Abstract
Stiff person syndrome (SPS) is a rare condition that causes rigidity in the muscles of the body and extremities, difficulty in walking, episodic spasms and progressive disability. SPS is generally seen together with autoimmune disorders such as diabetes mellitus, thyroiditis, vitiligo and pernicious anaemia. Rarely, it may develop as a paraneoplastic condition. SPS cases associated with breast cancer, small cell lung carcinoma, thymoma, Hodgkin's lymphoma and colorectal cancer have been reported in the literature. We present a case of a 58-year-old female patient who had malignant mesothelioma-associated SPS. Patients who have muscle spasms and difficulty in movement of joints should be evaluated for SPS before diagnosis of Parkinson's or other neurological disorders, and possible underlying malignancies should be excluded.
Background
Stiff person syndrome (SPS) is a rare condition that causes rigidity in the muscles of the body and extremities, difficulty in walking, episodic spasms and progressive disability. In addition, autonomic symptoms including excessive sweating/hyperhidrosis, elevated pulse, changes in blood pressure, constipation and urinary retention may accompany the clinical condition.1 2 The insufficient synthesis of γ-aminobutyric acid (GABA) or the toxic effects of antiglutamic acid decarboxylase (GAD) antibodies on the GABAergic neurons through an autoimmune process is considered to play a role in the development of this disease.2 SPS is generally seen together with autoimmune disorders such as diabetes mellitus, thyroiditis, vitiligo and pernicious anaemia. Rarely, it may develop as a paraneoplastic condition. SPS cases associated with breast cancer, small cell lung carcinoma, thymoma, Hodgkin's lymphoma and colorectal cancer have been reported in the literature.3–6 We present a case of a 58-year-old female patient who had malignant mesothelioma associated with SPS, together with literature findings.
Case presentation
A 58-year-old female patient was admitted to the clinic with rigidity in the upper and lower extremities and abdominal muscles, painful muscle spasms and difficulty walking. According to the medical history, the patient was being followed up with mesothelioma diagnosis, and was on carboplatin (AUC 5)+gemcitabine (1000 mg/m2) treatment once every 3 weeks. The patient did not have a history of stroke, Parkinson's disease, epilepsy, multiple sclerosis, carbon monoxide poisoning, exposure to toxic substances, encephalitis, spinal cord injury, peripheral nerve lesion, diabetes mellitus, hypertension, trauma or arthritis. Physical examination showed that the patient's body mass index was 22 kg/m2. Lumbar lordosis was elevated in the posture examination, and the upper extremities were in flexion from the elbow (figure 1). Gait was possible with a bilateral elbow crutch; flexion in the hips and knees was limited during gait, and gait was with small steps and was antalgic. In addition, the rhythmic swinging of the bilateral upper extremity was reduced during walking. The joint movement spacing in the bilateral upper and lower extremities was open, but was resistant and painful at the end of the movement. The motor, sensory and deep tendon reflexes were normal. The plantar response was flexion, and there was no clonus. The cranial nerves were intact.
Figure 1.
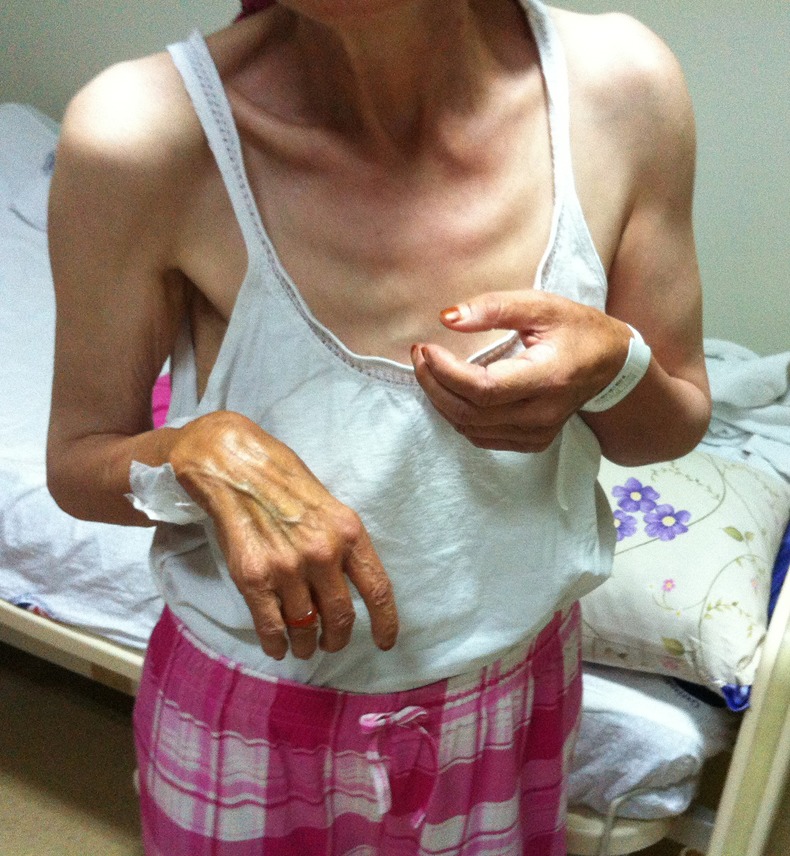
The elbows of the patient seem to be in flexion position.
Investigations
The patient's previous thorax CT showed linear densities extending to the pleura that were more prominent in the lower lobe of the right lung, which were accompanied by minimal ground glass densities, a thickening in the right pleura, low-density pleural effusion and bilateral axillary millimetric lymph nodes (figure 2A). The histopathological evaluation of the biopsy material collected from the pleura was reported as ‘epithelial malignant mesothelioma’ (figure 2B).
Figure 2.
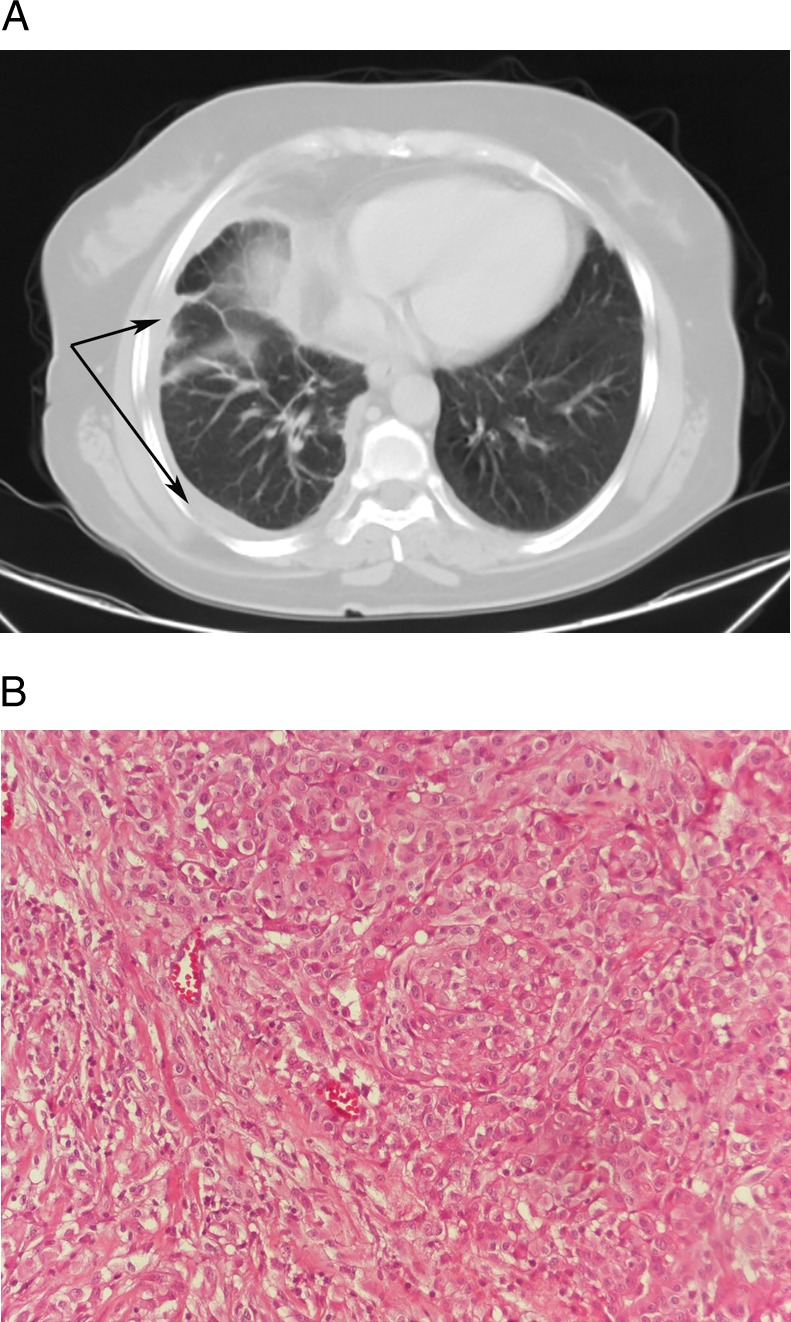
(A) A case with malignant mesothelioma: linear densities extending to the pleura that are more prominent in the lower lobe of the right lung, accompanied by minimal ground glass densities and a thickening in the right pleura. (B) Tumoural structure consisting of epithelioid cells that has a vesicular nucleus, locally distinct nucleoli, papillary structures and glandular organisation (×200, H&E).
The patient's whole blood count, erythrocyte sedimentation rate, C reactive protein, fasting glucose level, thyroid, liver and kidney functions, electrolyte panel, vitamin B12, folic acid, vitamin D levels, creatine kinase, whole urine examination, rheumatoid factor, antinuclear antibody, human leucocyte antigen B27 and brucella tube agglutination test results were normal. The urine and blood cultures were negative. In addition, ocular examination, sacroiliac graphy, whole abdominal ultrasonography, respiratory function test, electrocardiography and MRI of the brain and spinal colon were normal (figure 3). Cerebrospinal fluid was clear and had normal pressure (120 cm H2O); protein and glucose levels were normal, and there was no oligoclonal band. Electroneuromyography showed synchronous, hyper and involuntary firing of the motor units in agonist and antagonist muscles. The patient responded to an unexpected auditory stimulant by jumping. A normal interference pattern was determined during the spasms. The nerve transmission rates were normal. There was a normalisation in the EMG following the administration of 10 mg of intravenous diazepam. In addition, serum anti-GAD antibodies were positive (140 IU/mL, normal range <1.5). However, amphiphysin and gephyrin antibodies could not be evaluated due to the limited laboratory conditions. Given the existing evaluation and the examination findings, the patient's symptoms related to the musculoskeletal system were considered to be associated with SPS that developed secondary to malignant mesothelioma.
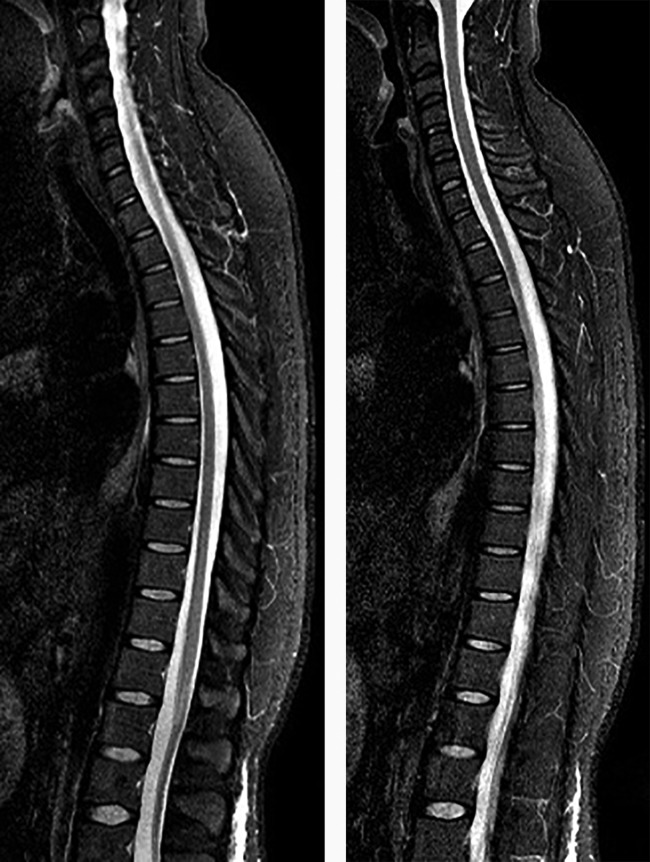
Figure 3.
The spinal MRI of the patient evaluated as normal.
Treatment
The patient was started on 10 mg of benzodiazepine (2×1), 10 mg of baclofen (4×1; started with 2×1/2 and increased gradually), 0.5 mg/kg/day of methylprednisone (oral) and 100 mg of tramadol (1×1/day). In addition, the patient was started on a rehabilitation programme that consisted of exercises towards joint movement spacing in the lumbar area, upper and lower extremities, stretching–relaxing and isometric exercises and mobilisation training.
Outcome and follow-up
Painful muscle spasms continued in the control evaluations, while their severity and frequency decreased. The patient was admitted to the intensive care unit in the third follow-up month due to respiratory failure and overall condition, and was declared deceased despite all treatments.
Discussion
SPS is a rare neurological disorder with autoimmune features. According to the literature, SPS has been reported to develop insidiously in the middle ages, and especially after emotional stress. It has an incidence of 1/1 000 000, with a female/male ratio of 2/1.7 While SPS is usually seen with autoimmune disorders, it may appear as a paraneoplastic condition in 5% of the cases.8 Paraneoplastic SPS is reported to usually present with breast cancer, small cell lung carcinoma, thymoma and Hodgkin's lymphoma.8 Moreover, two cases related to colorectal cancer have been reported.9 10
The Dalakas criteria are used worldwide to diagnose SPS11
Stiffness in the axial muscles, prominently in the abdominal and thoracolumbar paraspinal muscle leading to a fixed deformity (hyperlordosis);
Superimposed painful spasms precipitated by unexpected noises, emotional stress, tactile stimuli;
Confirmation of the continuous motor unit activity in agonist and antagonist muscles by electromyography;
Absence of neurological or cognitive impairments that could explain the stiffness;
Positive serology for GAD65 (or amphiphysin) autoantibodies, assessed by immunocytochemistry, western blot or radioimmunoassay;
Response to diazepam.
Patients not meeting these criteria (eg, without the classical axial distribution of stiffness and rigidity) are described as atypical. In general, our case meets most of the diagnostic criteria for SPS.
Malignant mesothelioma is a mortal disease that originates from mesothelial cells, has an insidious course and presents itself with local symptoms such as chest pain and difficulty in breathing. The mean annual incidence of mesothelioma is 1.3/100 000 for men, and 0.2/100 000 for women, while the mean age is 55.6 years.12 The present case is important as it is the first reported case of SPS that is associated with malignant mesothelioma.
Determining the antibodies against the GAD enzyme in majority of SPS cases has contributed significantly to our understanding of SPS's pathophysiological and specific immunological background. GAD is an enzyme present in the presynaptic terminals that play a role in GABA synthesis, as well as GABAergic inhibitory synapses. The insufficient synthesis of GABA or the toxic effects of GAD antibodies on the GABAergic neurons through an autoimmune process is considered to play a role in the development of this disease. Anti-GAD antibodies are found in only 70% of the cases.3 Progressive variants of SPS, which have less association with GAD antibodies, and which have limited involvement with the stiff-limb syndrome, encephalomyelitis, rigidity and myoclonus have been also reported.13 Antibodies against amphiphysin have been detected in the paraneoplastic variants of SPS, especially those associated with breast cancer. Similar to GAD, these substances play a role in the regulation of neural transmission.5 Moreover, antibodies against gephyrin have been detected in a case with a mediastinal tumour.14 In the present case, amphiphysin and gephyrin antibodies were not analysed; however, the finding that GAD antibodies were positive was one of the factors supporting the SPS diagnosis.
Since the agonist and antagonist muscles in the lower extremities are usually contracted in SPS, sufficient hip and knee flexion is not observed during walking, and the step length is shortened. At the same time, there are painful spasms in the abdominal and lumbar muscles. The muscles of the upper extremity are rarely involved.8 Symptoms of autonomic dysfunction may be observed in some patients. In the present case, the involvement of the lower extremities and body muscles, and muscle spasms in the upper extremities, while being more prominent in the flexor muscles, were interesting.
Intravenous immunoglobulin (IVIG), plasmapheresis, anxiolytics, myorelaxants, steroids and anticonvulsants, which are suggested for SPS treatment, ameliorate the symptoms but do not cure the disease.8 IVIG and plasmapheresis treatments were not performed as the current case had muscoskeletal symptoms continuing for 1 year. On the other hand, there had been only a partial improvement in her situation by other medical treatments and rehabilitation programmes.
Parkinson's disease (PD) is the second most common neurodegenerative disease. Current studies estimate that 1–2 million people in the USA suffer from PD. Multiple system atrophy, progressive supranuclear palsy and corticobasal degeneration are the most common nonparkinsonian disorders and account for almost 15% of patients with parkinsonism evaluated in academic subspecialty movement disorder clinics.15 SPS is rare, although its true frequency has not been fully ascertained. The British Neurological Surveillance Unit identified 119 cases among the UK population over 5 years (2000–2005), implying a prevalence of 1–2 cases/million.11
Considering that GAD antibodies may be negative in approximately one-third of the patients, patients who have muscle spasms and difficulty in movement of joints should be evaluated for SPS before diagnosis of Parkinson's or other neurological disorders, and possible underlying malignancies should be excluded. In this context, in our opinion horses are the first thought but one must not forget the zebras even if they are rare.
Learning points.
The present case is important as it is the first reported case of Stiff person syndrome (SPS) that is associated with malignant mesothelioma.
Determining the antibodies against the antiglutamic acid decarboxylase enzyme in the majority of SPS cases has contributed significantly to our understanding of SPS's pathophysiological and specific immunological background.
Patients who have muscle spasms and difficulty in movement of joints should be evaluated for SPS before diagnosis of Parkinson's or other neurological disorders, and possible underlying malignancies should be excluded.
Parkinson's disease is the second most common neurodegenerative disease, whereas, SPS is very rare. So in our opinion, horses are the first thought but one must not forget the zebras even if they are rare.
Footnotes
Contributors: All the authors have contributed to the case report as related to the manuscript's conception and design, interpretation of data, revising it critically for important intellectual content and final approval.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Toro C, Jacobowitz DM, Hallett M. Stiff-man syndrome. Semin Neurol 1994;14:154–8 [DOI] [PubMed] [Google Scholar]
- 2.Fatima A, Merrill R, Bindu J, et al. Stiff-person syndrome (SPS) and anti-GAD related CNS degenerations: protean additions to the autoimmune central neuropathies. J Autoimmun 2011;37:79–87 [DOI] [PubMed] [Google Scholar]
- 3.Dalakas MC, Fujii M, Li M, et al. The clinical spectrum of anti-GAD antibodypositive patients with stiffperson syndrome. Neurology 2000;55:1531–5 [DOI] [PubMed] [Google Scholar]
- 4.Rosin L, DeCamilli P, Butler M, et al. Stiff-man syndrome in a woman with breast cancer: an uncommon central nervous system paraneoplastic syndrome. Neurology 1998;50:94–8 [DOI] [PubMed] [Google Scholar]
- 5.Nguyen-Huu BK, Urban PP, Schreckenberger M, et al. Antiamphiphysin-positive stiff-person syndrome associated with small cell lung cancer. Mov Disord 2006;21:1285–7 [DOI] [PubMed] [Google Scholar]
- 6.Sommer C, Weishaupt A, Brinkhoff J, et al. Paraneoplastic stiff-person syndrome: passive transfer to rats by means of IgG antibodies to amphiphysin. Lancet 2005;365:1406–11 [DOI] [PubMed] [Google Scholar]
- 7.Bilic E, Bilic E, Sepec BI, et al. Stiff-person syndrome, type 1 diabetes, dermatitis herpetiformis, celiac disease, microcytic anemia and copper deficiency. Just a coincidence or an additional shared pathophysiological mechanism? Clin Neurol Neurosurg 2009;111:644–5 [DOI] [PubMed] [Google Scholar]
- 8.Hadavi S, Noyce AJ, Leslie RD, et al. Stiff person syndrome. Pract Neurol 2011;11:272–82 [DOI] [PubMed] [Google Scholar]
- 9.Badzek S, Miletic V, Prejac J, et al. Paraneoplastic stiff person syndrome associated with colon cancer misdiagnosed as idiopathic Parkinson's disease worsened after capecitabine therapy. World J Surg Oncol 2013;11:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu YL, Lo WC, Tseng CH, et al. Reversible stiff person syndrome presenting as an initial symptom in a patient with colon adenocarcinoma. Acta Oncol 2010;49:271–2 [DOI] [PubMed] [Google Scholar]
- 11.Dalakas MC. Stiff person syndrome: advances in pathogenesis and therapeutic interventions. Curr Treat Options Neurol 2009;11:102–10 [DOI] [PubMed] [Google Scholar]
- 12.Metinta S, Metinta M, Ucgun I, et al. Malignant mesothelioma due to environmental exposure to asbestos: follow up of a Turkish cohort living in a rural area. Chest 2002;122:2224–9 [DOI] [PubMed] [Google Scholar]
- 13.Barker RA, Revesz T, Thom M, et al. Review of 23 patients affected by the stiff man syndrome: clinical subdivision into stiff trunk (man) syndrome, stiff limb syndrome, and progressive encephalomyelitis with rigidity. J Neurol Neurosurg Psychiatry 1998;65:633–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler M, Hayashi A, Ohkoshi N, et al. Autoimmunity to gephyrin in stiff-man syndrome. Neuron 2000;26:307–12 [DOI] [PubMed] [Google Scholar]
- 15.De Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol 2006;5:525–35 [DOI] [PubMed] [Google Scholar]