Abstract
Objective: To compare two short protocols for ovarian stimulation in IVF cycles using an antagonist and an agonist short protocol. The outcomes studied were dosis rec FSH needed, days of stimulation, number of oocytes retrieved and pregnancy outcome.
Methods: A prospective randomised study design. Inclusion criteria: first or second IVF attempt in women younger than 40 years. In the agonist protocol (Suprefact®) nasal spray was used. In the antagonist protocol (Orgalutran)® was started as soon as at least 1 follicle of 12 mm was visualized on ultrasound.
Results: 160 cycles were included in the study: 80 in the antagonist group and 80 in the agonist group. A higher dosis of recombinant FSH (rec FSH) was used for stimulation in the antagonist group (1897 IU versus 1655 IU). Pregnancy rate per ET in the antagonist group was 37% with an ongoing pregnancy rate of 21%/ET and an implantation rate of 22%; versus respectively 39%, 20% and 22% in the agonist treated group. Live birth rate per started cylce was 19% in the antagonist group versus 20% in the agonist group.
Conclusion: This study shows that implantation rates, ongoing pregnancy rates and live birth rates are equal in both groups. An identical number of oocytes was retrieved, with no difference in duration of the stimulation although a higher dosis of rec FSH was needed in the antagonist group.
Keywords: GnRH-Agonist, GnRH-antagonist, IVF, infertility, live birth rate, ovarian stimulation, pregnancy outcome
Introduction
To suppress ovulation and luteinization a long GnRH agonist regimen is one of the most commonly used protocols by many IVF programs. Such a protocol requires frequently a longer course of ovarian stimulation, usually with higher exogenous gonadotropin requirements (Scott and Navot, 1994; Hugues and Cedrin Durnerin, 1998).
In the short GnRH agonist flare-up protocol, gonadotropin stimulation is started at day 3 of the menstrual cycle. Different studies (Frydman et al., 1988a, 1988b; Ho et al., 2008) showed no difference in delivery rate between short and long GnRH agonist protocols.
More recently, GnRH antagonists have become available. GnRH antagonists competitively block pituitary GnRH receptors, inducing a rapid, reversible suppression of gonadotropin secretion. Due to their distinct pharmacological mode of action, GnRH antagonists can be administered at mid-cycle to prevent a premature LH surge while not causing any suppression in the early follicular phase, which is a crucial time for follicular recruitment.
An initial meta-analysis in the Cochrane database reported that GnRH antagonists are associated with a shorter duration of stimulation, a reduced gonadotropin consumption and a reduced ovarian hyperstimulation incidence than long GnRH agonist protocols. In the study of Shanbhag a lower pregnancy rate compared with the GnRH agonist long protocol was observed (Shanbhag et al., 2007).Up till now more than 30 randomised controlled trials were performed comparing different stimulation protocols in different IVF-populations.
The meta-analysis of Kolibianakis (Kolibianakis et al., 2006) included 22 RCT. No statistical significant difference was observed in live birth rate between GnRH antagonist and GnRH agonists. This meta-analysis included 18 studies with a long-agonist protocol and 4 studies with a short-agonist protocol. They found no statistically significant difference on live birth rate according to the type of agonist protocol used. These studies using short-agonist protocol (Akman et al., 2001; Malmusi et al., 2005; Schmidt et al., 2005)were all performed in a subgroup of poor responders.
Still the best protocol for the IVF patient is widely debated in the literature. As the optimal protocol remains inconclusive, a wide variation in physician preferences remains.
This is by our knowledge the first report of a prospective, randomized, controlled trial comparing a GnRH antagonist protocol with a short GnRH agonist protocol in a general IVF population.
Material and methods
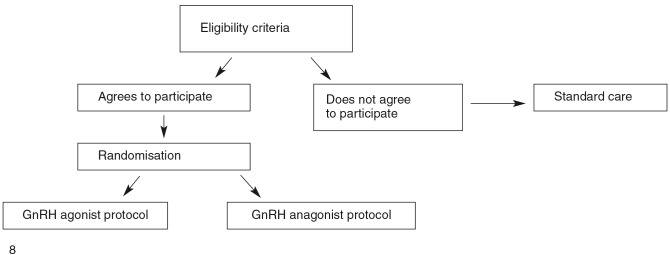
A prospective randomised study design comparing a short GnRH agonist protocol and a GnRH antagonist protocol. 160 patients were included in the study between january 2009 and july 2010. Inclusion criteria were: first or second IVF attempt in women younger than 40 years. Exclusion criteria were: 2 or more failed IVF attempts, 40 years or older, pre-implantation genetic diagnosis cycles and cycles with use of testicular sperm extraction. Randomisation was performed using a blinded envelope system (Fig. 1).
Fig. 1. Randomisation proces.
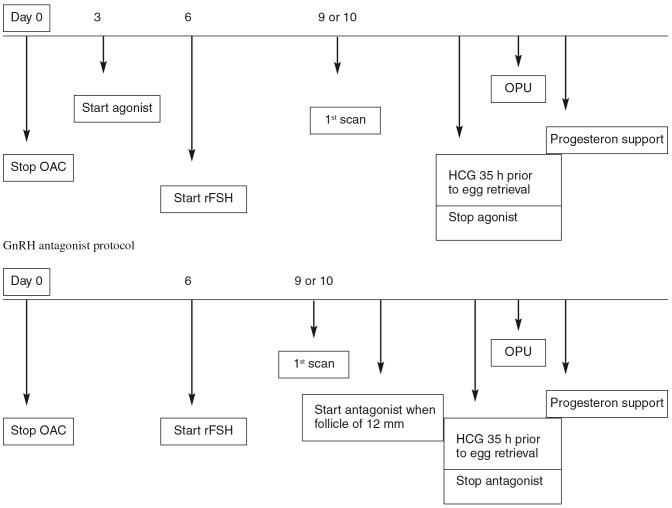
All patients received oral contraceptives the cycle preceeding the IVF treatment for organizational reasons (Fig. 2). Rec-FSH (Puregon®, MSD) was used in both groups at an initial dosis of 150 IU for patients younger than 36 years and 200 IU for patients of 36 years and older. In both groups administration of rec FSH was started 6 days after stop of oral contraceptives. In the agonist protocol (Suprefact®, Aventis Pharma) nasal spray (3 puffs, 3×/day) was initiated 3 days after stop of oral contraceptive pill up till the day of hCG injection. In the antagonist protocol (Orgalutran®, MSD) was used and started as soon as at least 1 follicle of 12 mm was visualized on ultrasound. When at least two follicles reached a diameter of 18 mm or more, 5000 IU hCG (Pregnyl®, MSD) was used in both groups. Ultrasound guided oocyte aspiration was performed 35 hours after hCG administration. For luteal support micronised progesteron was used vaginally at a dosage of 3 × 200 mg/day and started on day 1 after egg retrieval. Embryo transfer took place on day 2 or 3 after oocyte pick-up. The difference of day of embryotransfer was made for pratical reasons and was depending on the day of oocyte pick-up. A pregnancy (serum hCG) test was performed on day 14 after egg retrieval.
Fig. 2. Treatment regimes.
The primary endpoint was live birth rate (LBR). Secondary endpoints were ongoing pregnancy rate (OPR), number of oocytes retrieved at oocyte pick-up, days of stimulation, dosage of rec FSH needed and implantation rate.
Proportions were compared with the Fisher’s exact test or the chi-square test, where appropriate. Continuous variables were compared with the t-test for independent samples or the Mann-Whitney depending on the normality of their distribution. Statistical significance was accepted when P < 0.05.
Results
Between January 2009 and July 2010 160 patients were included in the study: 80 in the antagonist group and 80 in the agonist group. Both groups were comparable for age, number of IVF attempts, IVF or ICSI treatment, day of embryotransfer (Table I) The mean age was 31,79 years (± SD 3.9) in the antagonist group and 31.3 years (± SD 4.5) in the agonist group. In the antagonist group 74% were 1st IVFattemps versus 79% in the agonist group. ICSI was performed in 79% of antagonist cycles versus 73% in agonist cycles. One cycle was cancelled in the agonist group and 6 cycles were cancelled in the antagonist group. All cancellations were due to poor respons. Poor respons was defined as less than 3 mature follicles. The mean number of days for ovarian stimulation needed was 10.9 in the antagonist group and 10.2 in the agonist group and was not different between the two groups. A higher dosis of FSH was used for stimulation in the antagonist group (1897 IU versus 1655 IU). This is a statistically significant difference (p = 0,02) (Table II). The level of Oestradiol at the day of hCG administration was statistically significantly different between both groups. Oestradiol levels were lower in the antagonist group, 1087 versus 2457 pg/ml. There was no difference in the mean number of retrieved oocytes between the antagonist and the agonist group (11 versus 11,2 oocytes). In the antagonist group no oocytes were recruited at the moment of oocyte pick-up in 1 cycle. In the agonist group fertilization failed in 2 cycles. In total 149 embryotransfers were performed, 73 in the antagonist group and 76 in the agonist group. Embryotransfer was performed on day 2 or day 3 depending on practical reasons. In the antagonist group 64% of embryotransfers were performed on day 2, versus 70% in the agonist group. Embryotransfer was performed according to the Belgian legislation (Ombelet et al., 2005). This means that under the age of 36 years, all patients received a single embryo transfer in their first and second IVF attempt. If however in the second attempt, no top quality embryo was available, double embryo transfer was allowed. Double embryotransfer was allowed for all patients of 36 years and older, regardless the number of attempts.The mean number of embryos transferred in both groups was 1,2. Pregnancy rate per ET (HCG +) in the antagonist group was 37% with an ongoing pregnancy rate per ET of 21% and an implantation rate of 22%; versus respectively 39%, 20% and 22% in the agonist treated group. Ongoing pregnancy rate per started cycle was 19%in the antagonist gorup and 21% in the agonist group. Live birth rate per started cylce was 19% in the antagonist group versus 20% in the agonist group. In both groups a mean of 3,5 embryos could be cryopreserved.
Table I. Comparison of both groups.
| Antagonist | Agonist | p-values | |
| Mean Age (years) | 32 +/- 3.9 | 31.3 +/- 4.5 | NS |
| Nb. IVF | 15* | 21 | NS |
| Nb. ICSI | 58* | 58 | NS |
| Day 2 transfer | 47 (64%) | 53 (70%) | NS |
| Day 3 transfer | 26 | 23 | NS |
| 1st IVF attempt | 59 (74%) | 63 (79%) | NS |
NS = Not statistically significantly different
* In 1 patient split IVF/ICSI was performed.
Table II. Results of the study.
| Antagonist | Agonist | p-value | |
| Number of oocyte pick-up | 74 | 79 | NS |
| Cancellation rate | 7.5 % | 1.2 % | NS |
| Mean duration of stimulation (days) | 10.9 | 10.2 | NS |
| Mean dosage of rFSH needed (IU) | 1897 | 1655 | P = 0.02 |
| Mean Oestradiol levels (pg/ml) | 1087 | 2457 | P < 0.01 |
| Mean number of oocytes | 11.0 | 11.2 | NS |
| Mean number of embryos | 6.5 | 6.3 | NS |
| Fertilization rate | 59% | 56% | NS |
| Mean number embryos/transfer | 1.2 | 1.2 | NS |
| Positive hCG | 37 % | 39 % | NS |
| Implantation rate | 22 % | 22 % | NS |
| Ongoing pregnancy rate | 21 % | 20 % | NS |
| Live Birth Rate | 19% | 20% | NS |
| Mean number of embryos cryopreserved | 3.5 | 3.6 | NS |
NS = Not statistically significantly different.
Pregnancy rate was slightly higher in the group who received embryotransfer on day 3: 43% versus 36%, but this difference is not statistically significant (p = 0,476). This difference was only seen in the antagonist group (46% on day 3 versus 32% on day 2). In the agonist group no difference in pregnancy rate according to day of transfer was found (39% versus 40%).
The study design did not exclude the possibility for patients to be included 2 times in the study. This is a weakness of this study. 19 patients participated 2 cycles in the study. 5 of them were pregnant in the first cycle, these pregnancies were not ongoing. The distribution of these patients was equally in both study groups (16 cycles in the antagonist group and 22 in the agonist group).
In both groups a rather high rate of biochemical pregnanies and miscarriages was observed. In the agonist group 10 biochemical pregnancies (33%) were noted versus 6 in the antagonist group (22%). Miscarriage rate was 29% in the antagonist group and 15% in the agonist group. Ongoing pregnancy rate (21% in the antagonist group versus 20% in the agonist group) and live birth rate (19% in the antagonist group versus 20% in the agonist group) per started cylce were comparable in both groups.
Discussion
To our knowledge this is the first study comparing the use of a short agonist protocol with the antagonist protocol in an overall IVF-population.
We observed an ongoing pregnancy rate, implantation rate, number of oocytes retrieved and duration of stimulation that is comparable in both groups. The only difference found between the 2 groups is the dosage of rec FSH needed for stimulation which was significantly higher in the antagonist group and the oestradiol levels which were lower in the antagonist group. This is somewhat surprising as it is the general opinion that the antagonist protocol resulted in a lower administration of rec FSH units, but most of the studies were comparing with agonist long protocols.
An initial meta-analysis in the Cochrane database reported that GnRH antagonists are associated with a shorter duration of stimulation, a reduced gonadotropin consumption and reduces ovarian hyperstimulation incidence in comparison to a long GnRH agonist protocol.
Although initially the antagonist protocol was used in poor responders hoping for a better ovarian response, these studies mostly compared an antagonist protocol to an agonist long protocol (Akman et al., 2000; Marci et al., 2005; Franco et al., 2006). We observed in our study a higher cancellation rate of 8% in the antagonist treated group due to low ovarian reaction versus 1 % in the agonist protocol, this despite a slightly higher mean age in the agonist group. This difference however was not significant.
Our data could not confirm the findings of recent studies comparing both protocols in poor responders, showing different results in number of oocytes retrieved and pregnancy rates. The study of De Placido (De Placido et al., 2006) in a subgroup at risk for poor ovarian respons showed an increase in mature oocytes and oocyte quality in the antagonist group.
Lainas et al. (2008) showed an increase in pregnancy rate in the antagonist group compared to a short flare-up protocol in poor responders.
Other studies (Malmusi et al., 2005; Mohamed et al., 2005; Demirol and Gurgan, 2009) have shown, that the flare-up protocol is more effective than the GnRH-antagonist protocol in terms of retrieved oocytes and top-quality embryos in poor responder patients.
Bodri et al. (2006, 2011) used oocyte donation cycles to compare both protocols. They observed that in oocyte donation cycles, both the short GnRH agonist and antagonist protocols appear to be similar in ovarian response and embryo quality and comparable in terms of recipients’ pregnancy and implantation rates.
A possible explanation of the adverse effect of antagonists in some studies could be based due to an effect on the endometrium. The study of Rackow showed that endometrial receptivity is decreased in cycles with GnRH antagonist use (Rackow et al., 2008). This could also explain why this effect is not observed in oocyte donation cycles.
Lainas et al. (2009) reported the results of a study comparing the antagonist protocol to GnRH agonist long protocol in patients with polycystic ovary syndrome. They concluded that the incidence or OHSS was lower in the antagonist group.
In 2011 another cochrane review was published including 45 RCT comparing GnRH anatagonist to GnRH agonist long protocol. According to this review a reduction in incidence of OHSS can be observed in the GnRH antagonist group (Al-Inany 2011).
Our results are difficult to compare with the previous mentionned studies as our study was performed in a group of non selected patients referred to our IVF program not taking into account a possible poor ovarian response.
Conclusion
This prospective randomized study shows that live birth rate, implantation rates and evolutive pregnancy rates are equal for the short agonist protocol and the antagonist protocol in an overall IVF-population. An identical number of oocytes was retrieved in both groups, with no difference in duration of the stimulation although a higher dosis of FSH was used in the antagonist group and lower oestradiol levels were noted in the antagonist group.
References
- Akman MA, Erden HF, Tosun SB, et al. Addition of GnRH antagonist in cycles of poor responders undergoing IVF.(2000) Hum Reprod. 2000;15(10):2145–2147. doi: 10.1093/humrep/15.10.2145. [DOI] [PubMed] [Google Scholar]
- Akman MA, Erden HF, Tosun SB, et al. Comparison of agonistic flare-up-protocol and antagonistic multiple dose protocol in ovarian stimulation of poor responders: results of a prospective randomized trial. Hum Reprod. 2001;16(5):868–870. doi: 10.1093/humrep/16.5.868. [DOI] [PubMed] [Google Scholar]
- Al-Inany HY, Youssef MA, Aboulghar M, et al. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2011;5(CD001750) doi: 10.1002/14651858.CD001750.pub3. [DOI] [PubMed] [Google Scholar]
- Bodri D, Sunkara SK, Coomarasamy A. Gonadotropin-releasing hormone agonists versus antagonists for controlled ovarian hyperstimulation in oocyte donors: a systematic review and meta-analysis. Fertil Steril. 2011;95(1):164–169. doi: 10.1016/j.fertnstert.2010.06.068. [DOI] [PubMed] [Google Scholar]
- Bodri D, Vernaeve V, Guillen JJ, et al. Comparison between a GnRH antagonist and a GnRH agonist flare-up protocol in oocyte donors: a randomized clinical trial. Hum Reprod. 2006;(9):2246–2251. doi: 10.1093/humrep/del152. [DOI] [PubMed] [Google Scholar]
- De Placido G, Mollo A, Clarizia R, et al. Gonadotropin-releasing hormone (GnRH) antagonist plus recombinant luteinizing hormone vs. a standard GnRH agonist short protocol in patients at risk for poor ovarian response. 2006;85(1):247–250. doi: 10.1016/j.fertnstert.2005.07.1280. [DOI] [PubMed] [Google Scholar]
- Demirol A, Gurgan T. Comparison of microdose flare-up and antagonist multiple-dose protocols for poor-responder patients: a randomized study. 2009;92(2):481–485. doi: 10.1016/j.fertnstert.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Franco JG, Jr. , Baruffi RL, Mauri AL, et al. GnRH agonist versus GnRH antagonist in poor ovarian responders: a meta-analysis. Reprod Biomed Online. 2006;13(5):618–627. doi: 10.1016/s1472-6483(10)60651-7. [DOI] [PubMed] [Google Scholar]
- Frydman R, Allart J, Parneix I, et al. Comparison between flare up and down regulation effects of luteinizing hormone-releasing hormone agonists in an in vitro fertilization program. Fertil Steril. 1988;50(3):471–475. doi: 10.1016/s0015-0282(16)60135-8. [DOI] [PubMed] [Google Scholar]
- Frydman R, Parneix I, Allart J, et al. LHRH agonists in IVF: different methods of utilization and comparison with previous ovulation stimulation treatments. Hum Reprod. 1988;3(4):559–561. doi: 10.1093/oxfordjournals.humrep.a136744. [DOI] [PubMed] [Google Scholar]
- Ho CH, Chen SU, Peng FS, et al. Prospective comparison of short and long GnRH agonist protocols using recombinant gonadotrophins for IVF/ICSI treatments. Reprod Biomed Online. 2008;16(5):632–639. doi: 10.1016/s1472-6483(10)60476-2. [DOI] [PubMed] [Google Scholar]
- Hugues JN, Cedrin Durnerin IC. Revisiting gonadotrophin-releasing hormone agonist protocols and management of poor ovarian responses to gonadotrophins. Hum Reprod Update. 1998;4:83–101. doi: 10.1093/humupd/4.1.83. [DOI] [PubMed] [Google Scholar]
- Kolibianakis EM, Collins J, Tarlatzis BC, et al. Among patients treated for IVF with gonadotrophins and GnRH analogues, is the probability of live birth dependent on the type of analogue used? A systematic review and meta-analysis. Hum Reprod Update. 2006;12(6):651–671. doi: 10.1093/humupd/dml038. [DOI] [PubMed] [Google Scholar]
- Lainas TG, Sfontouris IA, Papanikolaou EG, et al. Flexible GnRH antagonist versus flare-up GnRH agonist protocol in poor responders treated by IVF: a randomized controlled trial. Hum Reprod. 2008;23(6):1355–1358. doi: 10.1093/humrep/den107. [DOI] [PubMed] [Google Scholar]
- Lainas TG, Sfontouris IA, Zorzovilis IZ, et al. Flexible GnRH antagonist protocol versus GnRH agonist long protocol in patients with polycystic ovary syndrome treated for IVF: a prospective randomised controlled trial (RCT) Hum Reprod. 2009;25(3):683–689. doi: 10.1093/humrep/dep436. [DOI] [PubMed] [Google Scholar]
- Malmusi S, La Marca A, Giulini S, et al. Comparison of a gonadotropin-releasing hormone (GnRH) antagonist and GnRH agonist flare-up regimen in poor responders undergoing ovarian stimulation. Fertil Steril. 2005;84(2):402–406. doi: 10.1016/j.fertnstert.2005.01.139. [DOI] [PubMed] [Google Scholar]
- Marci R, Caserta D, Dolo V, et al. GnRH antagonist in IVF poor-responder patients: results of a randomized trial. Reprod Biomed Online. 2005;11(2):189–193. doi: 10.1016/s1472-6483(10)60957-1. [DOI] [PubMed] [Google Scholar]
- Mohamed KA, Davies WA, Allsopp J, et al. Agonist “flare-up” versus antagonist in the management of poor responders undergoing in vitro fertilization treatment. Fertil Steril. 2005;83(2):331–335. doi: 10.1016/j.fertnstert.2004.07.963. [DOI] [PubMed] [Google Scholar]
- Ombelet W, De Sutter P, Van der Elst J, et al. Multiple gestation and infertility treatment: registration, reflection and reaction: The Belgian project. Hum Reprod Update. 2005;11:3–14. doi: 10.1093/humupd/dmh048. [DOI] [PubMed] [Google Scholar]
- Rackow BW, Kliman HJ, Taylor HS. GnRH antagonists may affect endometrial receptivity. Fertil Steril. 2008;89:1234–1239. doi: 10.1016/j.fertnstert.2007.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt DW, Bremner T, Orris JJ, et al. A randomized prospective study of microdose leuprolide versus ganirelix in in vitro fertilization cycles for poor responders. Fertil Steril. 2005;83(5):1568–1571. doi: 10.1016/j.fertnstert.2004.10.053. [DOI] [PubMed] [Google Scholar]
- Scott RT, Navot D. Enhancement of ovarian responsiveness with microdoses of gonadotropin-releasing hormone agonist during ovulation induction for in vitro fertilization. Fertil Steril. 1994;61:880–885. doi: 10.1016/s0015-0282(16)56700-4. [DOI] [PubMed] [Google Scholar]
- Shanbhag S, Aucott L, Bhattacharya S, et al. Interventions for ‘poor responders’ to controlled ovarian hyperstimulation (COH) in in-vitro fertilisation (IVF) Cochrane database of systematic reviews. 2007;(CD004379) doi: 10.1002/14651858.CD004379.pub2. [DOI] [PubMed] [Google Scholar]