Abstract
We report on a patient with chronic herpes simplex virus-2 encephalitis who was characteristic for concomitantly having chronic or recurrent posterior uveitis. A 66-year-old immunocompetent man suffering from a 6-month refractory posterior uveitis developed a 1-month history of impaired short-term memory and orientation. Brain MRI demonstrated hyperintense lesions in the right parietal lobe in diffusion and fluid attenuated inversion recovery (FLAIR) sequences. Cerebrospinal fluid (CSF) examination showed mild pleocytosis and increased protein concentration. Quantitative PCR for HSV-2 DNA was positive in CSF. Treated with acyclovir, his cognitive functions gradually improved and the posterior uveitis was cured. Clinicians must be aware that HSV-2 should be considered in the aetiological investigation of chronic encephalitis in an immunocompetent patient. HSV-2 is well known for its ability to cause unilateral chronic or recurrent posterior uveitis. Therefore, posterior uveitis should be considered as an associated feature of HSV-2 encephalitis.
Background
Herpes simplex virus (HSV) is one of the main pathogens causing infection in the central nervous system (CNS). The HSV encephalitis (HSE) predominantly affects the limbic system and the adjacent areas of the frontotemporal lobe. HSE is caused mainly by HSV type 1 (HSV-1), while HSV type 2 (HSV-2) accounts for 1.6–6.5% of all HSE in adults.1 In contrast to HSV-1 acute encephalitis, a chronic or fluctuating course may be observed in HSV-2 encephalitis.2 We report a case of chronic HSV-2-induced encephalitis that was characteristic for concomitantly having chronic or recurrent posterior uveitis.
Case presentation
A 66-year-old immunocompetent man was referred with a 1-month history of impaired short-term memory and orientation. Six months before admission, he showed generalised convulsion first, and a brain MRI indicated an abnormality in the right parietal lobe (figure 1A,B). Around the same time, he developed red eye and impaired visual acuity of the right eye. Then, the ophthalmological examinations indicated that he developed posterior uveitis, inflammation of the choroid layer behind the retina, based on the funduscopy findings showing multiple white-coloured lesions (figure 1C). Although he has treated with antibiotic or corticosteroid eyedrops for 2 months, the posterior uveitis was not improved (figure 2A). On admission, he was alert but suffered disorientation, impairment of short-term memory, visuospatial agnosia indicative of a considerable cognitive dysfunction. The Mini-Mental State Examination (MMSE) score was 20. There were no meningitic symptoms. He had no skin rash and no lymphadenopathy. Routine laboratory tests, including complete blood count and blood chemistry, were normal. Autoantibodies including antineuronal antibodies were negative. MRI of the brain demonstrated developed hyperintense lesions in the right parietal lobe in diffusion and fluid-attenuated inversion recovery (FLAIR) sequences (figure 2B,C). However, lesions of orbitofrontal or mesial temporal lobes were not revealed. Cerebrospinal fluid (CSF) examination showed 15 cells/mm3 (mononuclear), protein concentration of 47 mg/dL and a glucose level of 66 mg/dL. Oligoclonal IgG bands were detected in the CSF. Quantitative PCR for HSV-2 DNA was positive in CSF (920 000 copies/mL). Thus, the patient was diagnosed as having HSV-2-induced chronic encephalitis and posterior uveitis. Treated with acyclovir (30 mg/kg/day for 28 days), his symptoms gradually disappeared, and the posterior uveitis was cured (figure 3A). Follow-up CSF examination showed 5 cells/mm3. The CSF protein concentration also decreased (32 mg/dL), and HSV-2 DNA changed to negative. Although increased FLAIR signal remained, restricted diffusion had disappeared (figure 3B,C). Corticosteroid was not offered during the course of the disease.
Figure 1.
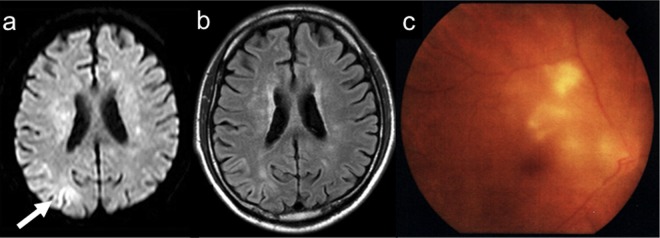
Brain images of diffusion-weighted imaging (A) and fluid-attenuated inversion recovery (B) at 6 months before admission. Restricted diffusion was demonstrated in the right parietal lobe. (C) Funduscopy revealed multiple white-coloured lesions at 2 months before admission.
Figure 2.
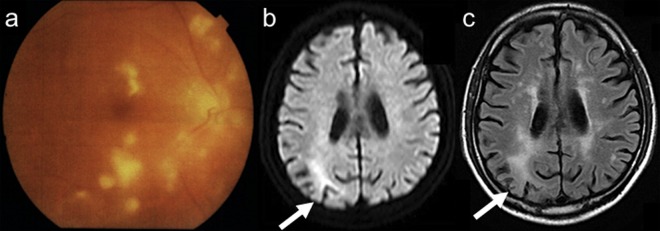
Follow-up of funduscopy examinations (A). Diffuse white-coloured lesions were extended despite antibiotics or corticosteroids. Follow-up images of diffusion-weighted imaging (B) and fluid-attenuated inversion recovery (FLAIR) (C). Restricted diffusion and increased FLAIR signal were seen in the right parietal lobe on admission.
Figure 3.
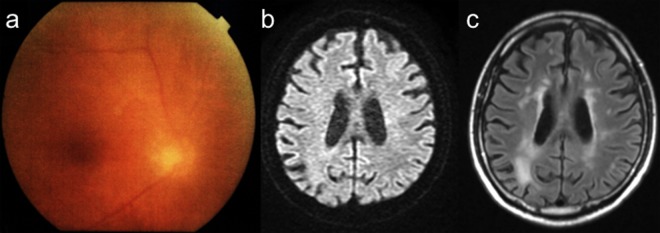
Follow-up of funduscopy examinations (A). Diffuse white-coloured lesions were resolved after treatment with acyclovir. Follow-up images of diffusion-weighted imaging (B) and fluid-attenuated inversion recovery (FLAIR) (C). With the treatment of acyclovir, although increased FLAIR signal remained, restricted diffusion disappeared.
Discussion
In contrast to encephalitis due to HSV-1, a chronic or a fluctuating course may be observed in HSV-2 encephalitis. Cases of HSV encephalitis due to HSV-1 have been associated with lesions of orbitofrontal and mesial temporal lobes. In those of HSV-2 encephalitis, however, MRI may reveal non-specific white matter lesions or normal findings.3 4 As recurrent CNS infectious disease, recurrent aseptic meningitis, called Mollaret’s syndrome is identified. The causative agent of this condition is generally considered to be HSV-2, which is readily detectable in CSF.5 Recent report indicated that recurrent HSV-2 meningitis patients lacked antibodies to HSV-1,6 suggesting that a major risk factor for developing meningitis was the absence of prior HSV-1 infection and, hence, absence of a prior immune response to HSV. Then, similar immune functional deficiencies may contribute to this chronic HSV-2 encephalitis.
Posterior uveitis is inflammation that affects the posterior part of the eye. It can affect the choroid, the head of the optic nerve or the retina. Autoimmune diseases including Behçet’s disease and rheumatoid arthritis are well known to be associated with uveitis. Although infections are a rare cause of uveitis, various infections including bacterial, fungal and viral can cause uveitis. HSV is among the most common causes of infectious uveitis, and it may affect healthy as well as immunocompromised hosts. Posterior uveitis caused by HSV may appear as part of HSV disease elsewhere (skin, brain and anterior segment of the eye). HSV can cause inflammation of the eyes, uveitis and acute retinal necrosis. Previous case reports have been presented where HSE preceded acute retinal necrosis.7–10 In those cases, HSV-1 was mainly detected in the brain and the eye by PCR, and brain-to-eye transmission of HSV-1 by the transaxonal route was suggested.7–9 The present case was characteristic in that encephalitis and posterior uveitis caused by HSV-2 occurred simultaneously, and in addition, both conditions recovered with acyclovir treatment. We need to be aware of the association of encephalitis and uveitis due to HSV-2.
Learning points.
Herpes simplex virus (HSV)-2 should be considered in the aetiological investigation of chronic encephalitis in an immunocompetent patient.
MRI of HSV-2 encephalitis may not reveal lesions of orbitofrontal and mesial temporal lobes suggestive of HSV-1 but non-specific white matter lesions.
HSV-2 is well known for the ability to cause unilateral chronic or recurrent posterior uveitis. Therefore, posterior uveitis should be considered as an associated feature of HSV-2 encephalitis.
Our findings indicated that utilising HSV-2 PCR in the cerebrospinal fluid should be an important diagnostic consideration. HSV-2 encephalitis and concomitant posterior uveitis were successfully treated with acyclovir.
Footnotes
Contributors: All authors have actively participated in the management of this patient and in preparation, editing and critically appraising this manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Aurelius E, Johansson B, Sköldenberg B, et al. Encephalitis in immunocompetent patients due to herpes simplex virus type 1 or 2 as determined by type-specific polymerase chain reaction and antibody assays of cerebrospinal fluid. J Med Virol 1993;39:179–86 [DOI] [PubMed] [Google Scholar]
- 2.Berger JR, Houff S. Neurological complications of herpes simplex virus type 2 infection. Arch Neurol 2008;65:596–600 [DOI] [PubMed] [Google Scholar]
- 3.Urban PP, Jöhnck W, Pohlmann C, et al. Reversible progressive cognitive decline due to herpes simplex type 2 encephalitis with normal MR imaging. J Neurol 2008;255:1975–7 [DOI] [PubMed] [Google Scholar]
- 4.Harrison NA, MacDonald BK, Scott G, et al. Atypical herpes type 2 encephalitis associated with normal MRI imaging. J Neurol Neurosurg Psychiatry 2003;74:974–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kupila L, Vainionpaa R, Vuorinen T, et al. Recurrent lymphocytic meningitis: the role of herpesviruses. Arch Neurol 2004;61:1553–7 [DOI] [PubMed] [Google Scholar]
- 6.Aurelius E. Neurological disease in herpes simplex virus type 2 infection. In: Studahl M, Cinque P, Bergström T. Herpes simplex viruses. New York: Taylor and Francis Group, 2006:317–38 [Google Scholar]
- 7.Maertzdorf J, Van der Lelij A, Baarsma GS, et al. Herpes simplex virus type 1 (HSV-1)-induced retinitis following herpes simplex encephalitis: indications for brain-to-eye transmission of HSV-1. Ann Neurol 2001;49:104–6 [DOI] [PubMed] [Google Scholar]
- 8.Gain P, Chiquet C, Thuret G, et al. Herpes simplex virus type 1 encephalitis associated with acute retinal necrosis syndrome in an immunocompetent patient. Acta Ophthalmol Scand 2002;80:546–9 [DOI] [PubMed] [Google Scholar]
- 9.Vandercam T, Hintzen RQ, de Boer JH, et al. Herpetic encephalitis is a risk factor for acute retinal necrosis. Neurology 2008;71:1268–74 [DOI] [PubMed] [Google Scholar]
- 10.Kianersi F, Masjedi A, Ghanbari H. Acute retinal necrosis after herpetic encephalitis. Case Rep Ophthalmol 2010;1:85–9 [DOI] [PMC free article] [PubMed] [Google Scholar]


