Abstract
African Americans and Hispanics are disproportionately affected by the HIV/AIDS epidemic. Within the most heavily affected cities, a few neighborhoods account for a large share of new HIV infections.
Addressing racial and economic disparities in HIV infection requires an implementation program and research agenda that assess the impact of HIV prevention interventions focused on increasing HIV testing, treatment, and retention in care in the most heavily affected neighborhoods in urban areas of the United States.
Neighborhood-based implementation research should evaluate programs that focus on community mobilization, media campaigns, routine testing, linkage to and retention in care, and block-by-block outreach strategies.
Although HIV incidence in the United States has remained relatively stable since the mid-1990s, rates among African Americans and Hispanics are 8 and 3 times those among Whites, respectively.1 Approximately 65% of new HIV infections in the United States occur in non-White populations. Individual behavioral risk factors, including unprotected sex and substance use, do not fully explain racial disparities in HIV infection; minority populations do not engage in higher rates of HIV risk behaviors than individuals of other races.2
GEOGRAPHIC AND RACIAL DISPARITIES IN HIV INFECTION
New research underscores the pivotal role that sexual networks, structural factors, and geography play in potentiating HIV risks; a recent study published in Morbidity and Mortality Weekly Report revealed strong associations between HIV and poverty, low socioeconomic status (SES), unemployment, and lower educational attainment in 24 US cities.3 A subsequent article published in the same journal showed that AIDS prevalence was 2.3% overall in urban census tracts with high poverty rates.4 Similarly, new mapping tools (for examples, see www.aidsvu.org) help visualize associations between low SES, race, and geographic clustering of HIV infections in these same heavily affected communities. HIV prevalence rates in certain urban neighborhoods rival those of some sub-Saharan African countries. Within the most highly affected US cities, a discrete number of specific neighborhoods account for a large share of HIV infections and AIDS-related mortality.
For example, in Washington, DC, 2.7% of the general population is infected with HIV, but the epidemic is most heavily concentrated in wards 5, 6, 7, and 8, where residents are predominantly African American and of low SES, and where the HIV prevalence rate is as high as 3.1%. This is a stark contrast with ward 3, where residents are predominantly White and of higher SES, and the HIV prevalence rate is 0.4%.5
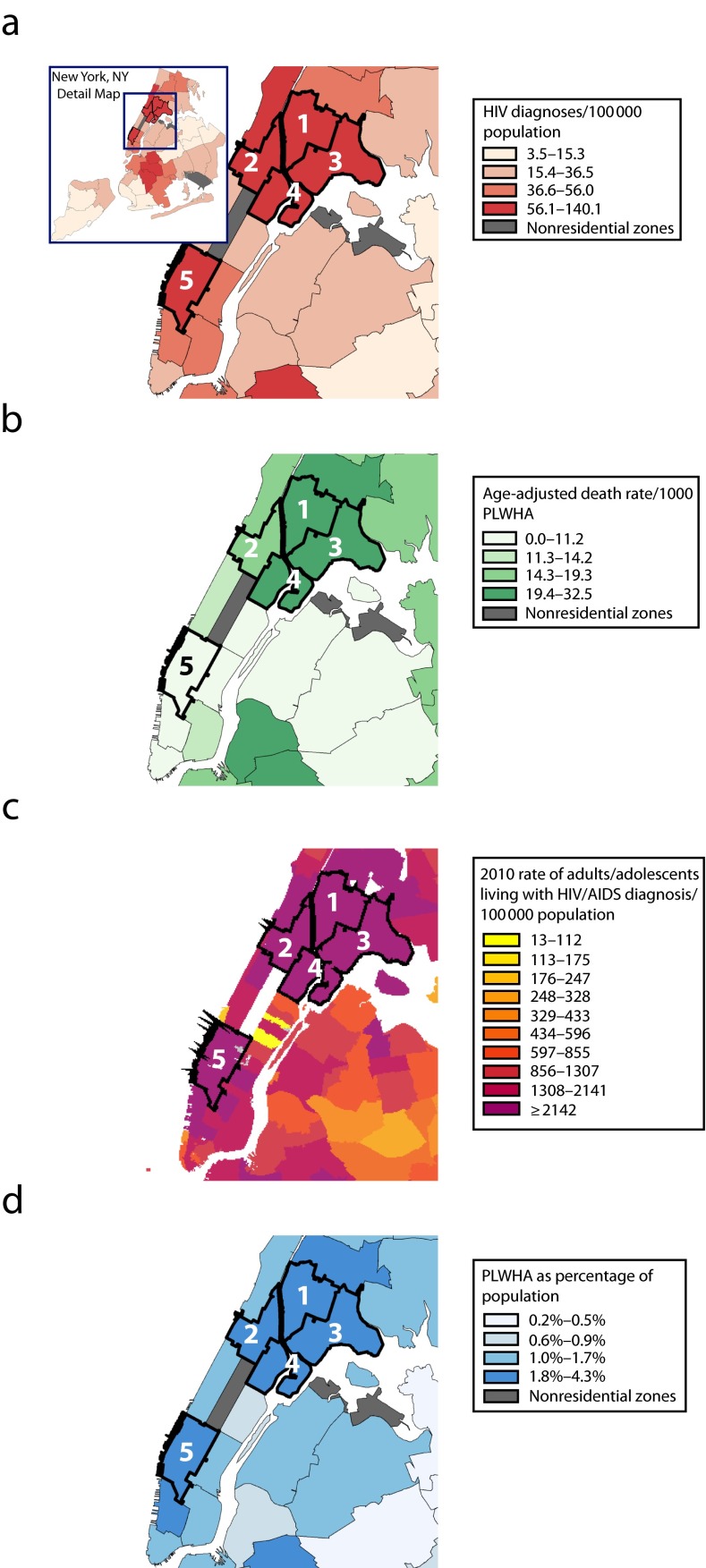
Similarly, although New York City has an overall HIV prevalence rate of 1.4%, the predominantly African American and Hispanic neighborhoods of East Harlem, Central Harlem, High-Bridge Morrisania, and Hunts Point–Mott Haven, as well as predominantly White Chelsea, have rates ranging from 2.4% to 4.5% (Figure 1). However, AIDS-related mortality rates in the predominantly White neighborhood of Chelsea, which has a large gay population, are far lower than those in other predominantly African American and Hispanic neighborhoods with high infection rates.
FIGURE 1—
Racial and geographic disparities in HIV/AIDS outcomes in New York City neighborhoods for (a) HIV diagnoses (b) age-adjusted death rate (c) 2010 rate of adults/adolescents living with HIV/AIDS diagnosis and (d) PLWHA as percentage of population: 2012.
Note. PLWHA = people living with HIV/AIDS. Marked neighborhoods are (1) High-Bridge Morrisania, (2) Central Harlem–Morningside Heights, (3) Hunts Point–Mott Haven, (4) East Harlem, and (5) Chelsea–Clinton.
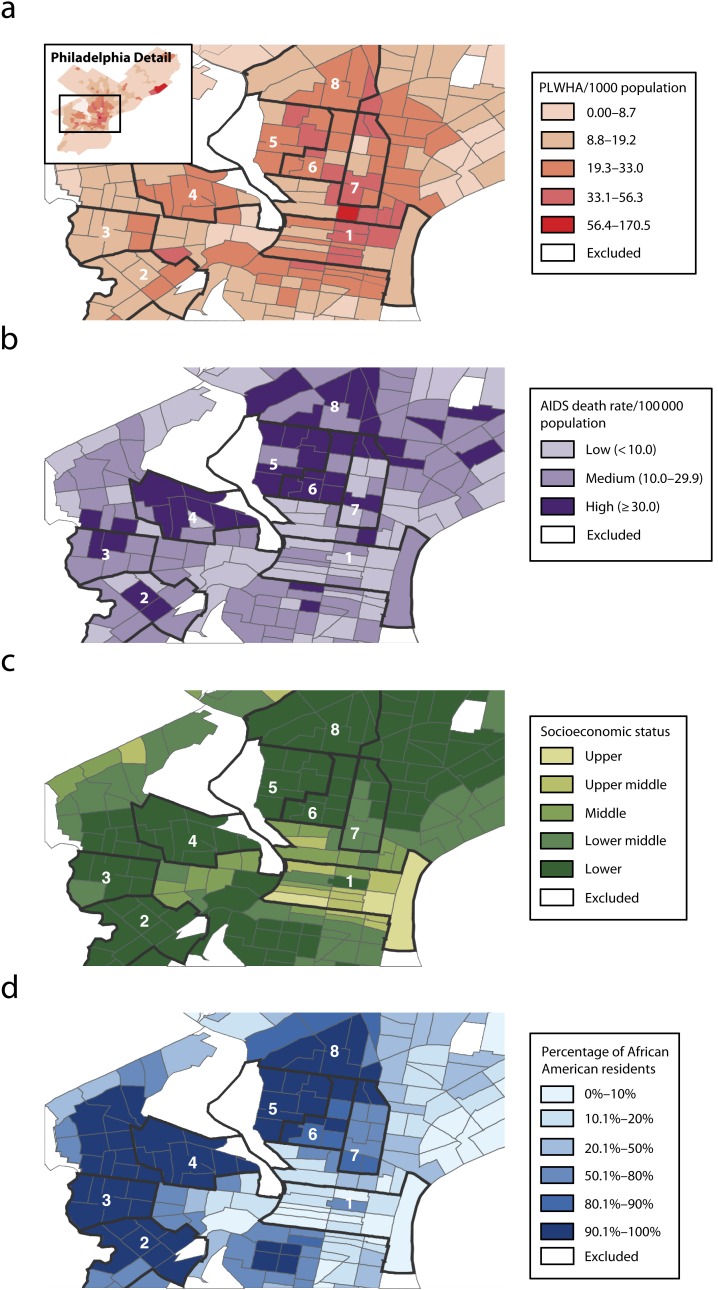
Finally, Philadelphia’s HIV infection rate of 114 per 100 000 is five times the national average. Although HIV prevalence in Philadelphia is high among residents of Center City, an affluent, predominantly White neighborhood with a large gay community, AIDS-related mortality in Center City is far lower than that in predominantly African American neighborhoods with high rates of infection (Figure 2).6 These higher rates of HIV infection and AIDS-related mortality in inner-city communities exemplify many of the public health challenges our nation faces in addressing domestic HIV/AIDS microepidemics.
FIGURE 2—
Racial and socioeconomic disparities in AIDS-related mortality, by census tract and neighborhood in Philadelphia, PA, for (a) PLWHA (b) AIDS death rate (c) socioeconomic status, and (d) percentage of African American Residents: 2011.
Note. PLWHA = people living with HIV/AIDS. Marked neighborhoods are (1) Center City, (2) Paschall–Kingsessing, (3) Cobbs Creek, (4) Mill Creek–Parkside, (5) Strawberry Mansion, (6) Shaswood–tanton, (7) Poplar–Temple, and (8) Nicetown–Tioga.
Source. Data were derived from Office of HIV Planning of Philadelphia.6
The National HIV/AIDS Strategy (NHAS) calls for reducing HIV incidence, increasing access to care, reducing HIV-related health disparities, and distributing resources to the most heavily affected communities. To accomplish these objectives, the Centers for Disease Control and Prevention (CDC) has allocated $64 million for Enhanced Comprehensive HIV Prevention Planning demonstration project grants for the 12 metropolitan areas with the largest number of people living with AIDS; together, these municipalities account for 44% of the nation’s AIDS cases.
HIV infection often clusters in neighborhoods that already experience significant social and economic burdens; thus, if the NHAS is to be maximally effective, public health officials will need to address these microepidemics by concentrating resources, program evaluation efforts, and implementation research in the most heavily affected communities. We outline an implementation research agenda and strategy that prioritizes a neighborhood-centered approach to HIV prevention.
THE CASE FOR A RESEARCH IMPLEMENTATION AGENDA
HIV testing and treatment are among the most effective HIV prevention interventions, given that individuals with positive HIV test results tend to reduce their risk behaviors7 and individuals with HIV who adhere to highly active antiretroviral therapy can dramatically decrease their chances of transmitting HIV to others.8 However, despite advances in testing, approximately 18% of Americans with HIV are unaware of their infection, only 50% are enrolled in treatment and care, and fewer than 30% of the 1.2 million with HIV in the United States are virologically suppressed.9 Suppressing HIV plasma viremia improves the health of those living with HIV and has salutary public health ripple effects in that it dramatically reduces the chances of the virus being passed to others.
African Americans and Hispanics have lower rates of timely HIV diagnosis, linkage to and retention of care, and suppressed HIV infection than people of other races and ethnicities.10 Many of these individuals reside in inner-city communities. Increasing the number of HIV-positive individuals with virologically suppressed HIV has become a public health priority; the National Institutes of Health (NIH) is now focusing greater efforts on implementation research, defined by NIH as
research that will identify, develop, and refine effective and efficient methods, systems, infrastructures, and strategies to disseminate and implement evidence-based health behavior change interventions, prevention, early detection, diagnosis, treatment, symptom management, and quality of life improvement interventions, clinical guidelines, policies, and data monitoring and surveillance reporting tools into public health and clinical practice settings.11
In the context of HIV/AIDS, implementation research could be used to develop and determine the best ways to scale programmatic efforts to increase the number of people living with HIV/AIDS who are virologically suppressed. Given the focal nature of the US epidemic, the aforementioned racial disparities suggest that culturally tailored and geographically circumscribed efforts are needed to promote more widespread uptake of testing and treatment in neighborhoods with high HIV prevalence rates.
Although recent analyses show that many federal HIV/AIDS expenditures are allocated to metropolitan areas where large numbers of individuals are living with HIV,12 these data do not clarify whether resources are reaching neighborhoods with the highest rates of new HIV infections. Given that many new HIV infections are concentrated in a few neighborhoods within much larger jurisdictions, concentrating HIV prevention efforts on neighborhoods, rather than cities or individual behaviors, presents an opportunity to begin to address geographic and racial disparities in HIV testing, treatment, and care. These efforts should focus on increasing the number of individuals who know their HIV status, enhancing and hastening linkage to HIV/AIDS care, retaining individuals in care, and suppressing HIV viral loads. Focusing on neighborhoods may also create a more inclusive and efficient strategy for achieving the NHAS goal of reducing HIV/AIDS-related racial disparities.
In an era of scarce resources and fiscal challenges, our most effective HIV prevention resources should be deployed to the most heavily affected communities. One way to enhance the impact in these communities would be piloting implementation research in which neighborhoods are the unit of intervention and analysis. A neighborhood-based implementation research agenda could start by developing and evaluating programs that
engage local community leaders to help design culturally appropriate initiatives that encourage HIV testing and treatment and address stigma,
create widespread media messages featuring local community leaders promoting HIV testing and treatment in the most heavily affected neighborhoods,
make routine testing readily accessible to all residents,
enhance efforts to link and retain people in care, and
increase local access to HIV testing and care through neighborhood, block-by-block, and even door-to-door testing, engagement, and linkage to care programs.
Each of these elements has been deployed in a limited number of settings in the United States, including the Bronx, New York, and Philadelphia, Pennsylvania, as well as in Thailand and Kenya. We argue that additional efforts should be made to use this implementation strategy in other highly affected communities in the United States. Such programs could help normalize HIV testing, identify undiagnosed infections, link and retain individuals in treatment and care, and provide uninfected persons with prevention interventions.
Community mobilization and leadership are critical in raising awareness, fighting stigma, tailoring programs to local contexts, and stimulating demand for testing and treatment services. New HIV prevention models in developing countries that combine community mobilization with promotion of HIV testing in narrowly defined geographic catchment areas have led to increased testing uptake.13 Domestic programs also offer important lessons.
The Black AIDS Institute’s Black Treatment Advocates Network has trained and mobilized a team of advocates nationwide to test and link more African Americans living with HIV to care. In New York City, the Bronx Knows HIV Testing Initiative, which brought together more than 75 local partners to scale up HIV screening in that highly affected borough, conducted 607 570 HIV tests between June 2008 and June 2011; the overall seropositivity rate was 0.9%, and 84% of newly diagnosed individuals were linked to care within one year of their diagnosis (see the case study in the next section). The Philadelphia Do One Thing campaign employs a social marketing and community mobilization strategy to promote HIV testing and treatment, routine testing in a federally qualified health center, and door-to-door testing outreach, all in a highly affected neighborhood. The program, which has tested more than 2700 individuals for HIV with a 0.7% seropositivity rate, has drawn on community mobilization to increase initiation of testing and treatment.14
The San Diego Lead the Way program promotes universal HIV testing and compares testing acceptance among adults who self-select to be tested at community HIV testing sites and those who are approached in a door-to-door testing program; the program also focuses on testing an entire neighborhood. Harlem United’s block-by-block HIV testing program revealed high rates of HIV infection in Harlem. Finally, HPTN 065, an NIH-funded trial, seeks to expand the “test and treat” model in Washington, DC, and the Bronx via community engagement, HIV testing, engagement in care, and medication adherence interventions.
These testing and linkage-to-care programs have shown success in drawing on community mobilization to promote local ownership and sustainability of HIV testing and treatment programs; however, further evaluations are needed to assess the effectiveness of such programs and highlight opportunities for scaling up local efforts across the country. Although focused efforts in higher prevalence settings may engage fewer people than broader based campaigns, these initiatives are likely to be more cost-effective than other options.
In addition, home-based self-testing for HIV may provide an opportunity to increase testing among the approximately 18% of Americans with HIV who are estimated to be unaware of their status. Home-based HIV testing programs in Kenya have been effective in enrolling individuals in care services before they become ill.15 Preliminary data from the United States suggest that self-testing may increase the frequency of repeat testing among at-risk men who have sex with men.16 Home-based HIV testing may be a useful harm reduction tool, particularly for individuals who rarely use condoms. Moreover, given the high rates of stigma in many communities, home-based testing programs in nonclinical settings may be important new tools to use in neighborhood-based testing and treatment campaigns; implementation research programs could evaluate whether self- and home-based HIV testing increase the number of people who know their status in the most heavily affected neighborhoods of the United States.
Media engagement is also critical in addressing the overwhelming stigma associated with HIV in many communities and in stimulating demand for HIV testing and treatment. Several recent municipal media campaigns have promoted HIV testing, including Test Miami, Get Screened Oakland, and Come Together DC. The CDC’s Testing Makes Us Stronger campaign promotes HIV testing among African American men who have sex with men, and Greater Than AIDS promotes HIV/AIDS awareness nationwide. Although greater efforts are needed to evaluate the impact of these programs with respect to encouraging the demand for and acceptability of HIV testing and treatment, understanding how these campaigns have developed locally tailored, positive, destigmatizing media messages to promote HIV testing and treatment in the most highly affected neighborhoods should be an important component of a geographically focused HIV prevention strategy.
Reducing HIV/AIDS disparities requires far greater effort to design and evaluate programs that aim to enhance rates of engagement and retention in care within the most heavily affected neighborhoods in the United States. One component of such an effort is a microlevel analysis of trends in engagement and retention in care at the neighborhood level. Patient navigation programs designed for HIV-positive populations have been associated with improved health outcomes and HIV virological suppression.17 In addition, during HIV care visits, health system interventions should be coupled with interventions designed to promote safer sex among those living with HIV.18 A neighborhood-focused implementation research agenda should tailor these interventions to the most heavily affected communities.
CASE STUDY: BRONX KNOWS
In 2007, HIV prevalence in the Bronx was 1.7%, higher than the citywide prevalence of 1.3%, and more than one Bronx neighborhood had a prevalence rate as high as 2.6%,19 rivaling the rates observed in Haiti and Ethiopia. The Bronx had the highest HIV-related death rate in New York City. In June 2008, New York City piloted the Bronx Knows HIV Testing Initiative, which focused on many of the aforementioned elements of implementation research, including engaging local community leaders, developing media messages featuring local community leaders promoting HIV testing and treatment in the most heavily affected neighborhoods, offering routine HIV testing coupled with efforts to link people to and retain them in care, and conducting local outreach to promote testing and linkage to care in each neighborhood.
Bronx Knows aimed to test an estimated 250 000 Bronx residents who had never been tested for HIV and link those with positive results to HIV primary care. During the three-year initiative, more than 75 clinical and nonclinical partners joined the project, including all Bronx hospitals, major community health centers, community-based social service organizations, several colleges and universities, and prominent faith groups and commercial businesses. The health department provided financial support; a coordinated, borough-wide social marketing campaign; technical assistance on the logistics of scale-up, testing technologies, and billing; and a real-time Web-based reporting mechanism to capture testing data.
Bronx Knows partners conducted 607 570 HIV tests in three years, with 4820 confirmed positive results (0.8% seropositivity).19 According to self-reported data, at least 1731 of those testing positive during the initiative were individuals receiving a new diagnosis; by the end of the initiative, collaborating agencies reported that they had linked 76% of these newly diagnosed individuals to HIV primary care.
The percentage of Bronx adults aged 18 to 64 years who reported ever having been tested for HIV increased from 72% at baseline (2007) to 80% in 2010. HIV/AIDS registry data on linkage to care showed greater improvements among Bronx Knows partners than partners in other boroughs. Linkage to care within 12 months improved from 82% in the year preceding the program to 84% during the program’s three-year duration (2008–2011). The New York City Department of Health and Mental Hygiene has expanded this successful model to other highly affected boroughs and launched Brooklyn Knows in December 2010.
CONCLUSIONS
A robust set of HIV prevention tools are now available that can help reduce HIV transmission rates. These tools include condoms, syringe services, antiretroviral medications to promote primary and secondary HIV prevention, clinic-based interventions for HIV-positive individuals, behavioral interventions targeting those at high risk for HIV, and health system interventions designed to enhance linkage to and retention in care. However, in the absence of knowledge regarding how to optimally combine these tools to best identify and engage individuals with HIV and those at risk, their impact will be attenuated. Individuals’ risk of acquiring HIV infection is the result of a complex interplay between individual risk behaviors and community-level factors, making neighborhoods important settings for addressing microepidemics in the United States.
To date, many HIV prevention interventions have been studied and used independently. There is a need for implementation research programs that better assess the combined effects of much more intensive efforts focusing on HIV testing and treatment, engagement and retention in care, and community mobilization in neighborhoods with high rates of HIV infection. Each of these components will be critical in achieving higher rates of suppressed viral RNA among individuals with HIV, which in turn will reduce HIV transmission.8,9
In addition, coupling use of geospatial mapping with epidemiological analysis could help tailor a precise approach to each neighborhood, including developing door-to-door and home-based HIV testing and outreach efforts as well as patient navigation and linkage to care programs adapted to local communities’ needs. The Bronx Knows program improved rates of HIV testing, diagnosis, and linkage to care across an entire New York City borough, and its impact was assessed through implementation research. Similar initiatives are needed in other heavily affected neighborhoods around the country, including creative new strategies to diagnose individuals and link them to HIV care, as well as retain them in care. These campaigns may include door-to-door and home-based testing efforts to raise HIV awareness and testing rates, expansion and tailoring of patient navigation programs to enhance linkage to and retention in care in the most heavily affected neighborhoods, and safer sex interventions for individuals with HIV.
Public health officials and researchers must monitor intended, as well as unintended, consequences of geographically focused HIV prevention efforts. In addition to neighborhood programs designed to decrease stigma, concurrent efforts will be needed to ensure that neighborhoods identified for focused interventions are not further marginalized and to prevent community backlash. Tailoring interventions to local communities requires ongoing community participation in developing, implementing, and evaluating interventions; the example programs highlighted here have successfully engaged communities in HIV prevention efforts.
Focusing efforts on entire neighborhoods as the unit of intervention is more complex than focusing on individuals because of the scale of such interventions. However, geography should not determine destiny: an implementation research agenda that dramatically scales and evaluates this suite of interventions in the most highly affected US neighborhoods represents an important new strategy to reduce racial, geographic, and economic disparities in HIV infection rates.
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (grants K01 AA020228-01A1, AI043638, and AI74621), the Center for AIDS Research, National Institutes of Health (grant P30-AI-42853), the National Institutes of Health (grant P01 AA019072), the California HIV Research Program (grant RN07-SD-702), and an HIV FOCUS grant from Gilead Sciences.
Philadelphia maps were made by Lindsay Kinkade and New York City maps by MAPLARGE. We acknowledge Monica Sweeney, Ben Tsoi, Julie Myers, Colin Shepard, Sarah Braunstein, Andrea Mantsios, Graham Harriman, Kent Sepkowitz, and the Bronx Knows HIV Testing Initiative partners for analysis and implementation activities.
Note. None of the funding agencies were involved in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation of the article.
References
- 1.Prejean J, Song R, Hernandez A et al. Estimated HIV incidence in the United States, 2006-2009. PLoS One. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallfors DD, Iritani BJ, Miller WC, Bauer DJ. Sexual and drug behavior patterns and HIV and STD racial disparities: the need for new directions. Am J Public Health. 2007;97(1):125–132. doi: 10.2105/AJPH.2005.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Characteristics associated with HIV infection among heterosexuals in urban areas with high AIDS prevalence—24 cities, United States, 2006–2007. MMWR Morb Mortal Wkly Rep. 2011;60(31):1045–1049. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. HIV infection among heterosexuals at increased risk—United States, 2010. MMWR Morb Mortal Wkly Rep. 2013;62(10):183–188. [PMC free article] [PubMed] [Google Scholar]
- 5.HIV/AIDS, Hepatitis, STD, and TB Administration (HAHSTA) Annual Report 2011. Washington, DC: Department of Health, Government of the District of Columbia; 2011. [Google Scholar]
- 6.Comprehensive Prevention Plan for Philadelphia. Philadelphia, PA: Office of HIV Planning of Philadelphia; 2011. [Google Scholar]
- 7.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39(4):446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MS, Chen YQ, McCauley M et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahle Gray K, Tang T, Shouse L, Li J, Mermin J, Hall HI. Using the HIV surveillance system to monitor the National HIV/AIDS Strategy. Am J Public Health. 2013;103(1):141–147. doi: 10.2105/AJPH.2012.300859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institutes of Health. Frequently asked questions about implementation science. Available at: http://www.fic.nih.gov/News/Events/implementation-science/Pages/faqs.aspx. Accessed February 5, 2014.
- 12.Mansergh G, Valdiserri RO, Yakovchenko V, Koh H. Aligning resources to fight HIV/AIDS in the United States: funding to states through the US Department of Health and Human Services. J Acquir Immune Defic Syndr. 2012;59(5):516–522. doi: 10.1097/QAI.0b013e318245cc05. [DOI] [PubMed] [Google Scholar]
- 13.Sweat M, Morin S, Celentano D et al. Community-based intervention to increase HIV testing and case detection in people aged 16–32 years in Tanzania, Zimbabwe, and Thailand (NIMH Project Accept, HPTN 043): a randomised study. Lancet Infect Dis. 2011;11(7):525–532. doi: 10.1016/S1473-3099(11)70060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trooskin S, Feller S, Lee H, et al. Preliminary results from “Do One Thing”: a comprehensive neighborhood-based HIV and HCV testing, prevention and media campaign in southwest Philadelphia. Paper presented at: National Summit on HIV and Viral Hepatitis Diagnosis, Prevention and Access to Care; November 26–28, 2012; Washington, DC.
- 15.Wachira J, Kimaiyo S, Ndege S, Mamlin J, Braitstein P. What is the impact of home-based HIV counseling and testing on the clinical status of newly enrolled adults in a large HIV care program in western Kenya? Clin Infect Dis. 2012;54(2):275–281. doi: 10.1093/cid/cir789. [DOI] [PubMed] [Google Scholar]
- 16. Katz DGM, Hughes J, Farquhar C, Stekler J. Acceptability and ease of use of home self-testing for HIV among MSM. Paper presented at: 19th Conference on Retroviruses and Opportunistic Infections; March 5–8, 2012; Seattle, WA.
- 17.Bradford JB, Coleman S, Cunningham W. HIV system navigation: an emerging model to improve HIV care access. AIDS Patient Care STDS. 2007;21(suppl 1):S49–S58. doi: 10.1089/apc.2007.9987. [DOI] [PubMed] [Google Scholar]
- 18.Safren SA, O’Cleirigh CM, Skeer M, Elsesser SA, Mayer KH. Project Enhance: a randomized controlled trial of an individualized HIV prevention intervention for HIV-infected men who have sex with men conducted in a primary care setting. Health Psychol. 2013;32(2):171–179. doi: 10.1037/a0028581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.HIV/AIDS Annual Surveillance Statistics, 2010. New York, NY: New York City Department of Health and Mental Hygiene; 2011. [Google Scholar]