained diabetic
*P < 0.05 significantly different from SC, † P < 0.05 significantly different from SD (repeated measurement ANOVA followed by LSD multiple comparison tests)
3.3. Heart Rate and Systolic Blood Pressure
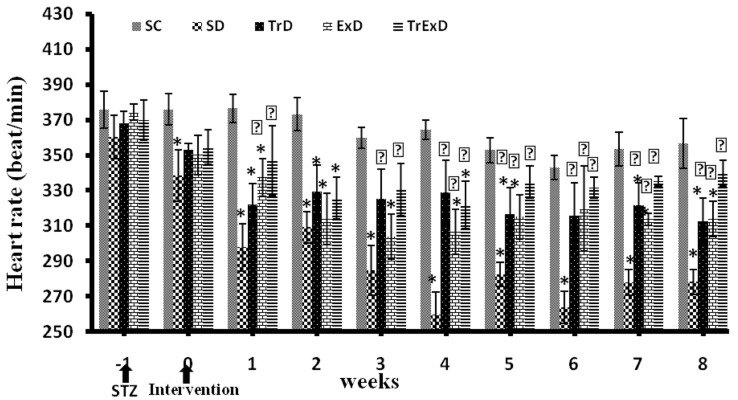
As shown in Figure 2, STZ-induced diabetes decreased heart rate in all the groups during the first 2 – 3 weeks post injection. However, the observed bradycardia was more severe in the sedentary diabetic group compared to the trained diabetic and GSE treated sedentary diabetic groups. At the end of the protocol, the heart rate in the sedentary diabetic group was significantly lower than that of the sedentary control group. Therefore, administration of ET or GSE partially and combination of ET and GSE completely improved the heart rate. However, no significant change was found in the control heart rate throughout the experiment.
Figure 2. Weekly Heart Rate Changes (Mean  ± SEM, n = 7 - 8) in Different Groups During 8 Weeks.
Abbreviations: SC, sedentary control, SD, sedentary diabetic, TrD, trained diabetic, ExD, mGSE treated sedentary diabetic, TrExD, GSE treated trained diabetic
*P < 0.05 significantly different from SC, †P < 0.05 significantly different from SD (repeated measurement ANOVA followed by LSD multiple comparison tests)
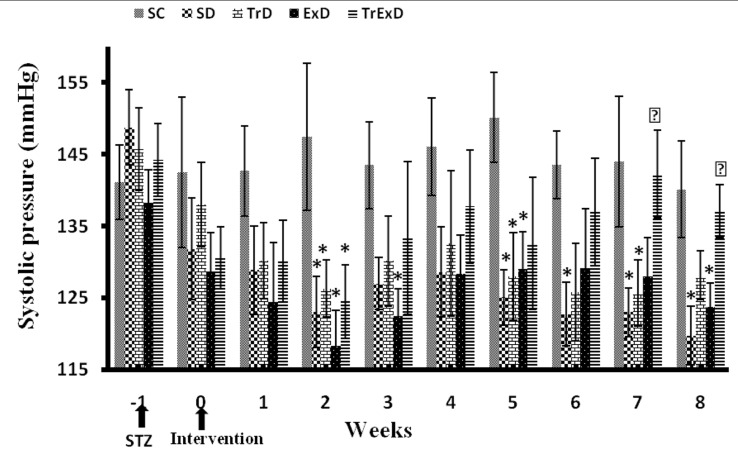
In comparison to the control group, the Systolic Blood Pressure (SBP) decreased in all the groups that received STZ in the first 2 weeks after diabetes induction (Figure 3). Although ET or GSE administration alone did not restore SBP, the combination of ET and GSE could restore it toward the control group.
Figure 3. Weekly Systolic Blood Pressure Changes (Mean  ± SEM, n = 7 - 8) in Different Groups During 8 Weeks.
Abbreviations: SC, sedentary control, SD, sedentary diabetic, TrD, trained diabetic, ExD, GSE treated sedentary diabetic, TrExD, GSE treated trained diabetic
*P < 0.05 significantly different from SC, †P < 0.05 significantly different from SD (repeated measurement ANOVA followed by LSD multiple comparison tests)
4. Discussion
As expected, body weight, HDL-c, heart rate, and SBP reduced and plasma TC, TG, LDL-c, and VLDL increased significantly in diabetic animals induced by STZ, which is in accordance with other studies conducted on the issue (2-4). Administration of ET or GSE alone improved heart rate partially, while the combination of GSE and ET improved them completely in conscious rats. On the other hand, administration of ET or GSE alone did not affect SBP and body weight, while their combination restored SBP completely and improved body weight partially. In general, control of blood lipid level is an important step towards decreasing the incidence of long-term complications of DM (19). In this study, combined ET and GSE were more effective than ET or GSE alone in improvement of lipid profiles in diabetic state induced by STZ.
In line with our study results, previous studies showed the positive effects of exercise on thelipid profiles of healthy rats (20,21) as well as type 2 (22) and type 1 (23) diabetic rats. Positive changes in lipid profile induced by ET in the trained diabetic animals may occur via an increase in triglyceride lipolysis (24), improvement of antioxidant/oxidant ratio (25), and altered synthesis of LDL-C or removal rate of LDL-C from the plasma by the tissues (20). In general, antioxidants play a major role in prevention of diabetes and its complications by free radicals scavenging (26). It has been shown that vitamin C (27), vitamin E (28), and α lipoic acid (19) could improve blood lipid profile in diabetic rats. Moreover, vitamin C was reported to prevent LDL-cholesterol from oxidative damage by scavenging the free radicals, helping the degradation of cholesterol, and directing the cholesterol towards bile acid synthesis (27). In the present study, GSE as an antioxidant significantly improved lipid profile in diabetic animals. One study also indicated that grape seed tannins enhanced reverse cholesterol transport and bile acid excretion and reduced intestinal cholesterol absorption in the rats fed by hypercholesterolemic diets (29).
The combination of these two factors could potentiate these favorable effects, but the exact mechanisms and the involved systems are needed to be determined.
Loss of weight induced by STZ could be related to the inability to metabolize the carbohydrates that shift fuel sources to fatty acids (3) and proteins (30) as an energy source. Therefore, wasting of proteins and fatty acid stores induced by insulin deficiency might lead to reduction of body weight. It seems that co-administration of ET and GSE increased body weight by improvement of carbohydrate metabolization although more studies are required to be conducted on the issue.
The mechanisms related to STZ induced bradycardia in diabetic rats may include dysfunction of intrinsic and/or extrinsic control mechanisms to the heart (3). Reduction in the heart rate following STZ injection in isolated perfused hearts shows defect in intrinsic mechanisms, while that in conscious rats shows defect in intrinsic and/or extrinsic mechanisms that control the heart rate (3). Studies on isolated cardiac preparation indicated that basal spontaneous pacemaker rate was reduced by STZ. Thus, the reduced rate may reflect changes in electrophysiological properties of the sino-atrial node, such as alteration in maximum diastolic potential or the threshold or slope of diastolic depolarization (31). The observed bradycardia could also be mediated in part by alteration in autonomic nervous system; an increase in vagal or a decline in sympathetic tone would diminish heart rate. Previous results suggested that both sympathetic and parasympathetic tone to the heart were reduced significantly in diabetic animals (31, 32). The individuals with parasympathetic dysfunction have a high resting heart rate most likely because of vagal neuropathy that results in unopposed increased sympathetic tone. Moreover, combined parasympathetic - sympathetic dysfunction causes slower heart rates. Yet, advanced nerve dysfunction fixes heart rate (33). Additionally, increased expression of inducible Nitric Oxide Synthase (iNOS) and oxidative stress by chronic diabetes may produce peroxynitrite/nitrotyrosine and cause nitrosative stress leading to cardiovascular depression, bradycardia, and hypotension in STZ-induced diabetic rats (34).
The decrease in blood pressure may be explained by a study by Jackson and Carrier (35). They suggested that the reduction in arterial pressure may be the result of a decreased cardiac output in diabetic sedentary rats due to hypovolemia caused by hyperglycemicosmotic diuresis. Furthermore, increased parasympathetic nervous system activity can cause hypotension in the diabetic group although De Angelis (36) demonstrated a decrease in vagal function suggesting that changes in arterial pressure are not related to an increase in parasympathetic activity. The hemodynamic effect could be associated with the rapid and steady increase in plasma concentrations of nitric oxide, one of the most potent endothelium- derived relaxing factors (37,38). Although exercise or grape seed could not significantly increase arterial pressure, the combination of exercise and grape seed extract attenuated the STZ- induced hypotension and improved it toward the values observed in the controls. These protective effects may be attributable to the improvement of nitrosative stress (34), amelioration of diabetic-induced oxidant/antioxidant levels (39), changes in peripheral resistance (40), a better ventricular contractility, enhanced resting HR (8, 36), improvement of glucose homeostasis (36), arterial compliance improvement (41), and conduit arterial elasticity (42).
In conclusion, the study findings indicated that STZ-induced diabetes significantly reduced body weight, high density lipoproteins, heart rate, and SBP and increased total cholesterol, triglyceride, low density lipoprotein, and very low density lipoprotein. Moreover, grape seed extract combined with exercise training had a more significant improving effect on theplasma lipid profile, body weight, heart rate, and SBP compared to exercise training or grape seed extract alone. Thus, it may constitute a convenient and inexpensive therapeutic approach to some diabetic complications.
Acknowledgments
This paper was extracted from the Ph.D. thesis written by Hassan Ali Abedi. The study was financially supported by Ahvaz Jundishapur University of Medical Sciences (grant No. PRC-66) and was conducted in Physiology Research Center. Hereby, the authors would like to thank Dr. EsrafilMansoori and all the staff of Physiology Research Center of Ahvaz Jundishapur University of Medical Sciences for their cooperation in the research.
Footnotes
Implication for health policy/ practice/ research/ medical education:Grape seed extract as an antioxidant combined with exercise training had more beneficial effects than each one alone on complications of STZ induced diabetes, such as disturbances in lipid profile, loss of weight, bradycardia, and hypotension.
Authors’ Contribution: Mohammad Badavi: Study concept and design, Analysis and interpretation of the data, Critical revision of the manuscript for important intellectual content, Statistical analysis; Hassan Ali Abedi: Study concept and design, Analysis and interpretation of the data, Drafting of the manuscript, Critical revision of the manuscript for important intellectual content; Mahin Dianat: Study concept and design, Critical revision of the manuscript for important intellectual content; Ali Reza Sarkaki: Study concept and design, Critical revision of the manuscript for important intellectual content, Drafting of the manuscript.
Financial Disclosure: The authors declare that they have no conflicts of interest.
Funding/Support: This research was supported by the Research Affaires of Ahwaz Jundishapour University of Medical Sciences (grant No. PRC-66) and is a part of the Ph.D. thesis written by Hassan Ali Abedi, Ph.D. student of physiology.
References
- 1.Rao VS, Santos FA, Silva RM, Teixiera MG. Effects of nitric oxide synthase inhibitors and melatonin on the hyperglycemic response to streptozotocin in rats. Vascul Pharmacol. 2002;38(3):127–30. doi: 10.1016/s1537-1891(02)00212-4. [DOI] [PubMed] [Google Scholar]
- 2.Dall’ago P, D’Agord Schaan B, da Silva VO, Werner J, da Silva Soares PP, de Angelis K, et al. Parasympathetic dysfunction is associated with baroreflex and chemoreflex impairment in streptozotocin-induced diabetes in rats. Auton Neurosci. 2007;131(1-2):28–35. doi: 10.1016/j.autneu.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Howarth FC, Jacobson M, Shafiullah M, Adeghate E. Long-term effects of streptozotocin-induced diabetes on the electrocardiogram, physical activity and body temperature in rats. Exp Physiol. 2005;90(6):827–35. doi: 10.1113/expphysiol.2005.031252. [DOI] [PubMed] [Google Scholar]
- 4.Jafarnejad A, Bathaie SZ, Nakhjavani M, Hassan MZ. Investigation of the mechanisms involved in the high-dose and long-term acetyl salicylic acid therapy of type I diabetic rats. J Pharmacol Exp Ther. 2008;324(2):850–7. doi: 10.1124/jpet.107.130914. [DOI] [PubMed] [Google Scholar]
- 5.Grijalva J, Hicks S, Zhao X, Medikayala S, Kaminski PM, Wolin MS, et al. Exercise training enhanced myocardial endothelial nitric oxide synthase (eNOS) function in diabetic Goto-Kakizaki (GK) rats. Cardiovasc Diabetol. 2008;7:34. doi: 10.1186/1475-2840-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansakul C, Hirunpan P. Effects of exercise training on responsiveness of the mesenteric arterial bed to phenylephrine and KCl in male rats. Br J Pharmacol. 1999;127(7):1559–66. doi: 10.1038/sj.bjp.0702697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riddell MC, Iscoe KE. Physical activity, sport, and pediatric diabetes. Pediatr Diabetes. 2006;7(1):60–70. doi: 10.1111/j.1399-543X.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 8.Harthmann AD, De Angelis K, Costa LP, Senador D, Schaan BD, Krieger EM, et al. Exercise training improves arterial baro- and chemoreflex in control and diabetic rats. Auton Neurosci. 2007;133(2):115–20. doi: 10.1016/j.autneu.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Keaney JF, Creager MA. Oral antioxidant therapy improves endothelial function in Type 1 but not Type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2003;285(6):H2392–8. doi: 10.1152/ajpheart.00403.2003. [DOI] [PubMed] [Google Scholar]
- 10.Cameron NE, Jack AM, Cotter MA. Effect of alpha-lipoic acid on vascular responses and nociception in diabetic rats. Free Radic Biol Med. 2001;31(1):125–35. doi: 10.1016/s0891-5849(01)00564-0. [DOI] [PubMed] [Google Scholar]
- 11.Inkster ME, Cotter MA, Cameron NE. Treatment with the xanthine oxidase inhibitor, allopurinol, improves nerve and vascular function in diabetic rats. Eur J Pharmacol. 2007;561(1-3):63–71. doi: 10.1016/j.ejphar.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 12.Palmer AM, Thomas CR, Gopaul N, Dhir S, Anggard EE, Poston L, et al. Dietary antioxidant supplementation reduces lipid peroxidation but impairs vascular function in small mesenteric arteries of the streptozotocin-diabetic rat. Diabetologia. 1998;41(2):148–56. doi: 10.1007/s001250050883. [DOI] [PubMed] [Google Scholar]
- 13.Vieira da Costa VA, Vianna LM. Effect of alpha-tocopherol supplementation on blood pressure and lipidic profile in streptozotocin-induced diabetes mellitus in spontaneously hypertensive rats. Clin Chim Acta. 2005;351(1-2):101–4. doi: 10.1016/j.cccn.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Shi J, Yu J, Pohorly JE, Kakuda Y. Polyphenolics in grape seeds-biochemistry and functionality. J Med Food. 2003;6(4):291–9. doi: 10.1089/109662003772519831. [DOI] [PubMed] [Google Scholar]
- 15.Badavi M, Mehrgerdi FZ, Sarkaki A, Naseri MK, Dianat M. Effect of grape seed extract on lead induced hypertension and heart rate in rat. Pak J Biol Sci. 2008;11(6):882–7. doi: 10.3923/pjbs.2008.882.887. [DOI] [PubMed] [Google Scholar]
- 16.Cotter MA, Jack AM, Cameron NE. Effects of the protein kinase C beta inhibitor LY333531 on neural and vascular function in rats with streptozotocin-induced diabetes. Clin Sci (Lond). 2002;103(3):311–21. doi: 10.1042/cs1030311. [DOI] [PubMed] [Google Scholar]
- 17.Badavi M, Abedi H, Dianat M, Sarkaki A. Dysfunction of Mesenteric Vascular Bed in STZ-induced Diabetic Rats. International Journal of Pharmacology. 2011;7(8):813–20. [Google Scholar]
- 18.Modi K, Santani DD, Goyal RK, Bhatt PA. Effect of coenzyme Q10 on catalase activity and other antioxidant parameters in streptozotocin-induced diabetic rats. Biol Trace Elem Res. 2006;109(1):25–34. doi: 10.1385/BTER:109:1:025. [DOI] [PubMed] [Google Scholar]
- 19.Balkis Budin S, Othman F, Louis SR, Abu Bakar M, Radzi M, Osman K, et al. Effect of alpha lipoic acid on oxidative stress and vascular wall of diabetic rats. Rom J Morphol Embryol. 2009;50(1):23–30. [PubMed] [Google Scholar]
- 20.Asha Devi S, Prathima S, Subramanyam MV. Dietary vitamin E and physical exercise: II. Antioxidant status and lipofuscin-like substances in aging rat heart. Exp Gerontol. 2003;38(3):291–7. [PubMed] [Google Scholar]
- 21.Wang JS, Lin CC, Chen JK, Wong MK. Role of chronic exercise in decreasing oxidized LDL-potentiated platelet activation by enhancing platelet-derived no release and bioactivity in rats. Life Sci. 2000;66(20):1937–48. doi: 10.1016/s0024-3205(00)00519-1. [DOI] [PubMed] [Google Scholar]
- 22.de Lemos ET, Reis F, Baptista S, Pinto R, Sepodes B, Vala H, et al. Exercise training is associated with improved levels of C-reactive protein and adiponectin in ZDF (type 2) diabetic rats. Med Sci Monit. 2007;13(8):BR168–74. [PubMed] [Google Scholar]
- 23.Hasliza A, Noordin M, Goh Y. Effects of exercise and dietary polyunsaturated fatty acid on blood lipid profiles of streptozotocin-induced diabetes in rats. Pertanika Journal of Tropical Agricultural Science. 2011;34(1):151–5. [Google Scholar]
- 24.Ravi Kiran T, Subramanyam MV, Prathima S, Asha Devi S. Blood lipid profile and myocardial superoxide dismutase in swim-trained young and middle-aged rats: comparison between left and right ventricular adaptations to oxidative stress. J Comp Physiol B. 2006;176(8):749–62. doi: 10.1007/s00360-006-0096-5. [DOI] [PubMed] [Google Scholar]
- 25.Burneiko RC, Diniz YS, Galhardi CM, Rodrigues HG, Ebaid GM, Faine LA, et al. Interaction of hypercaloric diet and physical exercise on lipid profile, oxidative stress and antioxidant defenses. Food Chem Toxicol. 2006;44(7):1167–72. doi: 10.1016/j.fct.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Ramachandran S, Asokkumar K, Uma Maheswari M, Ravi TK, Sivashanmugam AT, Saravanan S, et al. Investigation of Antidiabetic, Antihyperlipidemic, and In Vivo Antioxidant Properties of Sphaeranthus indicus Linn. in Type 1 Diabetic Rats: An Identification of Possible Biomarkers. Evid Based Complement Alternat Med. 2011;2011 doi: 10.1155/2011/571721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owu DU, Antai AB, Udofia KH, Obembe AO, Obasi KO, Eteng MU. Vitamin C improves basal metabolic rate and lipid profile in alloxan-induced diabetes mellitus in rats. J Biosci. 2006;31(5):575–9. doi: 10.1007/BF02708409. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki H, Nakamura J, Koh N, Hamada Y, Hara T, Chaya S, et al. Effect of vitamin E and allylamine on the proliferation of cultured aortic smooth muscle cells from streptozotocin-induced diabetic rats. Life Sci. 1999;64(25):2317–25. doi: 10.1016/s0024-3205(99)00185-x. [DOI] [PubMed] [Google Scholar]
- 29.Tebib K, Besancon P, Rouanet JM. Dietary grape seed tannins affect lipoproteins, lipoprotein lipases and tissue lipids in rats fed hypercholesterolemic diets. J Nutr. 1994;124(12):2451–7. doi: 10.1093/jn/124.12.451. [DOI] [PubMed] [Google Scholar]
- 30.Chen V, Ianuzzo CD. Dosage effect of streptozotocin on rat tissue enzyme activities and glycogen concentration. Can J Physiol Pharmacol. 1982;60(10):1251–6. doi: 10.1139/y82-183. [DOI] [PubMed] [Google Scholar]
- 31.Hicks KK, Seifen E, Stimers JR, Kennedy RH. Effects of streptozotocin-induced diabetes on heart rate, blood pressure and cardiac autonomic nervous control. J Auton Nerv Syst. 1998;69(1):21–30. doi: 10.1016/s0165-1838(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 32.Maeda CY, Fernandes TG, Lulhier F, Irigoyen MC. Streptozotocin diabetes modifies arterial pressure and baroreflex sensitivity in rats. Braz J Med Biol Res. 1995;28(4):497–501. [PubMed] [Google Scholar]
- 33.Maser RE, Lenhard MJ. Cardiovascular autonomic neuropathy due to diabetes mellitus: clinical manifestations, consequences, and treatment. J Clin Endocrinol Metab. 2005;90(10):5896–903. doi: 10.1210/jc.2005-0754. [DOI] [PubMed] [Google Scholar]
- 34.Nagareddy PR, Xia Z, MacLeod KM, McNeill JH. N-acetylcysteine prevents nitrosative stress-associated depression of blood pressure and heart rate in streptozotocin diabetic rats. J Cardiovasc Pharmacol. 2006;47(4):513–20. doi: 10.1097/01.fjc.0000211744.93701.25. [DOI] [PubMed] [Google Scholar]
- 35.Jackson CV, Carrier GO. Influence of short-term experimental diabetes on blood pressure and heart rate in response to norepinephrine and angiotensin II in the conscious rat. J Cardiovasc Pharmacol. 1983;5(2):260–5. doi: 10.1097/00005344-198303000-00016. [DOI] [PubMed] [Google Scholar]
- 36.De Angelis KL, Oliveira AR, Dall'Ago P, Peixoto LR, Gadonski G, Lacchini S, et al. Effects of exercise training on autonomic and myocardial dysfunction in streptozotocin-diabetic rats. Braz J Med Biol Res. 2000;33(6):635–41. doi: 10.1590/s0100-879x2000000600004. [DOI] [PubMed] [Google Scholar]
- 37.Benrezzak O, Marois H, Daull P, Blouin A, Lepage R, Sirois P, et al. Profile of endothelin isopeptides and markers of oxidative stress alongside the onset of streptozotocin-type I diabetes in rats. J Cardiovasc Pharmacol. 2004;44 Suppl 1:S168–72. doi: 10.1097/01.fjc.0000166244.51239.9e. [DOI] [PubMed] [Google Scholar]
- 38.Hopfner RL, McNeill JR, Gopalakrishnan V. Plasma endothelin levels and vascular responses at different temporal stages of streptozotocin diabetes. Eur J Pharmacol. 1999;374(2):221–7. doi: 10.1016/s0014-2999(99)00316-7. [DOI] [PubMed] [Google Scholar]
- 39.Chakraphan D, Sridulyakul P, Thipakorn B, Bunnag S, Huxley VH, Patumraj S. Attenuation of endothelial dysfunction by exercise training in STZ-induced diabetic rats. Clin Hemorheol Microcirc. 2005;32(3):217–26. [PubMed] [Google Scholar]
- 40.Brands MW, Cloud LJ. Control of arterial pressure by angiotensin II and nitric oxide at the onset of diabetes. Am J Hypertens. 2003;16(7):600–3. doi: 10.1016/s0895-7061(03)00902-6. [DOI] [PubMed] [Google Scholar]
- 41.Mourot L, Boussuges A, Campo P, Maunier S, Debussche X, Blanc P. Cardiovascular rehabilitation increase arterial compliance in type 2 diabetic patients with coronary artery disease. Diabetes Res Clin Pract. 2009;84(2):138–44. doi: 10.1016/j.diabres.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Loimaala A, Groundstroem K, Rinne M, Nenonen A, Huhtala H, Parkkari J, et al. Effect of long-term endurance and strength training on metabolic control and arterial elasticity in patients with type 2 diabetes mellitus. Am J Cardiol. 2009;103(7):972–7. doi: 10.1016/j.amjcard.2008.12.026. [DOI] [PubMed] [Google Scholar]