Abstract
In this paper we focus on preterm birth as a uterine contractility disorder caused by hypercontractility of the myometrium. We describe changes in uterine function during term and preterm labor and delivery. We also examine the usefulness of measurement of uterine electromyographic (EMG) activity, noninvasively monitored from the abdominal surface of pregnant patients. The use of progesterone treatment for preterm birth is discussed and we conclude that present therapies with progesterone could be improved by changing the route of administration. Finally we show the results of recent studies that show that progesterone injections completely inhibit uterine EMG activity when given several days to hours before normal delivery. These studies illustrate how progesterone suppresses labor at term or preterm, probably through repression of genes which control excitability and conduction of electrical activity. However, direct profusion of soluble progesterone into the uterine cavity has little immediate inhibitory action and this may demonstrate that progesterone has no direct, nongenomic effects, at least in the rat model used. Further studies are required to determine the effects of progesterone on human uterine EMG activity and whether progesterone treatments will prevent preterm birth.
Keywords: Preterm birth, preterm labor, progesterone, uterine contractility, uterine EMG, myometrial contractility
Introduction
Preterm birth, i.e. birth prior to 37 weeks gestation, might be considered a uterine contractility disorder. Uterine contractility disorders are major health problems resulting in deaths of large numbers of babies and women worldwide and discomfort and difficulties with reproduction in women. Uterine contractility disorders include hypercontractility such as preterm labor/birth and dysmenorrhea and hypocontractility such as prolonged labor and post partum uterine atony (lack of tonic uterine contractile activity). In addition contractility disorders may include infertility and other puzzles related to women’s reproductive function. In this brief review we will outline the basis of uterine contractions and methods for the diagnosis of problems and discuss possible treatment strategies using progesterone or progestin analogs and the basis for their use. We will also demonstrate that progesterone acts mainly by suppression of uterine contractility to inhibit birth.
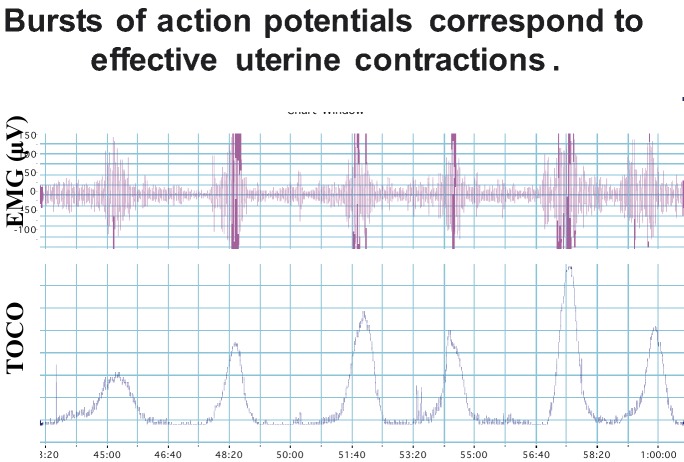
Csapo (1981) recognized the complex structure of the uterus and noted that it is composed of billions of small smooth muscle cells organized into bundles. Normally the uterus contracts in a phasic manner, relaxing and contracting with a certain characteristic periodicity. The fundamental basis for a uterine contraction is known to be produced by the underlying electrical activity of the muscle cells (Kuriyama, 1961; Kao, 1977; Marshall, 1982; Garfield et al., 1988). Just as in the heart the electrical activity (action potentials) which is generated in pacemaker areas produce a contraction by spreading over the heart, electrical activity of the uterus initiates and sustains contractions by conduction over the uterus. However, in the uterus a single action potential is not responsible for a strong contraction as in the heart, but rather bursts of action potentials generate contractions by changes in the influx of ions, particularly Na+ and Ca2+, into the muscle cells and relaxation is created by an efflux of ions, notably K+ and Ca2+, out of the cells. Figure 1 shows the relationship between electrical activity of the uterus organized into bursts and the mechanical, contraction, response of the muscle. The paper by Dr. Miyoshi in this monograph reviews studies of the electrical properties of the uterus and how tocolytics modify ion flow and contractility.
Fig. 1. Figure shows six bursts of uterine electrical myographic activity (EMG) recorded from the abdominal surface of a pregnant patient in labor (top of figure) and the corresponding intrauterine pressure recorded with a pressure transducer. Note the correspondence between the bursts and contractions.
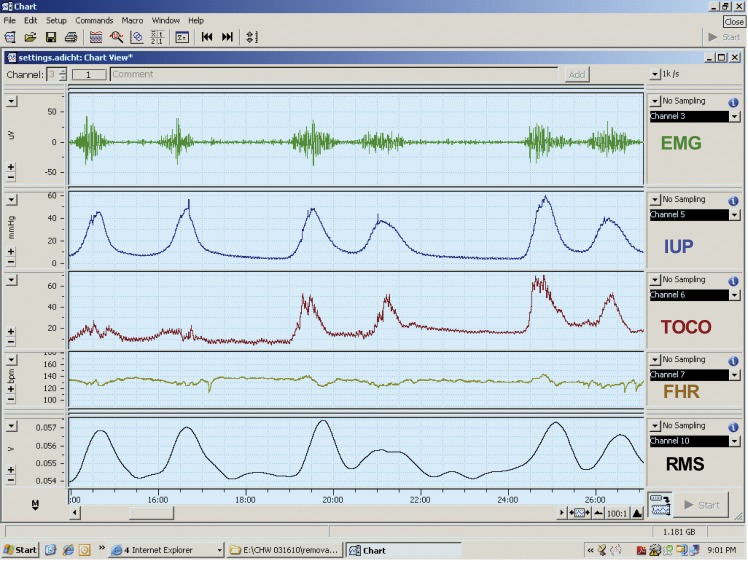
Some years ago we, and others, found that we could record electrical activity of the uterus by placing electrodes on the abdominal surface (Buhimschi and Garfield, 1996; Buhimschi et al., 1997; Buhimschi et al., 1998; Garfield et al., 1998; Garfield et al., 1998). This is similar to recording EKG of the heart by placing electrodes at specific locations on the body to record action potentials of cardiac muscle. We also noted that in humans contractions recorded with a tocodynamometer or intrauterine pressure device corresponded to the EMG signals recorded from the abdominal surface (Fig. 2). This technology allows one to noninvasively monitor uterine contractions and identify uterine contractility disorders. The use of abdominal surface measurements of uterine EMG activity allows for diagnosis of term and preterm labor (Garfield et al., 1998; Maner et al., 2003; Maul et al., 2004; Garfield et al., 2005; Maner and Garfield, 2007; Shi et al., 2008). The diagnosis of labor using EMG activity as recorded from the abdominal surface is discussed in more detail by Miha Lucovnik in this same issue. Lucovnik combined several parameters of uterine EMG to find high predictability for diagnosis of preterm and term labor (Lucovnik et al., 2011; Lucovnik et al., 2011). The EMG methods and analysis are powerful technology that will eventually replace uterine monitoring by the tocodynamometer and intrauterine pressure monitoring.
Fig. 2. Recording of EMG activity (top tracing) recorded from the abdominal surface, intrauterine pressure (IUP), and tocodynamometer pressure (TOCO), fetal heart rate (FHR) and root mean square of the EMG signals (RMS) in a patient in labor. Note that bursts of EMG activity correspond to changes in pressure recorded with IUP and TOCO and that the conversion of EMG signals to RMS resemble signals obtained with the IUP and TOCO. This shows that recording of EMG signals and conversion of these signals to RMS can be used to replace TOCO and IUP techniques for monitoring the contractions of a pregnant patient. Monitor from Reproductive Research Technologies, LLP, Houston, Texas.
Preterm Labor/Birth and Progestin Treatments
Preterm birth is one of the biggest health problems in the world and a challenge in obstetrics for physicians to identify early signs of labor and impending premature birth and define an appropriate treatment that will stop birth of a premature baby and allow a patient to proceed until term. Presently there are about 15 million babies born prematurely in the world among a total of about 150 million births per year (World Health Organization, 2012). Premature birth is the largest contributor to neonatal deaths in the world. Thus, finding ways to diagnose women that will deliver early and finding appropriate treatments to arrest premature birth should be one of the highest priorities in medicine today. Use of uterine EMG measurements may help in identifying patients that will deliver early and help in determining treatment strategies.
There have been many studies in animals and humans on the use of progesterone to inhibit labor and prevent premature birth. Csapo (1981) was a champion in this field and he first suggested that progesterone acts to inhibit uterine contractility. It is noteworthy that Csapo did not really consider the cervix important in the onset and maintenance of labor and delivery. Since then there have been numerous studies and this topic emerged again when in 2003 it was demonstrated in two important studies that progestins (Meis et al., 2003) and vaginal progesterone (da Fonseca et al., 2003) could reduce preterm birth in women with recurrent preterm birth or women at high risk for preterm birth. More recently these studies have been duplicated in a multicenter study with vaginal progesterone (Crinone® in Replens base, Columbia Laboratories) using patients with a short cervical length as a risk factor for preterm birth (Hassan et al., 2011). However, our studies suggested that vaginal progesterone is not the best route to administer progesterone as vaginal Crinone in rats does not inhibit birth whereas subcutaneous injections and transdermal progesterone completely block delivery (Kuon et al., 2010). Thus it was no surprise to us that the FDA recently rejected the approval of the use of vaginal progesterone for treatment of preterm birth declaring that there was no significant difference between the vaginal product and the placebo in clinical trials, but the FDA did conclude that such progesterone treatment posed no significant risk to the mother or child. The use of 17 alpha hydroxyprogesterone caproate has been approved by the US FDA and is marketed as Makena™.
Facchinetti (2007) indicated that 17 alpha hydroxyprogesterone caproate reduces cervical ripening if given to prevent preterm birth. Our studies on the effects of progestins on the uterus show that 17 alpha hydroxyprogesterone caproate does not inhibit human uterine contractility in vitro from tissues obtained during pregnancy, while authentic progesterone rapidly and reversible suppresses contractions (Ruddock et al., 2008). In addition 17 alpha hydroxyprogesterone caproate did not prevent delivery at term in rats while progesterone either topical or parenteral, but not vaginal application (Crinone®), prevented birth (Kuon et al., 2010; 2011). We concluded from these studies that 17 alpha hydroxyprogesterone caproate may not be the best treatment for prevention of preterm birth. Similarly, vaginal progesterone is unlikely to be successful in preventing preterm birth because the vaginal route is not the best way to administer progesterone and the vehicle used for Crinone (Repens) does not release progesterone effectively. 17 alpha hydroxyprogesterone is a weak progestin and unlikely to prevent preterm birth. On the other hand topical or parenteral treatments of progesterone may be much better treatments to reduce the incidence of preterm birth. These studies are outlined in more detail in the paper by Kuon and Garfield in this issue.
It is not exactly clear how progesterone prevents term or preterm birth. It is possible that progestins affect cervical ripening or uterine contractility or both. There is strong evidence that antiprogestins ripen the cervix and initiate preterm birth or abortion at almost any state of gestation in animals and humans (Chwalisz and Garfield 1994; Chwalisz et al., 1995; 1997; Shi et al., 2000). However, generally the cervix ripens slowly throughout pregnancy when progesterone levels are increasing in both animals and humans. Thus it is difficult to see how progesterone might regulate the cervix. On the other hand the uterus begins to contract normally only during a short time prior to birth. In other words the cervix and uterus change functionally in different time frames when progesterone and indeed estrogen levels are changing. From our early studies we found that electrical coupling between myometrial cells increases dramatically at term during birth in all species studied (Garfield et al., 1977; Garfield and Hayashi, 1981). In addition electrical coupling increases in the myometrium during treatments to initiate preterm birth (Garfield et al., 1982; Sims et al., 1982; Demianczuk et al., 1984; Cole et al., 1985; Garfield et al., 1987; 1988; Miller et al., 1989; Miyoshi et al., 1996; 1998). These observations are important because increased spread of current allows the uterus to contract more forcefully, by recruitment of large numbers of muscle cells, during term or preterm birth. Our studies included both structural studies as well as functional studies to measure coupling between myometrial cells. Recent studies indicate that progesterone treatment only slightly delays cervical ripening (Kuon et al., 2010; 2011). It was concluded that progesterone must prevent delivery by suppression of uterine contractility rather than have an action on the cervix to prevent delivery. In this paper we show that progesterone inhibits uterine electromyographic activity and thereby prevents delivery.
Progesterone inhibits uterine electromyographic activity and prevents preterm and term birth
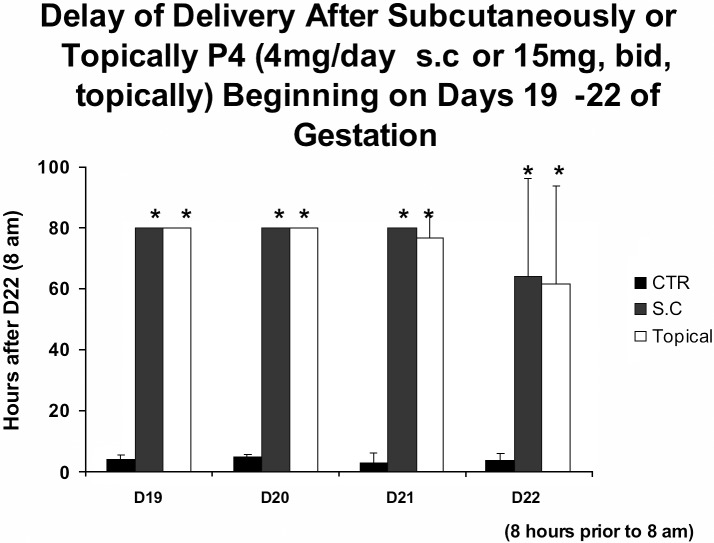
Previously we have measured EMG activity in rats, humans and other species during term and preterm birth. In all species there is a dramatic increase in the frequency, amplitude and power of the EMG signals during birth that underlie and are responsible for uterine contractions (Garfield et al., 1998; Shi et al., 2008; Lucovnik et al., 2011). Recently we have examined whether progesterone inhibits birth at term in rats. This is a well recognized model that has been used to bioassay the effects of progestins. We administered micronized progesterone topically and parenterally (subcutaneously, s.c.) daily beginning from day 19, day 20, or day 21 of gestation and 8 hours before normal delivery on day 22. All topical or parenteral (s.c.) treatments prevented birth up to 80 hours beyond the normal time of delivery observed in control, vehicle treated, rats (Fig. 3). The significance of these observations is that progesterone can prevent birth when given after the decline in progesterone levels in the blood and even after the cervix is already ripened and prepared for delivery. These studies suggest that topical or parenteral progesterone treatments, but not vaginal progesterone, inhibit uterine contractility to prevent birth. Why vaginal progesterone is not effective is not clear but it is possible that Replens, the base used by Columbia Laboratories for Crinone, does not release progesterone. The reason for suggesting this is because micronized progesterone in Replens base applied topically does not inhibit term birth in rats (Kuon et al., 2010; 2011). Although these early studies indicate that topical or parenteral progesterone, but not vaginal progesterone, can partially delay cervical ripening it does not prevent it from softening. Successful delay in delivery with only a few hours treatment with progesterone suggests that progesterone treatment might be used to prevent acute preterm birth.
Fig. 3. Figure 3 shows the delay in delivery in pregnant rats treated daily with parenteral (4 mg subcutaneous injection, S.C., of micronized progesterone) or topical progesterone (2 times daily, 15 mg micronized progesterone in fish oil) for 3 days (days 19-21), 2 days (days 20-21), 1 day (day 21) or 8 hours before normal delivery (day 22) on day 22 at 8 a.m. of gestation compared to controls (CTR). Note that all progesterone treatments substantially delay delivery, even when progesterone is given 8 hours prior to normally delivery. All animals treated with progesterone were sacrificed at 80 hours following 8 a.m. on day 22 of gestation. This study illustrates that progesterone can prevent delivery if given prior to the end of gestation at times when the cervix is already soft and prepared for delivery.
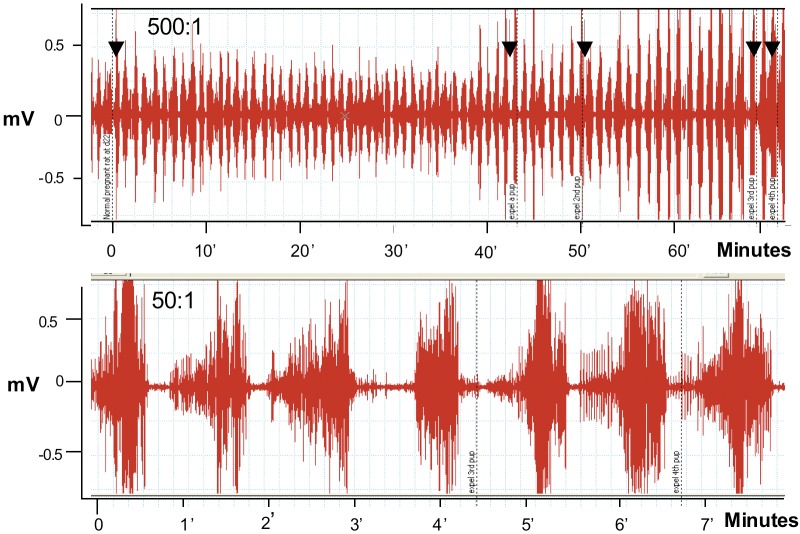
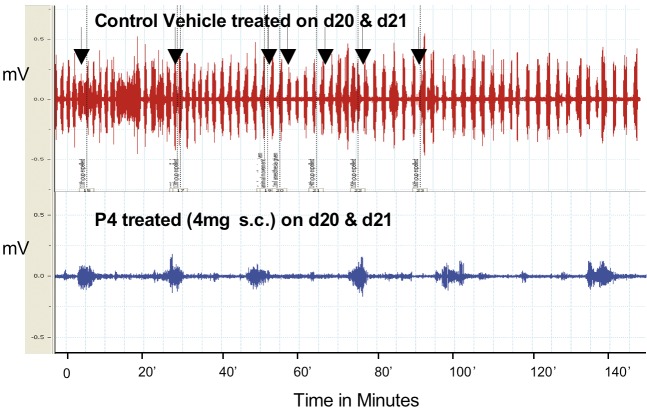
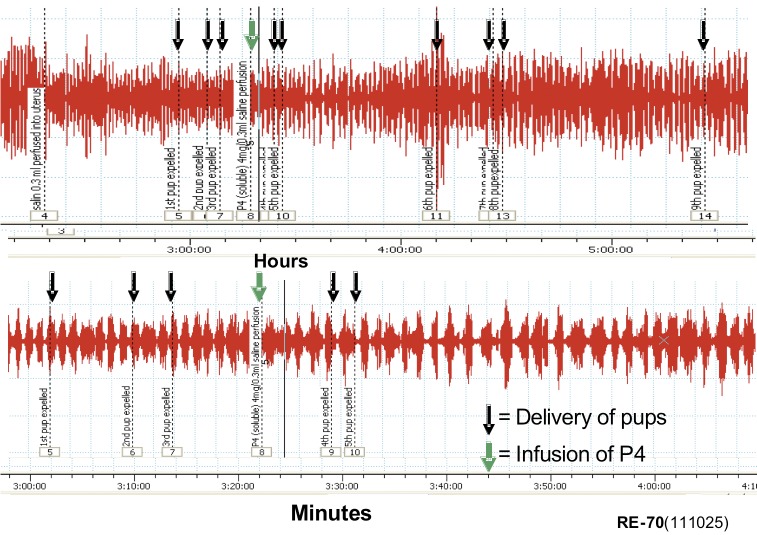
To examine the effects of progesterone on uterine EMG activity we used anesthetized rats at term and sometimes animals treated with antiprogestins which were delivering preterm. We placed electrodes directly on the uterus and recorded EMG activity by conventional recording equipment as we have done previously. We recorded EMG activity over several hours and remarkably these animals delivered fetuses, although sometimes the fetuses were not completely passed through the cervix, probably because the animals were under anesthesia and did not contract the abdominal muscles or push during delivery. We noted that the EMG activity was extremely high (Fig. 4) and very similar what we had observed previously in animals fitted with internal EMG devices to measure activity without anesthesia (Shi et al., 2008). Animals treated with progesterone 1 to 2 days prior to normal delivery had very low levels of EMG activity (Fig. 5). We repeated these experiments in many studies and noted the following: Progesterone (s.c.) significantly reduces the EMG burst frequency (bursts/30 min ± SEM: 1 day treatment =11.1 ± 1.7 vs. controls 23.2 ± 3.5, P < 0.01; 2 day treatment =10.5 ± 1.3 vs. controls 25.4 ± 4.4, P < 0.01). The EMG burst amplitudes are also significantly lower in the progesterone-treated animals (µV ± SEM: 1 day treatment = 74 ± 8 vs. controls 280 ± 58, P < 0.008; 2 day treatment = 127 ± 31 vs. controls 230 ± 14, P < 0.02). Thus the mean burst integrals (V2) are suppressed at 1 (P < 0.001) and 2 (P < 0.002) days after P4 treatment vs. controls, but not the burst duration (P > 0.05, ca. 30 seconds). This indicates that progesterone treatment suppresses uterine EMG activity and thereby inhibits birth. We also examined whether progesterone treatments given by infusion would inhibit uterine electrical activity and thereby prevent contractions and delivery at term. If so this could indicate that progesterone has a nongenomic action to inhibit uterine contractions and thus it might be used in case of acute preterm labor where a patient is already in established preterm labor that is likely to lead to preterm birth. Infusion of bolus injections of soluble progesterone through a catheter inserted into the uterine cavity in close proximity to the EMG recording electrodes did not produce an observable inhibition of contractions even at high progesterone doses, i.e. soluble progesterone equivalent to doses up to 5 mg of crystalline progesterone (Fig. 6). We also infused micronized progesterone dissolved in small amounts of ethanol and other solubilizing agents. These experiments also failed to show any inhibition but it was difficult to demonstrate because ethanol alone, and other agents used to solubilize progesterone, inhibited contractions by themselves. These studies, although somewhat incomplete and hard to interpret, suggest that progesterone does not exert an immediate, nongenomic action of the uterine muscle during delivery to suppress uterine EMG and contractile activity. Thus the question of whether progesterone treatment may immediately inhibit acute preterm labor by direct action on the uterus is not completely resolved.
Fig. 4. EMG recordings from a normal pregnant rat on day 22 of gestation during delivery (arrows indicate delivery of a fetus). Recordings showing > 60 minutes (top record) and for > 7 minutes (bottom). Note delivery of fetuses as marked with arrows. This indicates that the animals deliver their fetuses while under anesthesia and the uterine contractions which accompany birth consists of large bursts of EMG activity. This is an example from studies using more than 20 rats.
Fig. 5. Examples of EMG recordings from pregnant rats on day 22 of gestation from control rat (vehicle treated during delivery, top tracing, arrows indicate delivery of a fetus) and rat after two days treatment with P4 (4 mg micronized progesterone, P4, given subcutaneously for 2 days on day 20 and day 21 of gestation. Animals not delivering). Note the difference in the bursts (amplitude and frequency) of EMG activity in control rat recording compared to the rat treated with P4. Similar differences were observed following topical treatment of P4 but not after vaginal treatment.
Fig. W6. Examples of EMG records from delivering (pup delivery indicated with black arrows) rat perfused with soluble progesterone (equivalent to 4 mg of authentic progesterone, P4, 4 mg at green arrow) as shown during 4 hours recording (top) and the same record shown enlarged with recording time equal to about 70 minutes. Note the perfusion with progesterone had no effect on the EMG activity.
Discussion
Preterm birth and term delivery present enormous challenges to physicians, their patients and premature infants. We suggest that preterm labor and the resultant early birth are the consequence of premature uterine contractions and therefore can be considered as a uterine contractility disorder. Recently treatment of preterm birth has been attempted using weekly 17 alpha hydroxyprogesterone caproate injections and daily vaginal progesterone. Our studies indicate that neither of these progesterone preparations is useful to suppress term or preterm birth.We suggest that transdermal (topical) treatment of progesterone or progesterone injections may be useful. The transdermal route may be particularly helpful because progesterone, with a half-life of only about 30 hours, must be given daily and daily injections are not ideal. Progesterone inhibits uterine contractions through genomic mechanisms probably involving the classical progesterone receptors. Progesterone may not act through nongenomic mechanisms, although our studies with human myometrial tissues seems to indicate otherwise (Ruddock et al., 2008) and membrane progesterone receptors have been identified on human myometrial cells (Wu et al., 2011). The studies above involving infusion of progesterone into the uterine cavity indicate little effect but soluble progesterone and authentic progesterone in solvent may not be the best way to demonstrate this action.
Transabdominal measurement of EMG activity is a powerful tool to evaluate function of the uterus during pregnancy and identify true preterm or term labor. This method also could be useful to identify the best treatment strategies for preterm labor treatment. Lucovnik et al. (2011) estimate that less patients would be needed to examine the effectiveness of any treatment on preterm labor using EMG methods compared to other clinical determinates used to make clinical decisions on preterm birth treatment. Our studies above with progesterone treatment clearly show that progesterone suppresses uterine EMG activity after 1 to 2 days treatment and inhibits birth in rats even at 8 hours before normal delivery. This short time frame indicates that the site of progesterone action is not on the cervix. Ultimately analogs of progesterone might prove more effective for inhibition of preterm birth because 17 alpha hydroxyprogesterone caproate and authentic progesterone are both weak agonists for the progesterone receptor. Further studies are urgently needed to demonstrate better preparations of progesterone on human EMG activity and on preterm birth. This paper represents some Facts, our Views and Visions in ObGyn.
References
- Buhimschi C, Garfield RE. Uterine contractility as assessed by abdominal surface recording of electromyographic activity in rats during pregnancy. Am J Obstet Gynecol. 1996;174:744–753. doi: 10.1016/s0002-9378(96)70459-3. [DOI] [PubMed] [Google Scholar]
- Buhimschi C, Boyle MB, Garfield RE. Electrical activity of the human uterus during pregnancy as recorded from the abdominal surface. Obstet Gynecol. 1997;90:102–111. doi: 10.1016/S0029-7844(97)83837-9. [DOI] [PubMed] [Google Scholar]
- Buhimschi C, Boyle MB, Saade GR, et al. Uterine activity during pregnancy and labor assessed by simultaneous recordings from the myometrium and abdominal surface in the rat. Am J Obstet Gynecol. 1998;178:811–822. doi: 10.1016/s0002-9378(98)70498-3. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Garfield RE. Antiprogestins in the Induction of Labor. Ann N Y Acad Sci. 1994;734:387–413. doi: 10.1111/j.1749-6632.1994.tb21770.x. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Stockemann K, Fuhrmann U, et al. Mechanism of Action of Antiprogestins in the Pregnant Uterus. Ann N Y Acad Sci. 1995;761:202–224. doi: 10.1111/j.1749-6632.1995.tb31380.x. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Garfield RE. Regulation of the uterus and cervix during pregnancy and labor: Role of progeseterone and nitric oxide. Ann N Y Acad Sci. 1997;828:238–253. doi: 10.1111/j.1749-6632.1997.tb48545.x. [DOI] [PubMed] [Google Scholar]
- Cole WC, Garfield RE, Kirkaldy JS. Gap junctions and direct intercellular communication between rat uterine smooth muscle cells. Am J Physiol. 1985;249(18):C20–C31. doi: 10.1152/ajpcell.1985.249.1.C20. [DOI] [PubMed] [Google Scholar]
- Csapo AI. Principles and Practice of Obstetrics and Perinatology, Iffy I and Kamientsky HA, Eds. New York: Wiley; 1981. Force of labor; pp. 761–799. [Google Scholar]
- da Fonseca EB, Bittar RE, Carvalho MHB, et al. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: A randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419–424. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- Demianczuk N, Towell ME, Garfield RE. Myometrial electrophysiological activity and gap junctions in the pregnant rabbit. Am J Obstet Gynecol. 1984;149(5):485–491. doi: 10.1016/0002-9378(84)90021-8. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Paganelli S, Comitini G, et al. Cervical length changes during preterm cervical ripening: effects of 17-alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2007;196:453–e1-4. doi: 10.1016/j.ajog.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Garfield RE, Saade G, Buhimschi C, et al. Control and assessment of the uterus and cervix during pregnancy and labour. Hum Reprod Update. 1998;4:673–695. doi: 10.1093/humupd/4.5.673. [DOI] [PubMed] [Google Scholar]
- Garfield RE, Maner WL, MacKay LB, et al. Comparing uterine electromyography activity of antepartum patients versus term labor patients. Am J Obstet Gynecol. 2005;193:23–29. doi: 10.1016/j.ajog.2005.01.050. [DOI] [PubMed] [Google Scholar]
- Garfield RE, Chwalisz K, Shi L, et al. Instrumentation for the diagnosis of term and preterm labour. J Perinat Med. 1998;26:413–436. doi: 10.1515/jpme.1998.26.6.413. [DOI] [PubMed] [Google Scholar]
- Garfield RE, Sims S, Daniel EE. Gap junctions: Their presence and necessity in myometrium during parturition. Science. 1977;198:958–960. doi: 10.1126/science.929182. [DOI] [PubMed] [Google Scholar]
- Garfield RE, Hayashi RH. Appearance of gap junctions in the myometrium of women during labor. Am J Obstet Gynecol. 1981;140(3):254–260. doi: 10.1016/0002-9378(81)90270-2. [DOI] [PubMed] [Google Scholar]
- Garfield RE, Puri CP, Csapo AI. Endocrine structural and functional changes in the uterus during premature labor. Am J Obstet Gynecol. 1982;142(1):21–27. doi: 10.1016/s0002-9378(16)32279-7. [DOI] [PubMed] [Google Scholar]
- Garfield RE, Gasc JM, Baulieu EE. Effects of the antiprogesterone RU 486 on preterm birth in the rat. Am J Obstet Gynecol. 1987;157(5):1281–1295. doi: 10.1016/s0002-9378(87)80315-0. [DOI] [PubMed] [Google Scholar]
- Garfield RE, Blennerhassett MG, Miller SM. Control of myometrial contractility: Role and regulation of gap junctions. Oxf Rev Reprod Biol. 1988;10:436–490. [PubMed] [Google Scholar]
- Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in woman with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38:18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao CY. Biology of the Uterus. Wynn R.M., Ed. New York: Plenum Press; 1977. Electrical properties of uterine smooth muscle; pp. 423–496. [Google Scholar]
- Kuon RJ, Shi S, Maul H, et al. Pharmacologic actions of progestins to inhibit cervical ripening and prevent delivery depend on their properties, the route of administration, and the vehicle. Am J Obstet Gynecol. 2010;202:455–e1-9. doi: 10.1016/j.ajog.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuon RJ, Shi S-Q, Maul H, et al. A Novel Optical Method to Assess Cervical Changes During Pregnancy and Use to Evaluate the Effects of Progestins on Term and Preterm Labor. Am J Obstet Gynecol. 2011;205:82.e15–20. doi: 10.1016/j.ajog.2011.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H. In: Progesterone and the Defense Mechanism of Pregnancy. Ciba Foundation Study Group 9, Little Brown, Boston. 1961:51–70. [Google Scholar]
- Lucovnik M, Maner WL, Chambliss LR, et al. Noninvasive Uterine Electromyography For Prediction of Preterm Delivery. Am J Obstet Gynecol. 2011;204:228.e1–10.330. doi: 10.1016/j.ajog.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucovnik M, Kuon RJ, et al. Use of Uterine Electromyography to Diagnose Term and Preterm Labor. Acta Obstet Gynecol Scand. 2011;90:150–7. doi: 10.1111/j.1600-0412.2010.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucovnik M, Kuon RJ, Chambliss LR, et al. Use of Uterine Electromyography to Diagnose Term and Preterm Labor. Acta Obstet Gynecol Scand. 2011;90:150–157. doi: 10.1111/j.1600-0412.2010.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maner WL, Garfield RE, Maul H, et al. Predicting term and preterm delivery with transabdominal uterine electromyography. Obstet Gynecol. 2003;101:1254–1260. doi: 10.1016/s0029-7844(03)00341-7. [DOI] [PubMed] [Google Scholar]
- Maner WL, Garfield RE. Identification of human term and preterm labor using artificial neural networks on uterine electromyography data. Ann Biomed Eng. 2007;35:465–473. doi: 10.1007/s10439-006-9248-8. [DOI] [PubMed] [Google Scholar]
- Marshall JM. Regulation of activity in uterine smooth muscle. Physiol Rev. 1982;42:213–227. [PubMed] [Google Scholar]
- Maul H, Maner WL, Olson G, et al. Non-invasive transabdominal uterine electromyography correlates with the strength of intrauterine pressure and is predictive of labor and delivery. J Matern Fetal Neonatal Med. 2004;15:297–301. doi: 10.1080/14767050410001695301. [DOI] [PubMed] [Google Scholar]
- Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- Miller SM, Garfield RE, Daniel EE. Improved propagation in myometrium associated with gap junctions during parturition. Am J Physiol. 1989;256(25):C130–141. doi: 10.1152/ajpcell.1989.256.1.C130. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Boyle MB, MacKay L, et al. Voltage-Clamp Studies of Gap Junctions Between Uterine Muscle Cells During Term and Preterm Labor. Biophys J. 1996;71(3):1324–34. doi: 10.1016/S0006-3495(96)79332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, Boyle MB, MacKay LB, et al. Gap junction currents in cultured muscle cells from human myometrium. Am J Obstet Gynecol. 1998;178(3):588–593. doi: 10.1016/s0002-9378(98)70443-0. [DOI] [PubMed] [Google Scholar]
- Ruddock N, Shi S, Jain S, et al. Progesterone (P4), but not 17 alpha hydroxyprogesterone caproate (17P) inhibits uterine contractility during pregnancy. Am J Obstet Gynecol. 2008;199(4):391–e1-7. doi: 10.1016/j.ajog.2008.06.085. [DOI] [PubMed] [Google Scholar]
- Shi SQ, Maner WL, MacKay LB, et al. Identification of term & pre-term labor in rats by using artificial neural networks on uterine electromyography signals. Am J Obstet Gynecol. 2008;198:235.e1–235.e1-4. doi: 10.1016/j.ajog.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Shi SQ, Saade GR, et al. Studies of cervical ripening in pregnant rats:effects of various treatments. Mol Hum Reprod. 2000;6(4):382–389. doi: 10.1093/molehr/6.4.382. [DOI] [PubMed] [Google Scholar]
- Sims SM, Daniel EE, Garfield RE. Improved electrical coupling in uterine smooth muscle is associated with increased numbers of gap junctions at parturition. J Gen Physiol. 1982;80(3):353–375. doi: 10.1085/jgp.80.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Shi S-Q, Huang H, Balducci J, et al. Changes in PGRMC1, a Potential Progesterone Receptor, in Human Myometrium during Pregnancy and Labour at Term and Preterm. Mol Hum Reprod. 2011;17(4):233–242. doi: 10.1093/molehr/gaq096. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Born Too Soon: The Global Action Report on Preterm Birth. 2012. pp. 1–111. [Google Scholar]