Abstract
Preterm birth (PTB) is the leading cause of neonatal mortality and morbidity. Despite the current treatment procedures, the incidence of PTB has not changed in the past thirty years. Incomplete understanding of the biological and patophysiological mechanisms underlying preterm delivery is the major obstacle to prevent PTB. Cervical ripening is necessary for vaginal delivery and understanding of preterm cervical ripening is required for developing new treatment strategies. Several important substances such as HMGB1 and its receptors, CRH and its receptors and numerous cytokines are localized in the cervix and undergo distinct changes in labour. Other important molecules, such as CRH, CRH-BP, CRH-R1, CRH-R2, HMGB1, TLR2, TLR4, IL-10, IL-12, are localized in the cervical epithelium, also indicating their role in the process of cervical ripening during labour. Furthermore, CRH stimulates IL-8 secretion from both preterm and term cervical fibroblasts. Recent studies from our group show that major inflammatory changes occur in the cervix at labour irrespective of gestational age. This indicates that cervical ripening at both term and preterm is an inflammatory process even if no infection is present. However, preterm cervical ripening still entails some differences from term cervical ripening, for example in the down-regulation of mRNA expression of Toll-like receptors (TLR-2 and TLR-4) and IL-12, higher levels of IL-10 in cervical epithelium, and presents different secretion patterns of cervical fibroblasts. Moreover, preterm cervical ripening, like preterm delivery itself, is a multifactorial disorder with pathways which are partly different from those involved in PPROM and infected preterm labour.
Keywords: Cervical ripening, cytokines, CRH, cervical fibroblasts, HMGB1, preterm labour
Introduction
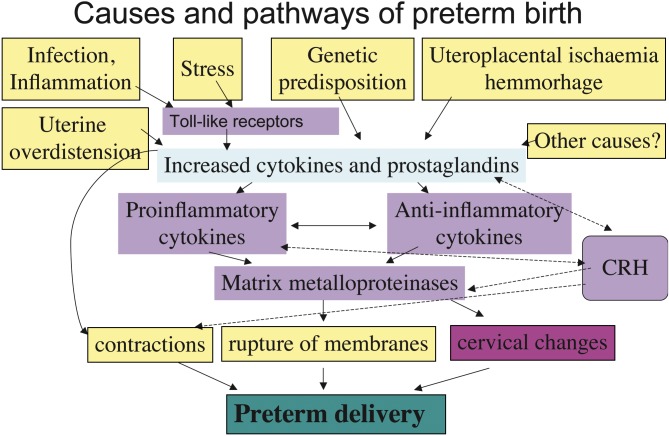
Preterm birth can be divided into elective deliveries due to maternal or foetal indications, and spontaneous preterm birth with or without preterm premature rupture of membranes (PPROM) (Goldenberg et al., 2008). Multiple causes i.e. infection, uteroplacental ischemia or haemorrhage, stress, endocrine factors, immunologically mediated processes may be associated with preterm birth (Challis et al., 2009) (Fig. 1).
Fig. 1. Causes and pathways of preterm birth.
Current tocolytic therapies have not reduced the incidence of preterm birth. Thus there is a need for new strategies for treatment and prevention of preterm birth. The current treatment strategies focus upon tocolytic agents and upon diminishing the uterine contractility. However, a preterm vaginal birth first requires cervical ripening after which myometrial contractions follow. Indeed, even painfully strong contractions in combination with stiff and closed cervix do not result in preterm delivery. In order to develop new strategies to diminish the preterm birth rate, it is therefore of major importance to improve our understanding of the physiology of preterm cervical ripening.
Composition of Cervix uteri
Danforth et al. (1947) stated that the human cervix consists mainly of fibrous connective tissue. The non-pregnant cervix is composed of 85% extracellular matrix (ECM) and 6-10% of muscle fibers (Rorie and Newton, 1967). The cervical ECM consists of collagens, proteoglycans, hyaluronan, and glycoproteins. Collagen I and collagen III are the major types and the dominating proteoglycan in non-pregnant cervix uteri is decorin. Fibromodulin and biglycan, and also large proteoglycans such as versican and heparan sulfate proteoglycan are present (Uldbjerg et al., 1983b; Norman et al., 1991; Westergren-Thorsson et al., 1998).
Cervical ripening at term
Cervical softening during pregnancy is characterized by a gradual decrease in the collagen concentration. Simultaneously the collagen extractability increases, suggesting changes in the organization of collagen fibrils (Uldbjerg et al., 1983a; Granstrom et al., 1989). The duration of cervical dilatation during labour correlates well with the collagen concentration and its solubility (Ekman et al., 1986). Immediately after vaginal delivery at term the mRNA levels for collagen I and III decrease by up to 60% compared to those in the non- pregnant cervix, but during involution (2-4 days after delivery) the levels are increased 2.5-fold and 3.5-fold respectively (Westergren-Thorsson et al., 1998).
The decorin concentration is decreasing with 50% until final ripening while that of versican, biglycan and the heparan sulfate proteoglycans increases (Norman et al. 1993; Westergren-Thorsson et al. 1998). Versican can attract water and bind hyaluronan (Wu et al., 2005), resulting in disintegration of the collagen bundles and a change in the physical properties to produce a soft and elastic tissue, thus facilitating cervical dilatation.
Gonadal steroids
Estrogen, progesterone and insulin-like growth factor-I (IGF-I) are involved in cervical ripening (Stjernholm et al., 1996; Stjernholm et al., 1997; Wang et al., 2001). ERα mRNA decreases in the ripe cervix at delivery, while ERβ mRNA levels are increased in the term pregnant cervix not in labour (Wang et al., 2001). The ERβ antigen is also co-localized with leukocyte markers in cervix (Stygar et al., 2001).
Cervical ripening – an inflammatory reaction
Further, the process of cervical ripening at labour can be regarded as an inflammatory reaction since the levels of IL-6, IL-8 increase at term labour (Sennstrom et al., 2000). This process is also associated with cervical leukocyte invasion (Young et al., 2002; Osman et al., 2003).
Cytokines recruit activated cells, which in turn secrete degradative enzymes such as matrix metalloproteinases (MMPs). Thus, increased levels of MMP-1, MMP-2, MMP-3, MMP-8 and MMP-9 have been observed during pregnancy and at the final cervical ripening (Stygar et al., 2002; Sennstrom et al., 2003).
Fibroblasts
Fibroblasts play a crucial role in the remodelling of the extracellular matrix (Larsen et al. 2006). Activated fibroblasts produce ECM components, cytokines and matrix metalloproteinases (Malmstrom et al., 2007; Akerud et al., 2008). Cervical fibroblast cultures established from the biopsies from non-pregnant women, term pregnant women at caesarean section before the onset of labour or parturient women, present different and stable phenotypes (Malmstrom et al., 2007). There is a decrease in proteoglycan secretion and an increase in IL-6, IL-8, MMP-1, MMP-3 production in the cultures from parturient donors (Malmstrom et al., 2007; Akerud et al., 2008).
Preterm cervical ripening
Prostaglandins
Prostaglandins, synthesized in foetal membranes and deciduas play an important role in parturition and cervical ripening. Local application of prostaglandin-E2 (PGE2) has become a routine treatment in inducing cervical ripening and labour both at term and at preterm (Ekman et al., 1983; Abelin Tornblom et al., 2002). Cervical ripening at preterm as well as at term is associated with decreased degradation of prostaglandins (Tornblom et al., 2004).
NO
Nitric oxide (NO) has been suggested as an active mediator in cervical ripening (Chwalisz and Garfield, 1998), and NO-donors induce ripening of the human cervix (Thomson et al., 1997). The expression of nitric oxide synthase (NOS) isoforms increases in the cervix in late pregnancy and parturition (Ledingham et al., 2000). Further, preterm labour is associated with higher mRNA expression of NOS isoforms in the cervix (Tornblom et al., 2005b). Cervical fluid nitric oxide metabolite (NOx) levels rise during labour, nitric oxide donor administration or cervical manipulation and are significantly related to cervical ripening. (Vaisanen-Tommiska et al., 2003).
Corticotropin-releasing hormone, CRH
CRH, also termed corticotropin-releasing factor (CRF) is the principal regulator of the hypothalamic-pituitary-adrenal (HPA) axis (Hillhouse and Grammatopoulos 2006).
CRH, CRH-BP, CRH-R1 and CRH-R2 have been identified at both mRNA and protein level in human placenta, deciduas, foetal membranes, endometrium and myometrium (Petraglia et al., 1992; Petraglia et al., 1993; Hillhouse and Grammatopoulos, 2002; Sehringer et al., 2004). Furthermore, CRH increases MMP-9 protein secretion by cultured cells from placenta and foetal membranes (Li and Challis, 2005). In addition, several studies have shown that CRH can stimulate the production of cytokines in different types of cells (Wang et al., 2007).
Klimaviciute et al (2006) found differences in CRH-BP, CRH-R1 and CRH-R2 mRNA expression in cervical tissue and myometrium.These changes seem to be related to pregnancy and labour but not to gestational age. CRH-BP, CRH-R1, CRH-R2 are down regulated during pregnancy. CRH-R2 in cervix and myometrium and CRH-BP in cervix are even more down regulated during labour.With immunohistochemistry CRH was localized in cervical epithelium with the highest concentration at term, while CRH-BP is decreased at labour, which shows possible involvement of CRH in cervical ripening (Klimaviciute et al., 2006).
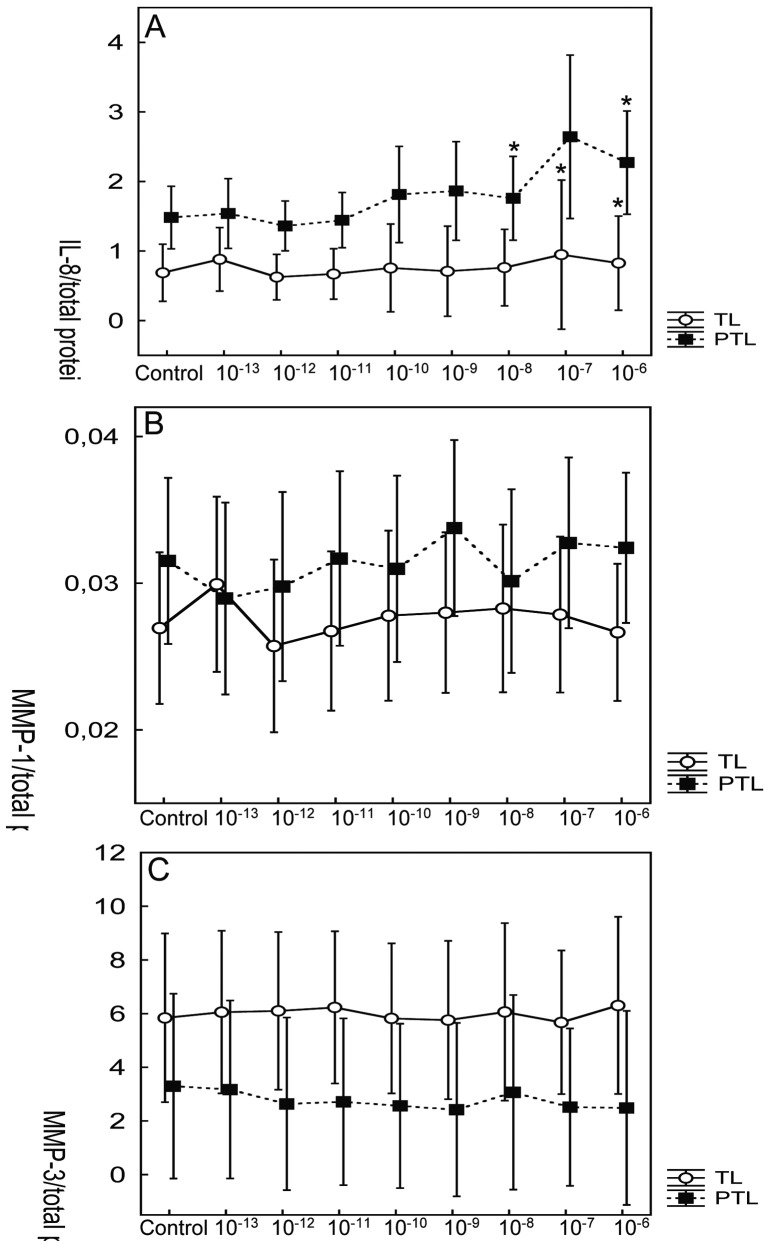
In vitro CRH stimulates IL-8 production in the cultures of preterm and term cervical fibroblasts, but doesn’t seem to have effect on the secretion of MMP-1 and MMP-3 (Dubicke et al., 2008) (Fig. 2).
Fig. 2. Effect of CRH on secretion of IL-8 (A), MMP-1 (B) and MMP-3 (C) by preterm (PTL) and term (TL) cervical fibroblasts. Different concentrations (M) of CRH indicated on x-axis. Data presented as mean ± SD. * p < 0.05 (compared with control). The secretion of IL-8 and MMP-1 was significantly higher in preterm cervical fibroblasts (p < 0.001 and p < 0.05, respectively). MMP-3 secretion was significantly higher in term cervical fibroblasts (p < 0.001).
High-mobility group box protein 1 (HMGB1)
HMGB1 is expressed by almost all cells (Bianchi and Manfredi, 2007). In 1999 it was discovered that activated macrophages secrete HMGB1 as a delayed mediator of inflammation (Wang et al., 1999). HMGB1 has also important extracellular cytokine-like functions mediating the late response to infection, injury and inflammation (Lotze and Tracey, 2005). HMGB1 induces NF-kB activation (Lotze and Tracey, 2005) and stimulates pro-inflammatory cytokine synthesis (Andersson et al., 2000).
Human term placenta expresses HMGB1. Labour does not influence its placental expression, although a tendency towards higher expression of extranuclear HMGB1 in placentas with preeclampsia has been observed (Holmlund et al., 2007). HMGB1 is expressed in human foetal membranes at term pregnancy (Ticconi et al., 2007).
A receptor for advanced glycation end-products (RAGE) and Toll-like receptor 2 (TLR2) and TLR4 are involved in HMGB1-mediated signalling (Bianchi and Manfredi, 2007).
Receptor for advanced glycation end-products (RAGE)
The receptor for HMGB1 is RAGE, a multiligand receptor of the immunoglobulin family. The soluble form of RAGE (sRAGE) receptor is considered to act as a regulator/inhibitor of HMGB1 action (Lotze and Tracey, 2005). RAGE is expressed in trophoblasts of first-trimester human chorionic villi from healthy women (Konishi et al., 2004). Human term placenta expresses RAGE, but labour does not influence this expression (Holmlund et al., 2007). RAGE staining is especially increased in the vasculature of myometrium in women with preeclampsia (Cooke et al., 2003). There are contradictory results concerning intra-amniotic infection/inflammation and amniotic fluid concentrations of sRAGE (Buhimschi et al., 2007; Romero et al., 2008). Women with threatening preterm birth had significantly higher serum sRAGE concentrations than healthy pregnant women.
Toll-like receptors (TLRs)
These receptors are expressed on different cell types belonging to the innate, adaptive immune system and non-immunological cells. TLR2 and TLR4 were the first TLRs to be demonstrated at the protein level in human term placenta (Holmlund et al., 2002). Expression of all ten TLRs has been described in human placenta and studied in pregnancy and in labour (Patni et al., 2007). Greater mRNA expression is seen of all TLR genes in the placenta during labour (Patni et al., 2009). TLRs in the cervix have been less extensively studied. In pregnant mice, increased mRNA expression of TLR2, TLR3 and TLR4 is seen (Gonzalez et al., 2007). TLRs 1 to 6 mRNA and the protein of TLR2 and TLR4 have been found in human non-pregnant cervix (Pioli et al., 2004). Up-regulation of mRNA expression of TLR2 and TLR4, but down-regulation of TLR3 and TLR5, were observed in the cervix during labour with microarray analysis. The down-regulation of TLR3 and TLR5, but not up-regulation of TLR2 and TLR4 was confirmed with real-time RT-PCR (Hassan et al., 2006).
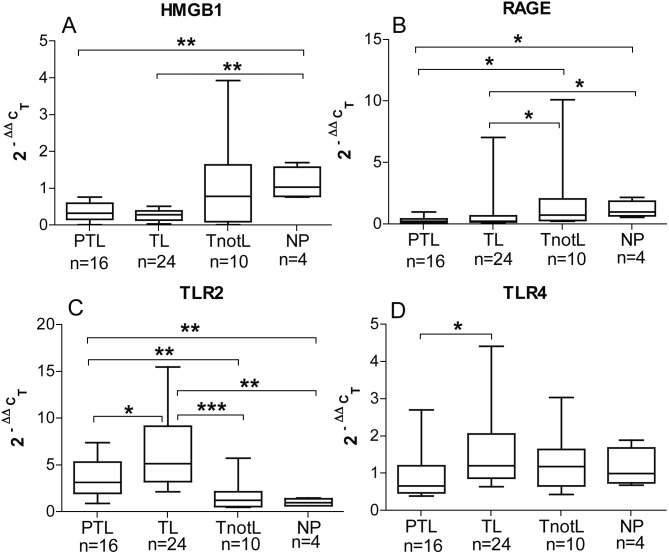
Dubicke et al found HMGB1, RAGE, TLR2 and TLR4 to be localized and to have an mRNA expression in the human cervix (Dubicke et al., 2010a) There was more extranuclear HMGB1 in the cervical epithelium and stroma in the preterm and term labour groups (Fig. 3). Extranuclear expression of HMGB1 during labour suggests a possible role of HMGB1 during the process of cervical ripening. There was a lower staining and tendency to lower mRNA expression for HMGB1 in the labouring groups. mRNA for RAGE was down regulated in the labouring groups, but there was higher concentration of soluble RAGE in labour. There was an up-regulation of TLR2 mRNA expression in labour. On the contrary, there were lower protein levels of TLR2 and TLR4 in labour (Fig. 4). The preterm group showed lower mRNA expression for TLR2 and TLR4 than term labour group (Fig. 4, C-D). Changes in mRNA expression of HMGB1, TLR2 and TLR4 in preterm labour, point towards possible differences in the mechanism of cervical ripening at preterm and term (Dubicke et al., 2010a).
Fig. 3. The immunohistochemical staining of HMGB1 in the squamous epithelium (column on the left) and in the stroma (column on the right) in the cervical tissue.
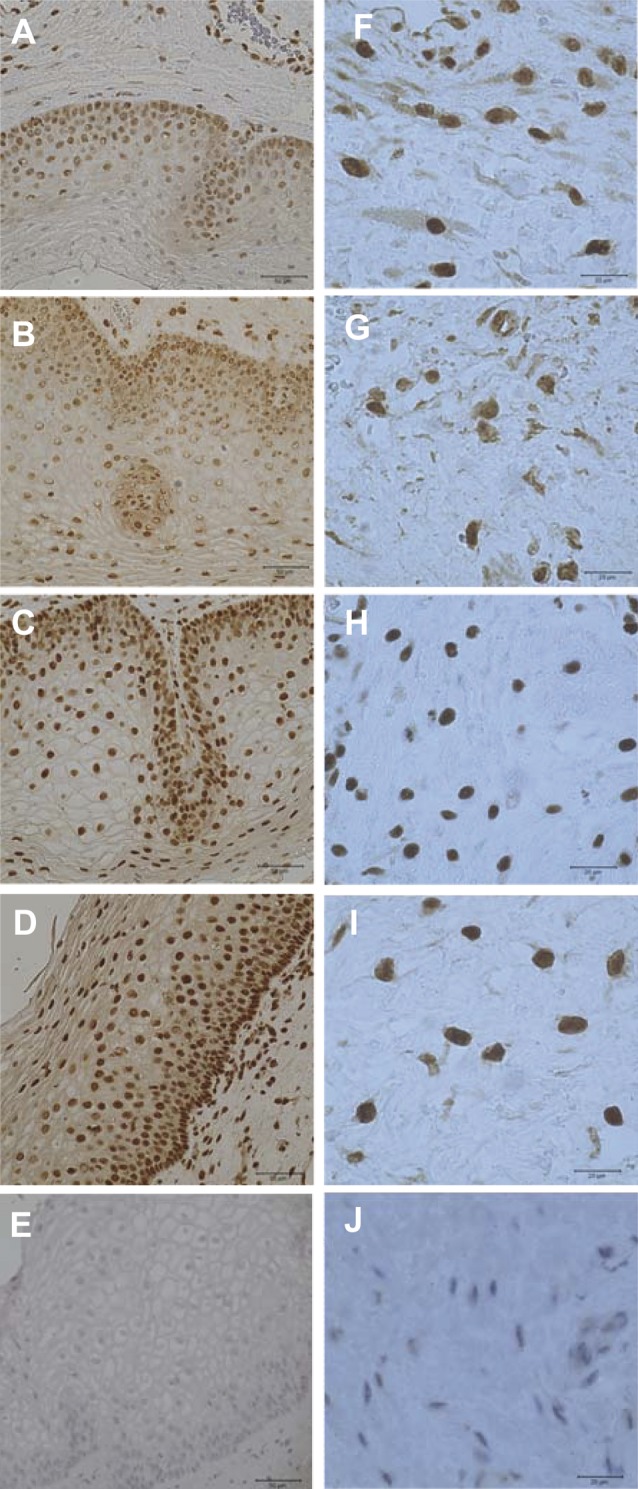
Fig. 4. mRNA expression of HMGB1 (A), RAGE (B), TLR2 (C) and TLR4 (D) in cervical tissue. Preterm labour (PTL), term labour (TL), term not in labour (TnotL), non-pregnant (NP). The box represents 25-75% of all data with the line through the box representing the median value. The whiskers extend to the range (min/max). *p < 0.05, **p < 0.01, ***p < 0.001.
Cytokines
Tornblom et al found in cervical biopsies obtained at preterm and term pregnancies the protein concentrations of IL-8, IL-6, and MCP-1 to be significantly increased during labour compared to non-labouring groups, whereas no changes were observed for RANTES and TNF-α. The mRNA levels of representative cytokines such as IL-8 and MCP-1 increased significantly whereas RANTES mRNA expression remained unchanged. WBC and CRP were significantly higher in the labouring groups as compared to groups not in labour. For neither of the analysed cytokines, WBC or CRP levels were there any changes between preterm and term groups (Tornblom et al., 2005a).
The role of IL-12 and IL-18 during pregnancy and parturition has attracted interest recently. IL-12 and IL-18 are important in regulating natural killer cell activities in early pregnancy, and are considered important for reproductive success. Higher IL-12 levels in mid-pregnancy are associated with preterm delivery with chorioamnionitis before 35 weeks of gestation (Gargano et al., 2008). Patients having low IL-18 and high IL-12 had a twofold-increased risk of delivering before 34 weeks of gestation (Ekelund et al., 2008). When infection is present in preterm labour, higher levels of IL-18 are found in amniotic fluid (Jacobsson et al., 2003). In animal studies, the frequency of foetal loss was significantly higher in IL-18 knock-out mice and in mice receiving IL-18 binding protein than in wild-type controls. IL-18 knock-out mice also present with elevated IL-12 expression in uterine tissues (Wang et al., 2006).
IL-10 is the most extensively studied of the anti-inflammatory cytokines. It decreases the production of pro-inflammatory cytokines such as IL-8, IL-6, TNF-α, IL-1β (Fortunato et al., 1998; Sato et al., 2003), matrix metalloproteinases (Fortunato et al., 2001) and prostaglandin E2 (Brown et al., 2000) in LPS-stimulated foetal membranes. The ratio of IL-10/IL-8 decreases in cervical secretions with advancing gestational age (Mondestin-Sorrentino et al., 2007). IL-10 was significantly reduced in placental tissues in chorioamnionitis-associated preterm labour as well as in term labour, compared with second-trimester normal pregnancy samples obtained from elective terminations (Hanna et al., 2006). However, patients who delivered preterm without intra-amniotic infection, had a significantly higher median amniotic fluid IL-10 concentration than those who delivered at term (Gotsch et al., 2008).
IL-4 and IL-13 have been less studied in pregnancy and labour. IL-4 is higher in cervical secretions in women with normal pregnancies not in labour compared to women with preterm labour (Hollier et al., 2004). Higher anti-inflammatory/pro-inflammatory cytokine ratio in cervical secretions during early pregnancy is associated with a higher risk of subsequent spontaneous preterm birth (Simhan and Krohn, 2009). In addition, IL-4, IL-10 and IL-13 gene polymorphism is associated with preterm delivery (Annells et al., 2004; Kerk et al., 2006; Heinzmann et al., 2009).
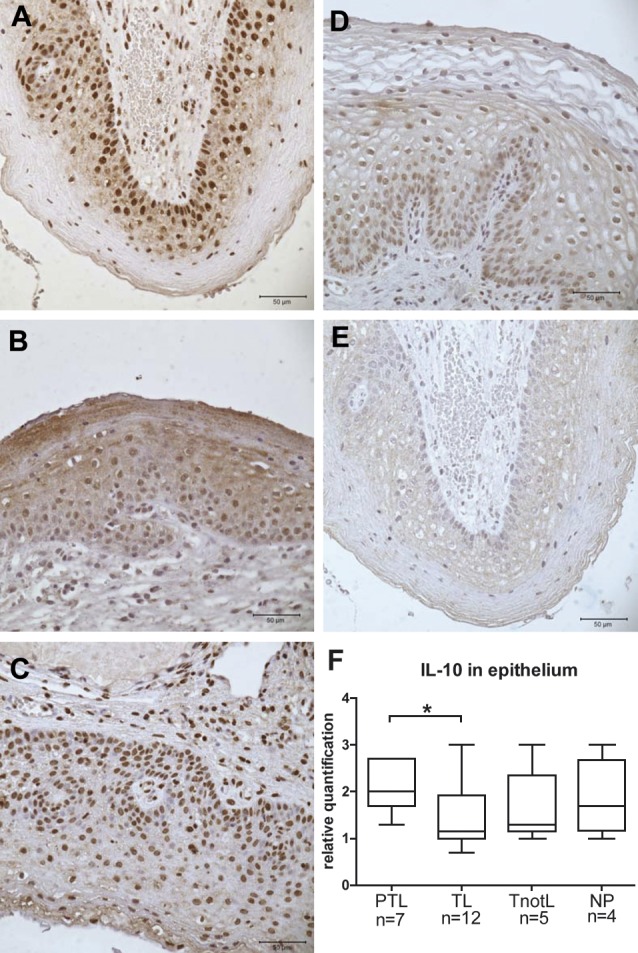
Dubicke et al. (2010b) reported an up-regulation of mRNA of IL-10, IL-1α, IL-1β, but down-regulation of IL-12 and IL-18 in the cervix of the labouring groups compared to the non- labouring group irrespective of gestational age. IL-4 mRNA was detected more frequently in the preterm than in the term labour group. IL-13 was detected more frequently in the labouring groups. IL-12 mRNA expression was lower in the preterm labour group than in the term labour group. The protein levels of IL-10 remained the same in all the groups, IL-4 and IL-12 decreased, while IL-18 increased in the labouring groups. IL-4 protein levels were significantly higher in the preterm subgroup with bacterial infection compared to non-infected group. IL-10 had higher expression in squamous epithelium at preterm labour than at term (Fig. 5) (Dubicke et al., 2010b).
Fig. 5. Immunohistochemical staining of IL-10 in cervical tissue.
Matrix metalloproteinases (MMPs)
The MMPs have broad and diverse substrate specificity: collagenases (MMP-1, -8 and -13) break down fibrillar and non-fibrillar collagens; stromelysins (MMP-3, -7 and -10) cleave proteoglycans, fibronectin, collagens IV, V and gelatins; gelatinases (MMP-2 and -9) target collagen IV, V, elastin, proteoglycan and fibronectin (Hulboy et al., 1997). Four tissue inhibitors of MMPs (TIMPs) have been described: TIMP-1, TIMP-2, TIMP-3 and TIMP-4. The second group of MMP inhibitors are plasma α-macroglobulins (Hulboy et al., 1997). The levels of MMPs in cervix, lower uterine segment, amniotic fluid, placenta, foetal membranes and maternal plasma increase during labour (Osmers et al., 1995; Stygar et al., 2002; Sennstrom et al., 2003). Also, polymorphism in MMP-1 and MMP-9 genes is associated with PPROM (Ferrand et al., 2002).
In preterm cervical ripening MMP-1, MMP-3 and MMP-9 mRNA expression was up-regulated in labour and a tendency towards higher protein levels of MMP-8 and MMP-9 in labour has been found (Dubicke et al., 2008). A different secretion pattern at preterm and term was registered in vitro in cervical cell culture raised from cervical samples. The secretion of IL-8 and MMP-1 was significantly higher (p < 0.001 and p < 0.05, respectively), but MMP-3 secretion significantly lower in preterm cervical fibroblasts compared to term fibroblasts (p < 0.001) (Dubicke et al., 2008) (Fig. 2).
Preterm cervical ripening
Although it is well established that intrauterine infection can lead to preterm labour, this does not appear to be the major cause of prematurity, since infection has been demonstrated in only 25-40% of preterm births (Slattery and Morrison, 2002; Goldenberg et al., 2008). Parturition itself is an inflammatory process. Inflammatory events can be observed in the myometrium, cervix, foetal membranes and peripheral blood (Tornblom et al., 2005a; Norman et al., 2007; Challis et al., 2009). Recent studies from our group indicate that cervical ripening at both term and preterm is an inflammatory process even if no infection is present (Tornblom et al., 2004; Tornblom et al., 2005a; Dubicke et al., 2008; Dubicke et al., 2010a; Dubicke et al., 2010b). The human cervix is dominated by ECM undergoing a pronounced remodelling both during preterm and term cervical ripening. Thus preterm cervical ripening seems to be a more relevant term than cervical insufficiency involved in preterm birth. The ultimate goal must be to develop new strategies to prevent preterm cervical ripening to reduce the number of spontaneous preterm births.
References
- Abelin Tornblom S, Ostlund E, Granstrom L, et al. Pre-term cervical ripening and labour induction. Eur J Obstet Gynecol Reprod Biol. 2002;104:120–123. doi: 10.1016/s0301-2115(02)00101-x. [DOI] [PubMed] [Google Scholar]
- Akerud A, Dubicke A, Sennstrom M, et al. Differences in heparan sulfate production in cervical fibroblast cultures from women undergoing term and preterm delivery. Acta Obstet Gynecol Scand. 2008;87:1220–1228. doi: 10.1080/00016340802460313. [DOI] [PubMed] [Google Scholar]
- Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annells MF, Hart PH, Mullighan CG, et al. Interleukins-1, -4, -6, -10, tumor necrosis factor, transforming growth factor-beta, FAS, and mannose-binding protein C gene polymorphisms in Australian women: Risk of preterm birth. Am J Obstet Gynecol. 2004;191:2056–2067. doi: 10.1016/j.ajog.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- Brown NL, Alvi SA, Elder MG, et al. The regulation of prostaglandin output from term intact fetal membranes by anti-inflammatory cytokines. Immunology. 2000;99:124–133. doi: 10.1046/j.1365-2567.2000.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhimschi IA, Zhao G, Pettker CM, et al. The receptor for advanced glycation end products (RAGE) system in women with intraamniotic infection and inflammation. Am J Obstet Gynecol. 2007;196:181. doi: 10.1016/j.ajog.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Challis JR, Lockwood CJ, Myatt L, et al. Inflammation and pregnancy. Reprod Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Garfield RE. Nitric oxide as the final metabolic mediator of cervical ripening. Hum Reprod. 1998;13:245–248. doi: 10.1093/humrep/13.2.245. [DOI] [PubMed] [Google Scholar]
- Cooke CL, Brockelsby JC, Baker PN, et al. The receptor for advanced glycation end products (RAGE) is elevated in women with preeclampsia. Hypertens Pregnancy. 2003;22:173–184. doi: 10.1081/PRG-120021068. [DOI] [PubMed] [Google Scholar]
- Danforth DN. The fibrous nature of the human cervix, and its relation to the isthmic segment in gravid and nongravid uteri. Am J Obstet Gynecol. 1947;53:541–557. doi: 10.1016/0002-9378(47)90273-1. [DOI] [PubMed] [Google Scholar]
- Dubicke A, Akerud A, Sennstrom M, et al. Different secretion patterns of matrix metalloproteinases and IL-8 and effect of corticotropin-releasing hormone in preterm and term cervical fibroblasts. Mol Hum Reprod. 2008;14:641–647. doi: 10.1093/molehr/gan060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubicke A, Andersson P, Fransson E, et al. High-mobility group box protein 1 and its signalling receptors in human preterm and term cervix. J Reprod Immunol. 2010a;84:86–94. doi: 10.1016/j.jri.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Dubicke A, Fransson E, Centini G, et al. Pro-inflammatory and anti-inflammatory cytokines in human preterm and term cervical ripening. J Reprod Immunol. 2010b;84:176–185. doi: 10.1016/j.jri.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Ekelund CK, Vogel I, Skogstrand K, et al. Interleukin-18 and interleukin-12 in maternal serum and spontaneous preterm delivery. J Reprod Immunol. 2008;77:179–185. doi: 10.1016/j.jri.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Ekman G, Malmstrom A, Uldbjerg N, et al. Cervical collagen: an important regulator of cervical function in term labor. Obstet Gynecol. 1986;67:633–636. [PubMed] [Google Scholar]
- Ekman G, Perssen PH, Ulmsten U, et al. The impact on labor induction of intracervically applied PGE2-gel, related to gestational age in patients with an unripe cervix. Acta Obstet Gynecol Scand Suppl. 1983;113:173–175. doi: 10.3109/00016348309155223. [DOI] [PubMed] [Google Scholar]
- Ferrand PE, Parry S, Sammel M, et al. A polymorphism in the matrix metalloproteinase-9 promoter is associated with increased risk of preterm premature rupture of membranes in African Americans. Mol Hum Reprod. 2002;8:494–501. doi: 10.1093/molehr/8.5.494. [DOI] [PubMed] [Google Scholar]
- Fortunato SJ, Menon R, Lombardi SJ. The effect of transforming growth factor and interleukin-10 on interleukin-8 release by human amniochorion may regulate histologic chorioamnionitis. Am J Obstet Gynecol. 1998;179:794–809. doi: 10.1016/s0002-9378(98)70085-7. [DOI] [PubMed] [Google Scholar]
- Fortunato SJ, Menon R, Lombardi SJ, et al. Interleukin-10 inhibition of gelatinases in fetal membranes: therapeutic implications in preterm premature rupture of membranes. Obstet Gynecol. 2001;98:284–298. doi: 10.1016/s0029-7844(01)01441-7. [DOI] [PubMed] [Google Scholar]
- Gargano JW, Holzman C, Senagore P, et al. Mid-pregnancy circulating cytokine levels, histologic chorioamnionitis and spontaneous preterm birth. J Reprod Immunol. 2008;79:100–110. doi: 10.1016/j.jri.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Xu H, Ofori E, et al. Toll-like receptors in the uterus, cervix, and placenta: is pregnancy an immunosuppressed state? Am J Obstet Gynecol. 2007;197:296. doi: 10.1016/j.ajog.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Gotsch F, Romero R, Kusanovic JP, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med. 2008;21:529–547. doi: 10.1080/14767050802127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granstrom L, Ekman G, Ulmsten U, et al. Changes in the connective tissue of corpus and cervix uteri during ripening and labour in term pregnancy. Br J Obstet Gynaecol. 1989;96:1198–1202. doi: 10.1111/j.1471-0528.1989.tb03196.x. [DOI] [PubMed] [Google Scholar]
- Hanna N, Bonifacio L, Weinberger B. Evidence for interleukin-10-mediated inhibition of cyclo- oxygenase-2 expression and prostaglandin production in preterm human placenta. Am J Reprod Immunol. 2006;55:19–27. doi: 10.1111/j.1600-0897.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- Hassan SS, Romero R, Haddad R, et al. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2006;195:778–786. doi: 10.1016/j.ajog.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Heinzmann A, Mailaparambil B, Mingirulli N, et al. Association of Interleukin-13/-4 and Toll-Like Receptor 10 with Preterm Births. Neonatology. 2009;96:175–181. doi: 10.1159/000210091. [DOI] [PubMed] [Google Scholar]
- Hillhouse EW, Grammatopoulos DK. Role of stress peptides during human pregnancy and labour. Reproduction. 2002;124:323–339. doi: 10.1530/rep.0.1240323. [DOI] [PubMed] [Google Scholar]
- Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev. 2006;27:260–286. doi: 10.1210/er.2005-0034. [DOI] [PubMed] [Google Scholar]
- Hollier LM, Rivera MK, Henninger E, et al. T helper cell cytokine profiles in preterm labor. Am J Reprod Immunol. 2004;52:192–206. doi: 10.1111/j.1600-0897.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- Holmlund U, Cebers G, Dahlfors AR, et al. Expression and regulation of the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 in the human placenta. Immunology. 2002;107:145–151. doi: 10.1046/j.1365-2567.2002.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmlund U, Wahamaa H, Bachmayer N, et al. The novel inflammatory cytokine high mobility group box protein 1 (HMGB1) is expressed by human term placenta. Immunology. 2007;122:430–7. doi: 10.1111/j.1365-2567.2007.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulboy DL, Rudolph LA, Matrisian LM. Matrix metalloproteinases as mediators of reproductive function. Mol Hum Reprod. 1997;3:27–45. doi: 10.1093/molehr/3.1.27. [DOI] [PubMed] [Google Scholar]
- Kerk J, Dordelmann M, Bartels DB, et al. Multiplex measurement of cytokine/receptor gene polymorphisms and interaction between interleukin-10 (-1082) genotype and chorioamnionitis in extreme preterm delivery. J Soc Gynecol Investig. 2006;13:350–366. doi: 10.1016/j.jsgi.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Klimaviciute A, Calciolari J, Bertucci E, et al. Corticotropin-releasing hormone, its binding protein and receptors in human cervical tissue at preterm and term labour in comparison to non-pregnant state. Reprod Biol Endocrinol. 2006;4:29. doi: 10.1186/1477-7827-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H, Nakatsuka M, Chekir C, et al. Advanced glycation end products induce secretion of chemokines and apoptosis in human first trimester trophoblasts. Hum Reprod. 2004;19:2156–2162. doi: 10.1093/humrep/deh389. [DOI] [PubMed] [Google Scholar]
- Ledingham MA, Thomson AJ, Young A, et al. Changes in the expression of nitric oxide synthase in the human uterine cervix during pregnancy and parturition. Mol Hum Reprod. 2000;6:1041–1048. doi: 10.1093/molehr/6.11.1041. [DOI] [PubMed] [Google Scholar]
- Li W, Challis JR. Corticotropin-releasing hormone and urocortin induce secretion of matrix metalloproteinase-9 (MMP-9) without change in tissue inhibitors of MMP-1 by cultured cells from human placenta and fetal membranes. J Clin Endocrinol Metab. 2005;90:6569–6574. doi: 10.1210/jc.2005-1445. [DOI] [PubMed] [Google Scholar]
- Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- Malmstrom E, Sennstrom M, Holmberg A, et al. The importance of fibroblasts in remodeling of the human uterine cervix during pregnancy and parturition. Mol Hum Reprod. 2007 doi: 10.1093/molehr/gal117. [DOI] [PubMed] [Google Scholar]
- Mondestin-Sorrentino M, Smulian JC, Vintzileos AM, et al. Variations in cervical IL-10 and IL-8 concentrations throughout gestation in normal pregnancies. Am J Reprod Immunol. 2007;57:482–497. doi: 10.1111/j.1600-0897.2007.00468.x. [DOI] [PubMed] [Google Scholar]
- Norman JE, Bollapragada S, Yuan M, et al. Inflammatory pathways in the mechanism of parturition. BMC Pregnancy Childbirth. 2007;7(Suppl 1):S7. doi: 10.1186/1471-2393-7-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman M, Ekman G, Malmstrom A. Changed proteoglycan metabolism in human cervix immediately after spontaneous vaginal delivery. Obstet Gynecol. 1993;81:217–223. [PubMed] [Google Scholar]
- Norman M, Ekman G, Ulmsten U, et al. Proteoglycan metabolism in the connective tissue of pregnant and non-pregnant human cervix. An in vitro study. Biochem J. 1991;275:515–520. doi: 10.1042/bj2750515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman I, Young A, Ledingham MA, et al. Leukocyte density and pro-inflammatory cytokine expression in human foetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod. 2003;9:41–55. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- Osmers RG, Grill BC, Rath W, et al. Biochemical events in cervical ripening dilatation during pregnancy and parturition. J Obstet Gynaecol. 1995;21:185–194. doi: 10.1111/j.1447-0756.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Patni S, Flynn P, Wynen LP, et al. An introduction to Toll-like receptors and their possible role in the initiation of labour. BJOG. 2007;114:1326–1334. doi: 10.1111/j.1471-0528.2007.01488.x. [DOI] [PubMed] [Google Scholar]
- Patni S, Wynen LP, Seager AL, et al. Expression and activity of toll-like receptors 1-9 in the human term placenta and changes associated with labour at term. Biol Reprod. 2009;80:243–248. doi: 10.1095/biolreprod.108.069252. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Potter E, Cameron VA, et al. Corticotropin-releasing factor-binding protein is produced by human placenta and intrauterine tissues. J Clin Endocrinol Metab. 1993;77:919–924. doi: 10.1210/jcem.77.4.8408466. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Tabanelli S, Galassi MC, et al. Human decidua and in vitro decidualized endometrial stromal cells at term contain immunoreactive corticotropin-releasing factor (CRF) and CRF messenger ribonucleic acid. J Clin Endocrinol Metab. 1992;74:1427–1431. doi: 10.1210/jcem.74.6.1375601. [DOI] [PubMed] [Google Scholar]
- Pioli PA, Amiel E, Schaefer TM, et al. Differential expression of Toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infect Immun. 2004;72:5799–5806. doi: 10.1128/IAI.72.10.5799-5806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Hassan S, et al. Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: modulation by infection and inflammation. J Perinat Med. 2008;36:388–398. doi: 10.1515/JPM.2008.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorie DK, Newton M. Histologic and chemical studies of the smooth muscle in the human cervix and uterus. Am J Obstet Gynecol. 1967;99:466–709. doi: 10.1016/0002-9378(67)90292-x. [DOI] [PubMed] [Google Scholar]
- Sato TA, Keelan JA, Mitchell MD. Critical paracrine interactions between TNF-alpha and IL-10 regulate lipopolysaccharide-stimulated human choriodecidual cytokine and prostaglandin E2 production. J Immunol. 2003;170:158–166. doi: 10.4049/jimmunol.170.1.158. [DOI] [PubMed] [Google Scholar]
- Sehringer B, Zahradnik HP, Simon M, et al. mRNA expression profiles for corticotrophin-releasing hormone, urocortin, CRH-binding protein and CRH receptors in human term gestational tissues determined by real-time quantitative RT-PCR. J Mol Endocrinol. 2004;32:339–348. doi: 10.1677/jme.0.0320339. [DOI] [PubMed] [Google Scholar]
- Sennstrom MB, Brauner A, Bystrom B, et al. Matrix metalloproteinase-8 correlates with the cervical ripening process in humans. Acta Obstet Gynecol Scand. 2003;82:904–911. doi: 10.1080/j.1600-0412.2003.00249.x. [DOI] [PubMed] [Google Scholar]
- Sennstrom MB, Ekman G, Thorsson G, et al. Human cervical ripening, an inflammatory process mediated by cytokines. Mol Hum Reprod. 2000;6:375–381. doi: 10.1093/molehr/6.4.375. [DOI] [PubMed] [Google Scholar]
- Simhan HN, Krohn MA. First-trimester cervical inflammatory milieu and subsequent early preterm birth. Am J Obstet Gynecol. 2009;200(4):377. doi: 10.1016/j.ajog.2008.10.038. [DOI] [PubMed] [Google Scholar]
- Slattery MM, Morrison JJ. Preterm delivery. Lancet. 2002;360:1489–1497. doi: 10.1016/S0140-6736(02)11476-0. [DOI] [PubMed] [Google Scholar]
- Stjernholm Y, Sahlin L, Akerberg S, et al. Cervical ripening in humans: potential roles of estrogen, progesterone, and insulin-like growth factor-I. Am J Obstet Gynecol. 1996;174:1065–1071. doi: 10.1016/s0002-9378(96)70352-6. [DOI] [PubMed] [Google Scholar]
- Stjernholm Y, Sahlin L, Malmstrom A, et al. Potential roles for gonadal steroids and insulin-like growth factor I during final cervical ripening. Obstet Gynecol. 1997;90:375–380. doi: 10.1016/s0029-7844(97)00245-7. [DOI] [PubMed] [Google Scholar]
- Stygar D, Wang H, Vladic YS, et al. Co-localization of oestrogen receptor beta and leukocyte markers in the human cervix. Mol Hum Reprod. 2001;7:881–906. doi: 10.1093/molehr/7.9.881. [DOI] [PubMed] [Google Scholar]
- Stygar D, Wang H, Vladic YS, et al. Increased level of matrix metalloproteinases 2 and 9 in the ripening process of the human cervix. Biol Reprod. 2002;67:889–894. doi: 10.1095/biolreprod.102.005116. [DOI] [PubMed] [Google Scholar]
- Thomson AJ, Lunan CB, Cameron AD, et al. Nitric oxide donors induce ripening of the human uterine cervix: a randomised controlled trial. BJOG. 1997;104:1054–1067. doi: 10.1111/j.1471-0528.1997.tb12066.x. [DOI] [PubMed] [Google Scholar]
- Ticconi C, Zicari A, Belmonte A, et al. Pregnancy-promoting actions of HCG in human myometrium and fetal membranes. Placenta. 2007;28:137–143. doi: 10.1016/j.placenta.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Tornblom SA, Klimaviciute A, Bystrom B, et al. Non-infected preterm parturition is related to increased concentrations of IL-6, IL-8 and MCP-1 in human cervix. Reprod Biol Endocrinol. 2005a;3:39. doi: 10.1186/1477-7827-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornblom SA, Maul H, Klimaviciute A, et al. mRNA expression and localization of bNOS, eNOS and iNOS in human cervix at preterm and term labour. Reprod Biol Endocrinol. 2005;3:33. doi: 10.1186/1477-7827-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornblom SA, Patel FA, Bystrom B, et al. 15-hydroxyprostaglandin dehydrogenase and cyclooxygenase 2 messenger ribonucleic acid expression and immunohistochemical localization in human cervical tissue during term and preterm labor. J Clin Endocrinol Metab. 2004;89:2909–15. doi: 10.1210/jc.2003-031149. [DOI] [PubMed] [Google Scholar]
- Uldbjerg N, Ekman G, Malmstrom A, et al. Ripening of the human uterine cervix related to changes in collagen, glycosaminoglycans, and collagenolytic activity. Am J Obstet Gynecol. 1983;147:662–6. doi: 10.1016/0002-9378(83)90446-5. [DOI] [PubMed] [Google Scholar]
- Uldbjerg N, Malmstrom A, Ekman G, et al. Isolation and characterization of dermatan sulphate proteoglycan from human uterine cervix. Biochem J. 1983b;209:497–503. doi: 10.1042/bj2090497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisanen-Tommiska M, Nuutila M, Aittomaki K, et al. Nitric oxide metabolites in cervical fluid during pregnancy: further evidence for the role of cervical nitric oxide in cervical ripening. Am J Obstet Gynecol. 2003;188:779–785. doi: 10.1067/mob.2003.161. [DOI] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- Wang H, Stjernholm Y, Ekman G, et al. Different regulation of oestrogen receptors alpha and beta in the human cervix at term pregnancy. Mol Hum Reprod. 2001;7:293–300. doi: 10.1093/molehr/7.3.293. [DOI] [PubMed] [Google Scholar]
- Wang W, Nan X, Ji P, et al. Corticotropin releasing hormone modulates endotoxin-induced inflammatory cytokine expression in human trophoblast cells. Placenta. 2007;28:1032–1038. doi: 10.1016/j.placenta.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Wang X, Hagberg H, Mallard C, et al. Disruption of interleukin-18, but not interleukin-1, increases vulnerability to preterm delivery and fetal mortality after intrauterine inflammation. Am J Pathol. 2006;169:967–976. doi: 10.2353/ajpath.2006.050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsson G, Norman M, Bjornsson S, et al. Differential expressions of mRNA for proteoglycans, collagens and transforming growth factor-beta in the human cervix during pregnancy and involution. Biochim Biophys Acta. 1998;1406:203–213. doi: 10.1016/s0925-4439(98)00005-2. [DOI] [PubMed] [Google Scholar]
- Wu YJ, La Pierre DP, Wu J, et al. The interaction of versican with its binding partners. Cell Res. 2005;15:483–494. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- Young A, Thomson AJ, Ledingham M, et al. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002;66:445–459. doi: 10.1095/biolreprod66.2.445. [DOI] [PubMed] [Google Scholar]