Abstract
The prenatal diagnosis of fetal coarctation is still challenging. It is mainly suspected by ventricular disproportion (smaller left ventricle than right ventricle). The sensitivity of ventricular discrepancy is however moderate for the diagnosis of coarctation and there is a high false positive rate. Prenatal diagnosis of coarctation is important because the delivery can be arranged in a centre with a pediatric cardiac intensive careand this reduces postnatal complications and longterm morbidity.
For many years the prenatal diagnosis of coarctation has been investigated to improve specificity and sensitivity by several of measurements. This article reviews all relevant articles from 2000 until 2011 searching pubmed and the reference list of interesting articles. An overview of specific measurements and techniques that can improve the diagnosis of coarctation has been made, such as the isthmus diameter, ductal diameter, isthmus/ductal ratio, z-scores derived from measurements of the distal aortic isthmus and arterial duct, the presence of a shelf andisthmal flow disturbance. Also 3-dimensional (3D) and 4-dimensional (4D) imaging with or without STIC has been suggested to be used as newer techniques to improve diagnosis of coarctation in fetal life. Although more methods regarding prenatal diagnosis of coarctationare being investigated, the ultrasound specialist remains challenged to correctly diagnose this cardiac anomaly in prenatal life.
Keywords: Prenatal diagnosis, coarctation, ventricular disproportion, isthmus/ductal ratio, discrepancy great vessels, isthmal z-scores, Doppler, flow disturbance, shelf
Introduction
Coarctation of the aorta is a common congenital heart defect. It accounts for approximately 8% of cardiac defects.
Coarctation of the aorta is characterized by narrowing of the distal aortic arch.This obstructive lesion may reduce the blood flow in the fetal aortic arch, leading to arch hypoplasia, although in some cases this may only be clinically evident after birth, or even in later life.
It remains one of the most difficult cardiac defects to diagnose before birth. Antenatal diagnosis of coarctation is critically important for early treatment of the neonate. Suspicion is usually raised when there is a ventricular disproportion, with a disproportionately smaller left ventricle than right ventricle. But a discrepant ventricular size has only a moderate sensitivity and a low specificity and low positive predictive value for the diagnosis. Other measurements such as the isthmus diameter, ductal diameter, isthmus/ductal ratio, z-scores derived from measurements of the distal aortic isthmus and arterial duct, the presence of a shelf and flow obstruction over the isthmus in a sagittal view can be of aid in the diagnosis of coarctation.
Literature sources
A literature search was conducted to identify all the published studies on fetal diagnosis of coarctation. Pubmed was searched using the following key words: antenatal diagnosis, coarctation, ventricular disproportion, isthmus/ductal ratio, aortic z-scores, aortic shelf and persistent left superior vena cava. All relevant studies between 2000-2011 have been selected. Furthermore we searched the reference list of all articles for more relevant information.
Coarctation of the aorta
Coarctation accounts for 8% of congenital heart diseases.
This congenital heart disease is characterized by a narrowing of the distal aortic arch and occurs in 0.2 to 0.62 per 1000 live births.
The narrowing in the descending aorta occurs at the insertion site of the ductus arteriosus (Fig. 1). It results from abnormalities in the development of the embryotic fourth and sixth aortic arches.
Fig. 1.
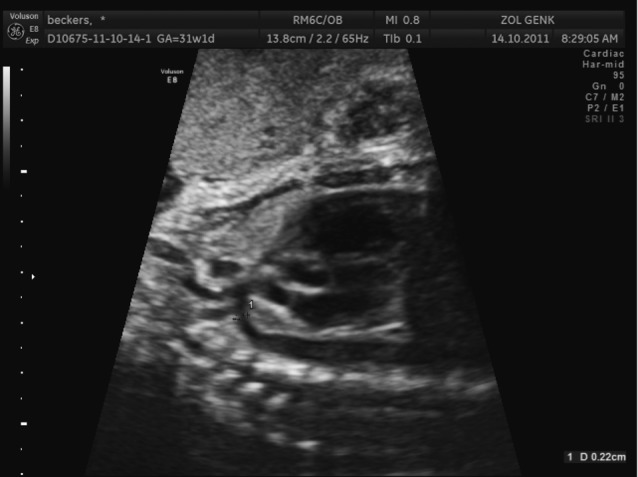
Sagittal view on the aortic arch with narrowing after left subclavian artery in coarctation aortae.
The underlying mechanism leading to coarctation is not fully understood but there are two important mechanisms proposed: the ductal tissue theory and the reduced flow theory. The ductal tissue theory suggests that ductal tissue invades the aortadistal to the isthmus (part of the aorta proximal of the ductus arteriosus and distal from the insertion of the left subclavian artery). The obstruction appears as an indentation (shelf) in the postero-lateral side of the descending aorta. The reduced flow theory suggests that obstruction develops secondary to haemodynamic disturbances. Normally in the fetus, the aortic isthmus only receives10% of the combined ventricular output, due to the bypass by the ductus arteriosus. If this amount is further reduced, the growth of the aortic isthmus can be compromised which can result in a narrowing of the isthmus.
In the fetus with coarctation, little haemodynamic consequences occur because the isthmus receives only 10% of the cardiac output. But after birth with ductal closure, a variety of haemodynamic effects can occur, depending on the severity of the obstruction. Clinical outcome varies from mild systemic hypertension to serious heart failure and death. The clinical manifestations are variable and so is the age of diagnosis. A rapid progression with heart failure can be seen in the neonate, but sometimesolder children are diagnosed, after a high blood pressure is detected. When fetal diagnosis of coarctation is made or suspected, delivery must be foreseen in a centre where specialised cardiac care can take place.
Intravenous Prostaglandines type 1 are used neonatally when an important coarctation is suspected to avoid closure of the ductus arteriosus immediately after birth and to gain time before surgery. For the surgical repair of coarctation several techniques are available of which end-to-end anastomosis is the most widely used and with the best long term results. Transcatheter treatment is also an accepted alternative for surgery with comparable results.
Despite excellent results overall for surgical and transcatheter treatment, longterm morbidity and mortality remain substantial. Early management by correct diagnosis, preferably during fetal life, can reduce perinatal mortality and long-term complications.
Ventricular disproportion
Suspicion for coarctation of the aorta is usually raised when there is ventricular disproportion in fetal life (with a smaller left than right ventricle) (Fig. 2). It is important to compare z-scores to ensure that the right ventricle is normal of size, and the left ventricle is smaller (and to exclude particular abnormalities where the disproportion is evoked by a larger right ventricle) (Doyle et al., 2005). In 1997 Brown et al. have already demonstrated a moderate sensitivity (62%) and a mediocre positive predictive value for coarctation (33%) for various forms of left-sided structural heart disease (Brown et al., 1997). A ventricular disproportion is the most sensitive in the second trimester, before 25 weeks of gestational age, and less in the third trimester. In the third trimester there is already a slight degree of fysiological disproportion (normal: LV/RV < 1.5) (Quarello et al., 2011). There is a high false positive rate, especially after 34 weeks (up to 80%) (Stos et al., 2007). False-positive diagnosis can result in parental anxiety and the differential diagnosis of isolated 4-chamber cardiac disproportion is also wide. In 2009 Quartermain et al. reviewed the echocardiograms of 35 fetuses evaluated in their centre with left-right ventricular disproportion that needed aortic arch intervention and compared the group that underwent neonatal intervention with the group that did not need intervention. Echocardiographic measurements were obtained at end diastole before atrioventricular valve closure and include long-axis dimensions and left and right mid-cavitary width dimension. The primary aim of this study was to identify prenatal echocardiographic markers that predict critical coarctation and the need for neonatal arch intervention in the fetus with isolated left-right ventricular disproportion. A secondary aim was to describe the spectrum of postnatal diagnosis and outcomes of fetuses referred for evaluation of isolated left-right ventricular disproportion. They showed no significant difference in the long axis dimension ratio, but the left ventricle mid-cavitary dimension to right mid cavitary dimension (LVmc/RVmc) was statistically significant lower in the intervention group. LVmc/RVmc < 0.6 has shown a sensitivity of 70%, a specificity of 67% and a positive predictive value of 73% for neonatal intervention or critical coarctation.
Fig. 2.
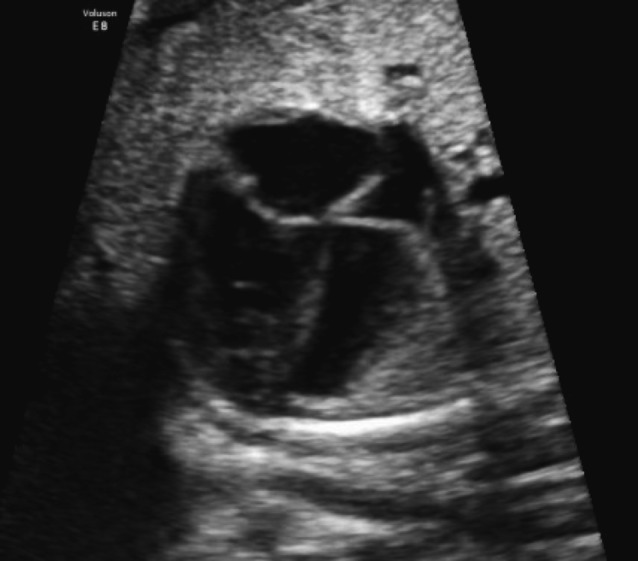
Four chamber view with ventricular disproportion (left side smaller than right side). The mid-caviary dimensions (white lines) can be measured and compared to normal sizes for gestational age.
Isthmus/ductal ratio and isthmus/ductal angle
The narrowest area of the aorta is at the aortic isthmus, the part of the aorta proximal to the insertion of the arterial duct in the descending aorta. The three vessel and tracheal view allow comparison of the aortic arch and the ductal arch and assessment of the fetal isthmus (Fig. 3). Pasquini et al. obtained z-scores for the aortic isthmus and ductal arch in normal fetuses by measuring the aortic arch and ductal diameter prospectively related to gestational age and to femur length (Pasquini et al., 2007). They also calculated the ratio of the isthmal to ductal diameters. Regression analysis of the ratio in normal fetuses against femur length and gestational age showed that it was very close to a constant value of 1, regardless of the value of femur length or gestational age. 95% of all ratio values lie within the range of 0.74 and 1.23. Results lower than 0.74 are suspicious for aortic coarctation and the further away from a value of 1 in negative sense, the more likely the presence of arch hypoplasia or coarctation.
Fig. 3.
Flowchart search strategy of databases
Matsui et al. measured the aortic isthmus and duct in the three vessel view of 200 fetuses referred for a cardiac scan with a normal cardiac anatomy and of 48 fetuses with a ventricular and/or arch disproportion and suspected isolated coarctation (Matsui et al., 2008). The isthmal to ductal ratio enabled good separation between the two groups. They used the95% reference range of 0.74 to 1.23 and found that of the 44 fetuses in the study group, 40 had an isthmal/ductal ratio < 0.74 and this included all fetuses who required postnatal surgery or surveillance. The 4 fetuses within the normal range were all normal postnatally.
Serial isthmus to ductal ratios can also help distinguish fetuses that would require surgery from those requiring surveillance. The higher the scores are (thus the higher the ratio), the less likely a coarctation of the aorta exists.
Quarello et al defined a standard view in order to obtain and evaluate the aortic/ductal angle. a sagittal view that includes the distal aortic and ductal arches and proximal descending aorta using power doppler 2D ultrasound. In normal fetuses the aortic/ductal angle ranges from 128.2° to 167 degrees and in fetuses with coarctation of the aorta from 82.2 to 125°. The aortic/ductal angle was significantly smaller in the coarctation group (Quarello et al., 2008).
Isthmal z-scores
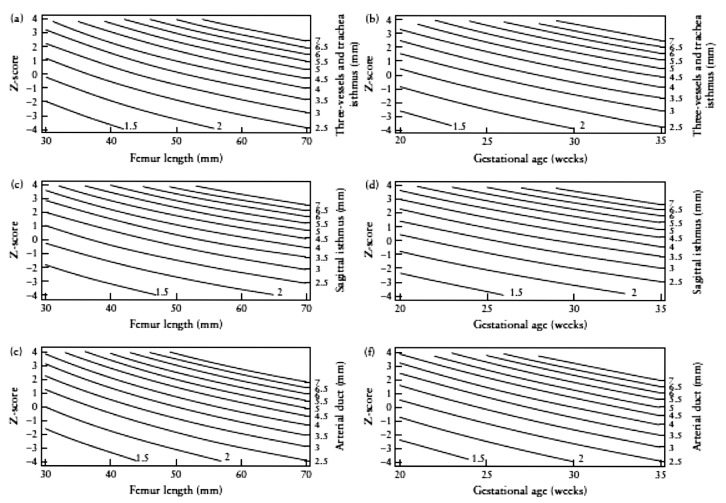
As described earlier, Pasquini et al developed z-scores for the aortic isthmus in normal fetuses as a reference for fetuses with suspected coarctation (Pasquini et al., 2007).They started from the formulae and nomograms produced by Schneider et al. in 2005 (Schneider et al., 2005). He calculated z-scores and compared cardiac measurements with non-cardiac fetal biometric parameters.The benefit of z-scores is that each measurement is reported as a multiple of standard deviationallowing examiners to report measurements that are above or below the 95% confidence intervals. Pasquini et al measured the aortic isthmus diameter immediately proximal to the insertion of the arterial duct in the transverse (three vessel) and the sagittal view. In neonatal coarctation, this is the narrowest portion of the arch. The ductal diameter was measured immediately before it entered the descending aorta in the three vessel and trachea view only (Fig. 4: Graphical displays of the z-scores). Z scores were created relating isthmal and ductal diameters to femur length and gestational age. Matsui et al.(2008) tested the applicability of published aortic arch and ductal z-scores on normal controls at a median of 22 weeks gestational age and tested the ability of serial z-scores to distinguish fetuses with coarctation within a cohort with ventricular and/or great arterial disproportion detected at screening. The aortic arch z-scores enabled good separation between the 200 fetuses in the control group and those with disproportion suspected to have coarctation at the first scan. As said earlier for the serial measurements of isthmus-ductal ratio, serial measurement of the aortic z-scores can contribute to make the difference between the fetuses that require surgery and those who can be observed.They also analysed the measurements made in the third trimester after 26 weeks and those turned out to be as good in separating the different groups.
Fig. 4.
Graphical display of the z-scores for isthmal diameter in the three vessels and trachea view based on femur length (a) and on gestational age (b), for isthmal diameter in the sagittal view based on femur length (c) and on gestational age (d), and for ductal diameter in the three vessels and trachea view based on femur length (e) and on gestational age (f).
Discrepancy of the great vessels
A coarctation of the aorta is almost always associated with a discrepancy of the great vessels where the diameter of the arteria pulmonalis is bigger than the diameter of the aorta during diastole.This is probably related to blood flow redistribution because of the increased resistance of the left ventricular outflow tract. It can be only a temporary redistribution that normalises after birth, or a permanent redistribution seen in cases requiring cardiac surgery after delivery.
Yagel et al. (2002) already proposed the value of analyzing 3 vessel and trachea view. Slodki et al. (2009) investigated the utility of analyzing prenatal mediastinal measures of the great arteries in the third trimester by measuring the diameters in the three vessel and trachea view.They found that the main PA:Ao diameter ratio can be a helpful tool for distinguishing true from false CoA. For a PA:Ao ratio of 1.60 or more, the sensitivity was 83%, specificity 85%, positive predictive value 62.5% and negative predictive value 94%.
Rizzo et al. (2010) proposed to measure the sizes of both pulmonary artery and aorta in the three vessel view by using four dimensional sonography with spatiotemporal image correlation (STIC). They found also that PA:Ao ratio was significantly higher in fetuses with CoA compared to those with a normal heart.
Doppler flow
Reversed blood flow across the foramen ovale and retrograde flow in the aortic arch have been shown to be sensitive predictors of severe forms of left-sided structural heart disease. Quartermain et al. (2009) found that an abnormal flow was seen in 12/28 fetuses, all of them required neonatal surgery and normal right-to-left atrial level shunting was seen in all normal fetuses, giving a sensitivity of 71%. Retrograde flow in the aortic arch is not always present and can be physiological in third trimester. Retrograde flow in patients with coarcatation is mainly observed during systole. Therefore retrograde flow in the fetal aortic arch combined with a small left heart is suspicious for coarctation (Quarello et al., 2011).
Persistent left superior vena cava
Persistent left superior vena cava (PLSVC) is present in about 0.3% of the population at necropsy. Its true incidence is unknown as it is most often asymptomatic and is difficult to detect during routine echocardiography. Pasquini et al. (2005) performed 1678 fetal echocardiograms on cardial referrals. 1230 were normal with a LSVC in 4 patients.Cardiac abnormality was present in 448 cases, with persistent PLSVC in 12 cases (2.7%). LSVC was seen in 5 of 10 fetuses with aortic arch hypoplasia of which nine required surgery for CoA. The odds ratio of the cardiac defects being CoA in fetuses with PLSVC is 61.57(Pasquini et al., 2005). Berg et al. (2006) evaluated the associated conditions of persistent left superior vena cava (PLSVC) by reviewing all cases between 1998 and 2004 in two tertiary referral centers. 82 Cases were studied and after exclusion of cases with heterotaxy syndrome, most common congenital heart defects were ventricular septum defects (41%) and coarctation (34%) (Berg et al., 2006). PLSVC detected in fetal life is not problematic given it remains an isolated condition without associated (left) heart anomalies.
Other associated factors
Fetal coarctation can be associated with bicuspid aortic valve, aortic valve stenosis, a large VSD and mitral stenosis (Abuhamad and Chaoui, 2010). The odds ratio for a coarctation when a bicuspid valve is detected is 53.5 (Stos et al., 2007). Bicuspid aortic valve is almost never detected prenatally, sometimes it can be suspected by a poststenotic dilatation or by abnormal Doppler flow.
Coarctation can also be associated with chromosomal abnormalities, especially when associated with other anomalies. In 14% of patients with Turner syndrome, a coarctation is present (Hamdan, 2006).
Other techniques
Yagel et al. (2002) described a case were the coarctation shelf was visualized using 3D ultrasound and static 3D volumes of the fetal heart with colour flow mapping. A localized posterior aortic shelf was demonstrated within the aortic isthmus.
Quarello and Trabbia (2009) described the visualization of a narrowing of the isthmus and tortuosity of the aortic arch using bidirectional high-definition flow combined with spatiotemporal image correlation (STIC). Thin multislices were generated by the tomographic ultrasound imaging technique (Quarello and Trabbia, 2009).
B-flow imaging has been used by Espinoza et al. (2009) to visualize the contraductal shelf. B flow is a display modality in 4D sonography that enhances signals from weak blood reflectors from vessels, suppressing strong signals from surrounding tissues, and is angle independent. Multiplanar imaging in a 3D and 4D sonography using a combination of spatiotemporal image correlation (STIC) and tomographic ultrasound imaging (TUI) is a novel display modality that allows for the simultaneous visualization of 3 orthogonal anatomic planes.The use the anatomal planes can help for the examination of the fetal heart with the possibility of off-line use and the reduction of operator dependency (Espinoza et al., 2009; Pooh, 2000). A sonographic finding described in neonates with coarctation of the aorta is the “contraductal shelf,” which is supposed to represent residual fibrous tissue derived from the ductus arteriosus.
B-flow imaging is potentially advantageous over color or power Doppler imaging when used in conjunction with STIC for the evaluation of the fetal vasculature. In B-flow imaging, echoes from the tissue and that of the blood flow can be displayed with high resolution and without the overlay that characterizes color Doppler imaging. A B-flow volume of the heart with off-lineM-mode tracing of the isthmic region (sampling at the level of the three vessel view on the isthmus and ductal arch) can detect a diastolic run-off that is present in fetal aortic coarctation. In normal hearts the isthmus will show absence of diastolic-run off with a pulsatile flow pattern while in coarctation diastolic run-off with continuous forward flow throughout the cardiac cycle is present. The evaluation of this pattern of flow remains subjective and the number of cases examined until now are still limited (Paladini et al., 2012).
Discussion
Coarctation of the aorta is a common congenital heart defect. It accounts for approximately 8% of cardiac defects with an incidence of 0.2 to 0.62 per 1000 live births.
Prenatal diagnosis of coarctation is still a difficult diagnosis to make accurately. Moreover the obstructive lesion occurring in coarctation may reduce the blood flow in the fetal aortic arch, leading to arch hypoplasia in severe cases, although in some cases this may only be clinically evident in the third trimester, after birth, or even in later life.
Detecting arch hypoplasia in fetal life is well feasible but it is also important to try to diagnose fetal coarctation because the prenatal diagnosis of this cardiac defect improves survival and reduces neonatal morbidity, at least if neonates are born in a center with specialised cardiac care. A false-positive diagnosis of fetal coarctation however creates unnecessary parental stress. Therefore it is necessary to have specialized ultrasonographic tools for the correct diagnosis of coarctation. The visualization of a small left ventricle with aortic hypoplasia at midgestation is easy, but the third trimester ventricular discrepancies are much more difficult in separating normal fetuses from those with coarctation.
The left ventricle mid-cavitary dimension during systole to right mid-cavitary dimension remains an important clue. LVmc/RVmc < 0.6 has shown a sensitivity of 70%, a specificity of 67% and a positive predictive value of 73% for neonatal intervention for critical coarctation. The three vessel and tracheal view allow comparison of the aortic arch and the ductal arch and assessment of the fetal isthmus. Isthmus/ductal diameter, isthmus/ductal angle and z-scores are important measurements to be made when coarctation is suspected. Doppler flow has been shown to be a useful tool in assessing retrograde blood flow through the foramen ovale and the isthmus. When there is a persistent left superior caval vein, it is important to keep in mind the association with aortal coarctation.Associated anomalies must at all times be excluded.
Other sonographic features to predict correctly coarctation prenatallyare isthmus diameter z-scores, isthmus to duct diameters, the visualization of a shelf and isthmal flow disturbance. In this prospective study Jowett et al. (2012) demonstrated that the use of four parameters (I, I :D, Shelf and Flow) in combination achieves superior diagnostic precision (86%) in the detection of true fetal CoA requiring perinatal surgery. Application of these sonographic criteria by the fetal cardiologist during serial review may increase diagnostic specificity and improve clinical management (Jowett et al., 2012).
Antenatal detection of coarctation still remains a challenging subject and newer techniques are constantly being developed. One of the newer techniques based on 3 and 4-dimensional imaging of the fetal heart with spaciotemporal imaging technique (STIC) and a B flow volume with afterwards offline M-mode modality to evaluate diastolic run-off known to be present in coarctation is promising. Referral to a specialized ultrosonographer is important when a coarctation is suspected because the diagnosis remains difficult and when suspected birth in a center with cardiac intensive care has to be arranged.
References
- Abuhamad A, Chaoui R. Practical Guide to Fetal Echocardiography: Normal and Abnormal Hearts. 2nd edn. Philadelphia: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- Berg C, Knuppel M, Geipel A, et al. Prenatal diagnosis of persistent left superior vena cava and its associated congenital anomalies. Ultrasound Obstet Gynecol. 2006;27:274–280. doi: 10.1002/uog.2704. [DOI] [PubMed] [Google Scholar]
- Brown DL, Durfee SM, Hornberger LK. Ventricular discrepancy as a sonographic sign of coarctation of the fetal aorta: how reliable is it? J Ultrasound Med. 1997;16:95–99. doi: 10.7863/jum.1997.16.2.95. [DOI] [PubMed] [Google Scholar]
- Doyle NM, Mastrobattista JM, Thapar MK, et al. Perinatal pseudocoarctation: echocardiographic findings in vein of Galen malformation. J Ultrasound Med. 2005;24:93–98. doi: 10.7863/jum.2005.24.1.93. [DOI] [PubMed] [Google Scholar]
- Espinoza J, Romero R, Kusanovic JP, et al. Prenatal diagnosis of coarctation of the aorta with the multiplanar display and B-flow imaging using 4-dimensional sonography. J Ultrasound Med. 2009;28:1375–1378. doi: 10.7863/jum.2009.28.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan MA. Coarctation of the aorta: a comprehansive review. J Arab Neonatal Forum. 2006;3:5–13. [Google Scholar]
- Jowett V, Aparicio P, Santhakumaran S, et al. Sonographic predictors of surgery in fetal coarctation of the aorta. Ultrasound Obstet Gynecol. 2012;40:47–54. doi: 10.1002/uog.11161. [DOI] [PubMed] [Google Scholar]
- Matsui H, Mellander M, Roughton M, et al. Morphological and physiological predictors of fetal aortic coarctation. Circulation. 2008;118:1793–1801. doi: 10.1161/CIRCULATIONAHA.108.787598. [DOI] [PubMed] [Google Scholar]
- Paladini D, Sorrentino M, Pastore G, et al. OC08.01: B-flow derived M-mode is a reliable tool to detect diastolic run-off in fetal aortic coarctation. Ultrasound Obstet Gynecol. 2012;40:15. [Google Scholar]
- Pasquini L, Fichera A, Tan T, et al. Left superior caval vein: a powerful indicator of fetal coarctation. Heart. 2005;91:539–540. doi: 10.1136/hrt.2004.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquini L, Mellander M, Seale A, et al. Z-scores of the fetal aortic isthmus and duct: an aid to assessing arch hypoplasia. Ultrasound Obstet Gynecol. 2007;29:628–633. doi: 10.1002/uog.4021. [DOI] [PubMed] [Google Scholar]
- Pooh RK. New application of B-flow sono-angiography in perinatology. Ultrasound Obstet Gynecol. 2000;15:163. doi: 10.1046/j.1469-0705.2000.00025.x. [DOI] [PubMed] [Google Scholar]
- Quarello AR, Ville Y, Carvalho JS. The aortic istmus-ductal angle: a novel measurement to diagnose fetal aortic coarctation. Ultrasound Obstet Gynecol. 2008;32:262–263. [Google Scholar]
- Quarello E, Stos B, Fermont L. Diagnostic prénatal dese coarctations de l’aorte. Gynecol Obstet Fertil. 2011;39:442–453. doi: 10.1016/j.gyobfe.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Quarello E, Trabbia A. High-definition flow combined with spatiotemporal image correlation in the diagnosis of fetal coarctation of the aorta. Ultrasound Obstet Gynecol. 2009;33:365–367. doi: 10.1002/uog.6270. [DOI] [PubMed] [Google Scholar]
- Quartermain MD, Cohen MS, Dominguez TE, et al. Left ventricle to right ventricle size discrepancy in the fetus: the presence of critical congenital heart disease can be reliably predicted. J Am Soc Echocardiogr. 2009;22:1296–1301. doi: 10.1016/j.echo.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Rizzo G, Arduini D, Capponi A. Use of 4-dimensional sonography in the measurement of fetal great vessels in mediastinum to distinguish true-from false-positive coarctation of the aorta. J Ultrasound Med. 2010;29:325–326. doi: 10.7863/jum.2010.29.2.325. [DOI] [PubMed] [Google Scholar]
- Schneider C, McCrindle BW, Carvalho JS, et al. Development of Z-scores for fetal cardiac dimensions from echocardiography. Ultrasound Obstet Gynecol. 2005;26:599–605. doi: 10.1002/uog.2597. [DOI] [PubMed] [Google Scholar]
- Slodki M, Rychik J, Moszura T, et al. Measurement of the great vessels in the mediastinum could help distinguish true from false-positive coarctation of the aorta in the third trimester. J Ultrasound Med. 2009;28:1313–1317. doi: 10.7863/jum.2009.28.10.1313. [DOI] [PubMed] [Google Scholar]
- Stos B, Le BJ, Fermont L, et al. Le diagnostic anténatal de la coarctation de l’aorte, est-il possible? Arch Mal Coeur Vaiss. 2007;100:428–432. [PubMed] [Google Scholar]
- Yagel S, Arbel R, Anteby EY, et al. The three vessels and trachea view (3VT) in fetal cardiac scanning. Ultrasound Obstet Gynecol. 2002;20:340–345. doi: 10.1046/j.1469-0705.2002.00801.x. [DOI] [PubMed] [Google Scholar]