Abstract
Objective
Diabetes among older adults causes many complications, including decreased lower extremity function and physical disability. Diabetes can cause peripheral nerve dysfunction, which might be one pathway through which diabetes leads to decreased physical function. The study aims were to determine: (1) whether diabetes and impaired fasting glucose are associated with objective measures of physical function in older adults, (2) which peripheral nerve function (PNF) tests are associated with diabetes, and (3) whether PNF mediates the diabetes-physical function relationship.
Research Design and Methods
This study included 983 participants, age 65 and older from the InCHIANTI Study. Diabetes was diagnosed by clinical guidelines. Physical performance was assessed using the Short Physical Performance Battery (SPPB), scored from 0-12 (higher values, better physical function) and usual walking speed (m/s). PNF was assessed via standard surface electroneurographic study of right peroneal nerve conduction velocity, vibration and touch sensitivity. Clinical cut-points of PNF tests were used to create a neuropathy score from 0-5 (higher values, greater neuropathy). Multiple linear regression models were used to test associations.
Results and Conclusion
12.8% (n=126) of participants had diabetes. Adjusting for age, sex, education, and other confounders, diabetic participants had decreased SPPB (β= −0.99; p< 0.01), decreased walking speed (β= −0.1m/s; p< 0.01), decreased nerve conduction velocity (β= −1.7m/s; p< 0.01), and increased neuropathy (β= 0.25; p< 0.01) compared to non-diabetic participants. Adjusting for nerve conduction velocity and neuropathy score decreased the effect of diabetes on SPPB by 20%, suggesting partial mediation through decreased PNF.
Diabetes is a common condition among older adults. The Center for Disease Control reported a 23.1% prevalence rate of diabetes, diagnosed and undiagnosed, amongst the United States’ 60 years of age and over population 1. There are many complications that occur as a result of diabetes, including diabetic neuropathies 2,3. Diabetic peripheral neuropathy (DPN) is one of the most severe complications of diabetes, occurring in 30%-50% of all diabetic patients 4-8. Both age and duration of diabetes are independent risk factors for DPN 8. Peripheral neuropathy is associated with increased pain, morbidity, and mortality 2. Diabetes is also associated with decreased function in the lower extremities 9. Many factors may contribute to decreased lower extremity function in diabetes, including nerve function 9-12. While previous studies have assessed the associations between diabetes, a specific aspect of peripheral nerve function, and lower extremity function, they have not assessed an overall measure of peripheral nerve function. The current study includes the analysis of a composite peripheral neuropathy score, including multiple measures of peripheral nerve function. Additionally, it remains unknown whether there is an effect of peripheral neuropathy on lower extremity function among older adults with impaired fasting glucose (IFG), which is also analyzed in the current study.
The aims of this study were to determine: 1) whether both diabetes and impaired fasting glucose are associated with objective measures of physical function in older adults, 2) which peripheral nerve function tests are associated with diabetes, and 3) whether peripheral nerve function mediates the relationship between diabetes and physical function.
Research Design and Methods
Study Population
The InCHIANTI Study (Invecchiare in Chianti, Aging in the Chianti Region) is a population-based cohort study conducted in the Chianti region of Italy that recruited 1453 participants at baseline. Baseline data collection occurred from September 1998 to March 2000. Initial interviews were conducted in the home. Following the interview, three additional appointments to take place in the study clinic were scheduled, during which the neuropathy test measures and physical performance measures were gathered. All participants gave informed consent. These current analyses excluded all participants under age 65 (n=298). Additionally, all remaining participants with incomplete baseline data on diabetes status, neuropathy tests, and performance measures were excluded (n=172) resulting in a final total of 983 men and women.
Exposure variable
Participants were classified with diabetes if they self-reported a physician’s diagnosis, used glucose-lowering medications, or had a fasting plasma glucose level >126 mg/dL. Participants who did not report a diagnosis of diabetes, but had a fasting plasma glucose level 100-125 mg/dL were considered to have impaired fasting glucose (IFG). Both fasting plasma glucose cutpoints follow the American Diabetes Association standards13.
Outcome variables
Two objective measures of lower extremity function were examined. The Short Physical Performance Battery (SPPB) is an objective measure of lower extremity function that includes 4-meter gait speed at usual pace, 3 standing balance tests, and time to complete 5 chair rises 14. Composite scores range from 0 to 12 with higher scores reflecting better performance. In addition, usual gait speed (in m/s) assessed over a distance of 4 meters was also examined.
Peripheral nerve function tests
A standard surface electroneurographic study of the right peroneal nerve was performed, which provided the amplitude of the distal compound muscle action potential (CMAP) and nerve conduction velocity (NCV) 15. Additionally, the clinical exam included a test of vibration sensitivity using 10 seconds of stimulation from a tuning fork set at 128 Hz at the level of the first metatarsal bone and a test of touch sensitivity of the external malleolus to 4.31 and 4.56 Semmes-Weinstein monofilaments testing 15. To assess the impact of diabetes status on nerve function collectively, a peripheral neuropathy score was calculated. The peripheral neuropathy score was a summation of abnormal performance on nerve function tests, specifically an NCV less than 40 (m/s), CMAP less than 3 (mV), inability to feel the 4.31 monofilament, inability to feel the 4.56 monofilament, and reduced or absent vibration sensitivity 16. Score values ranged from 0 to 5, with higher values indicating greater neuropathy.
Covariates
The following covariates were reported in the home interview and were included in analyses: age, sex, educational attainment, smoking status. In the clinic, trained geriatricians assessed the presence of medical conditions with standard algorithms using data gathered from medical history, prescription treatments, signs and symptoms, medical documents, and hospital discharge records 15. The medical conditions analyzed in this study included coronary heart disease, congestive heart failure, peripheral artery disease, hypertension, stroke, and chronic kidney disease (CKD), which was defined by the Cockroft-Gault equation of estimated glomerular filtration rate (eGFR) (CKD=eGFR<60mL/min) 17. Additional covariates analyzed were body mass index (BMI), the Mini-Mental State Examination (MMSE), and Center for Epidemiologic Studies-Depression (CES-D), all of which were assessed in the clinic.
Data Analysis
Differences in participant characteristics by diabetes status were assessed using analysis of variance and chi square statistics (Table 1). The association of diabetes with peripheral nerve function adjusting for age and sex was assessed using multiple linear regression (Table 2). To assess the association of diabetes with the objective measures of physical function and to assess the potential mediating effects of peripheral nerve function, four multiple linear regression models were performed with each outcome variable (Table 3). Indicator variables were used to compare diabetic older adults and IFG older adults to non-diabetic older adults (reference group). Model 1 includes age, sex, education, BMI, smoking status, and diabetes status as predictors. Model 2 additionally included NCV. Model 3 included the peripheral neuropathy score in place of NCV, while Model 4 was the full model, including NCV and the peripheral neuropathy score as well as medical conditions as predictors.
Table 1. Characteristics of Study Sample by Diabetes Status.
| Characteristics | No Diabetes n=750 |
IFG n=107 |
Diabetes n=126 |
p-value |
|---|---|---|---|---|
| Age in years, mean (SD) | 74.6 (7.4) | 74.8 (6.8) | 75.4 (7.5) | 0.50 |
| Women, % | 46.8 | 56.1 | 57.2 | 0.09 |
| Education in years, mean (SD) | 5.5 (3.4) | 4.9 (2.7) | 4.9 (3.2) | 0.05 |
| BMI in kg/m2, mean (SD) | 27.1 (4.0) | 29.1 (4.2) | 28.6 (4.2) | < 0.01 |
| < 22, % | 8.3 | 1.9 | 4.0 | < 0.01 |
| 22-24, % | 21.6 | 17.8 | 15.9 | |
| 25-29, % | 45.5 | 39.3 | 38.9 | |
| 30+, % | 19.7 | 41.1 | 34.9 | |
| BMI missing, % | 4.9 | 0.0 | 6.4 | |
| Medical Conditions | ||||
| CHD, % | 6.5 | 8.4 | 14.3 | 0.01 |
| CHF, % | 5.8 | 9.3 | 17.1 | < 0.01 |
| CKD, % | 27.8 | 32.7 | 20.0 | 0.10 |
| Hypertension, % | 63.3 | 71.8 | 69.7 | 0.10 |
| PAD, % | 10.9 | 15.8 | 15.0 | 0.20 |
| Stroke, % | 5.8 | 1.9 | 9.6 | 0.04 |
| Physical Performance Tests | ||||
| SPPB Score, mean (SD) | 9.8 (3.2) | 10.0 (2.9) | 8.7 (4.0) | < 0.01 |
| Walk Score, mean (SD) | 3.6 (1.0) | 3.6 (0.9) | 3.2 (1.3) | < 0.01 |
| Balance Score, mean (SD) | 3.3 (1.2) | 3.3 (1.2) | 2.9 (1.5) | 0.01 |
| Chair Score, mean (SD) | 3.0 (1.3) | 3.1 (1.2) | 2.5 (1.6) | < 0.01 |
| Usual Walking Speed in m/s, mean (SD) | 1.1 (0.3) | 1.0 (0.3) | 0.9 (0.3) | < 0.01 |
Abbreviations: IFG= Impaired Fasting Glucose; BMI = Body Mass Index; CHD = Coronary Heart Disease; CHF = Congestive Heart Failure; CKD = Chronic Kidney Disease; PAD = Peripheral Artery Disease; SPPB = Short Physical Performance Battery.
Table 2. Age and Sex Adjusted Peripheral Nerve Function Test Values by Diabetes Status.
| No Diabetes n=750 |
IFG n=107 |
Diabetes n=126 |
p-value | |
|---|---|---|---|---|
| Nerve Conduction Velocity in m/s, mean | 44.1 | 43.8 | 42.7 | < 0.01 |
| Nerve Conduction Velocity in m/s, % < 40 | 9.5 | 13.2 | 21.2 | < 0.01 |
| CMAP in mV, mean | 5.5 | 5.0 | 5.1 | 0.16 |
| CMAP in mV, % < 3 | 21.5 | 23.4 | 31.8 | 0.04 |
| 4.31 Monofilament, % unable to feel | 5.1 | 4.1 | 7.8 | 0.07 |
| 4.56 Monofilament, % unable to feel | 0.4 | 0.7 | 1.7 | 0.28 |
| Less Vibration Sensitivity, % reduced sensitivity | 82.9 | 83.0 | 85.6 | 0.50 |
| Neuropathy Score, %* | < 0.01 | |||
| 0-1 | 77.3 | 69.5 | 65.5 | |
| 2 | 18.5 | 21.9 | 21.2 | |
| ≥ 3 | 4.2 | 8.6 | 13.3 |
Abbreviations: IFG = Impaired Fasting Glucose
Not age and sex adjusted
Table 3. Association of Diabetes with Objective Measures of Physical Function.
| SPPB (N=896) | Usual Gait Speed in m/s (N=891) | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 β (SE) |
Model 2 β (SE) |
Model 3 β (SE) |
Model 4 | Model 1 β (SE) |
Model 2 β (SE) |
Model 3 β (SE) |
Model 4 | |
| Diabetes Status | ||||||||
| Non Diabetic | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| IFG | −.16 (.23) | −.13 (.23) | −.10 (.22) | −.05 (.21) | −.02 (.02) | −.02 (.02) | −.02 (.02) | −.01 (.02) |
| Diabetic | −.99 (.23) ** | −.91 (.23) ** | −.80 (.21) ** | −.67 (.21) ** | −.11 (.02) ** | −.10 (.02) ** | −.10 (.02) ** | −.10 (.02) ** |
| Age (years) | −.18 (.01) ** | −.18 (.01) ** | −.14 (.01) ** | −.11 (.01) ** | −.02 (.00) ** | −.02 (.00) ** | −.02 (.00) ** | −.01 (.00) ** |
| Women (vs. Men) | −.88 (.17) ** | −1.00 (.18) ** | −1.02 (.17) ** | −.72 (.17) ** | −.12 (.02) ** | −.13 (.02) ** | −.14 (.02) ** | −.11 (.02) ** |
| Education (years) | .03 (.02) | .03 (.02) | .04 (.02) | .01 (.02) | .01 (.00) ** | .01 (.00) ** | .01 (.00) ** | .01 (.00) ** |
| BMI | ||||||||
| < 22 | −.13 (.31) | −.08 (.31) | .12 (.30) | .14 (.29) | −.04 (.03) | −.04 (.03) | −.04 (.03) | −.01 (.03) |
| 22-24 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 25-29 | −.20 (.19) | −.22 (.19) | −.35 (.18) | −.40 (.17) * | −.05 (.02) ** | −.06 (.02) ** | −.06 (.02) ** | −.07 (.02) ** |
| 30+ | −.41 (.22) | −.45 (.22) * | −.45 (.21) * | −.53 (.20) ** | −.09 (.02) ** | −.09 (.02) ** | −.09 (.02) ** | −.09 (.02) ** |
| Smoking Status | ||||||||
| Never | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Former | −.14 (.20) | −.15 (.20) | −.11 (.19) | .01 (.18) | −.03 (.02) | −.03 (.02) | −.03 (.02) | −.03 (.02) |
| Current | −.16 (.23) | −.12 (.23) | −.00 (.22) | −.01 (.21) | −.02 (.02) | −.02 (.02) | −.00 (.02) | −.00 (.02) |
| NCV (m/s) | .05 (.02) * | −.02 (.02) | .00 (.00) | −.00 (.00) | ||||
| Neuropathy Score | ||||||||
| 0-1 | Ref | Ref | Ref | Ref | ||||
| 2 | −.94 (.18) ** | −.77 (.19) ** | −.10 (.02) ** | −.08 (.02) ** | ||||
| ≥ 3 | −2.42 (.31) ** | −1.66 (.33) ** | −.15 (.03) ** | −.10 (.04) * | ||||
| MMSE | .05 (.02) * | .01 (.00) ** | ||||||
| CES-D ≥ 16 (vs. < 16) | −.63 (.15) ** | −.07 (.02) ** | ||||||
| CHD | .11 (.25) | .01 (.03) | ||||||
| CHF | −.48 (.37) | −.07 (.04) | ||||||
| CKD | −.48 (.16) ** | −.06 (.02) ** | ||||||
| PAD | −.63 (.22) ** | −.06 (.02) * | ||||||
| Stroke | −1.55 (.33) ** | −.12 (.04) ** | ||||||
p < 0.05
p < 0.01
Abbreviations: BMI = Body Mass Index; IFG = Impaired Fasting Glucose; NCV = Nerve Conduction Velocity; MMSE = Mini Mental State Examination; CES-D = Center for Epidemiologic Studies Depression Scale; CHD = Coronary Heart Disease; CHF = Congestive Heart Failure; CKD = Chronic Kidney Disease; PAD = Peripheral Artery Disease
Results
The average age of the study sample was approximately 75 years old and did not vary significantly by diabetes status (Table 1). The percentage of women among the non-diabetic participants was lower (46.8%) than among the IFG (56.1%) and diabetic participants (57.2%). Mean years of education was higher among non-diabetic older adults (5.5, SD 3.4) compared to IFG (4.9, SD 2.7) and diabetic older adults (4.9, SD 3.2). Mean BMI was lowest in non-diabetic older adults (27.1 kg/m2, SD 4.0) and highest in IFG older adults (29.1 kg/m2, SD 4.2). The distribution of BMI was such that non-diabetic participants had the highest percentage of underweight, normal weight, and overweight older adults; however, the percentage of obese older adults was highest in the IFG group and lowest in the non-diabetic group. The prevalence of certain medical conditions differed significantly by diabetes status, with coronary heart disease, congestive heart failure, and stroke highest in the diabetic group. Chronic kidney disease, hypertension, and peripheral artery disease were highest among IFG participants; however, the difference in prevalence of these diseases across diabetes-status groups was not significant.
Both SPPB scores and usual gait speed were significantly different by diabetes status. Diabetic participants had a lower mean total SPPB score (8.7, SD 4.0) than both the IFG (10.0, SD 2.9) and non-diabetic (9.8, SD 3.2) participants. The mean scores of the three components of the SPPB were also significantly different by diabetes status, each being lowest in diabetic participants. Likewise, diabetic participants had the slowest mean (SD) usual gait speed of 0.9 m/s (0.3) compared to 1.0 m/s (0.3) and 1.1 m/s (1.1) for IFG and non-diabetic participants, respectively.
Diabetes was inversely significantly associated with NCV, adjusting for age and sex (Table 2). Non-diabetic participants had the highest mean NCV (44.1 m/s, SD 3.8) while diabetic participants had the lowest (42.7 m/s, SD 3.8). While there were no differences in mean CMAP or touch sensitivity seen according to diabetes status, diabetic participants had poorer results when cutpoints indicating low CMAP and reduced touch sensitivity were assessed. Neuropathy scores were significantly different by diabetes status, with the highest percentage of 0-1 scores among non-diabetic participants, and the highest percentage of scores of 3 or more among diabetic participants (Table 2). Mean (SD) neuropathy scores were 1.11(0.73), 1.23 (0.84), and 1.40 (0.89) for non-diabetes, IFG and diabetes groups, respectively.
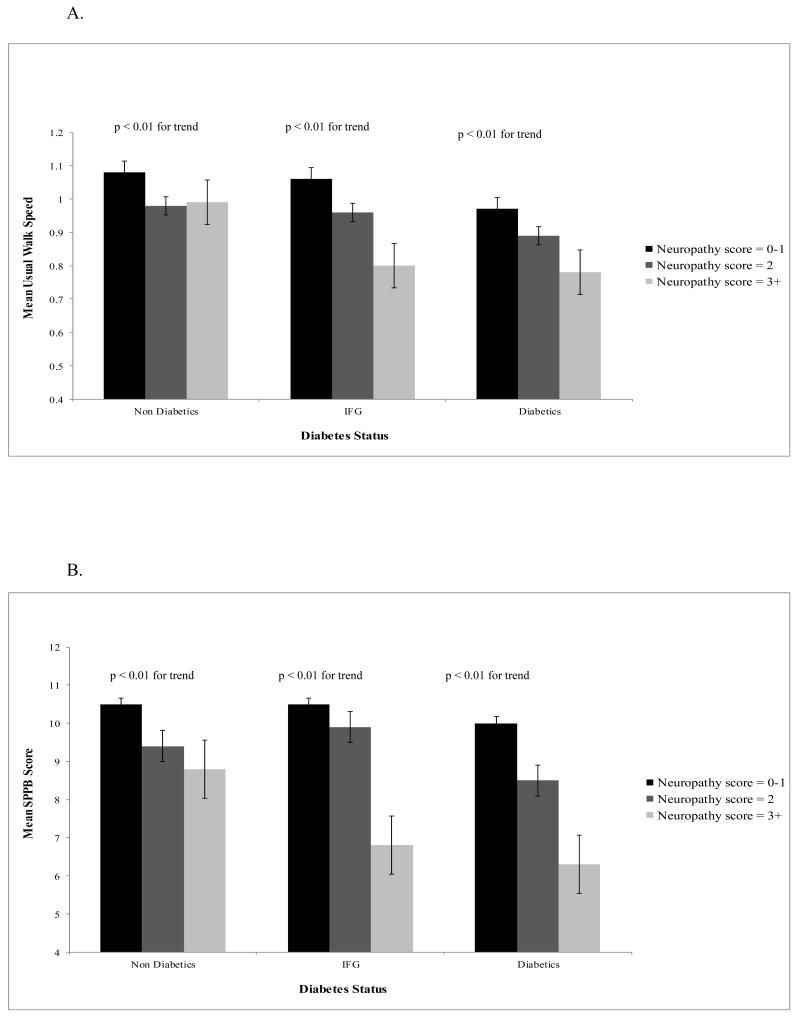
Mean usual gait speed and SPPB scores were assessed by diabetes status and peripheral neuropathy score, adjusting for age and sex (Figure 1). For each diabetes status group, a higher neuropathy score was significantly associated with a slower mean usual gait speed, although for non-diabetic participants, the mean usual gait speed was similar for participants with a neuropathy score of 2 and those with 3 or higher (Figure 1A). Likewise, for participants with a neuropathy score of 3 or higher, diabetic participants had a slower usual walk speed than non-diabetic participants (Figure 1A). Stronger associations were seen for mean SPPB score (Figure 1B). This seems to indicate that the addition of the chair and balance components of the SPPB are strongly associated with peripheral nerve function, contributing to the steeper gradients seen in Figure 1B among diabetic participants versus Figure 1A.
Figure 1.
A. Mean Usual Gait Speed by Neuropathy Score and Diabetes Status, adjusting for Age and Sex
B. Mean SPPB Scores by Neuropathy Score and Diabetes Status, adjusting for Age and Sex
The association between diabetes status and SPPB is shown in Table 3. After adjusting for age, sex, education, and BMI in model 1, diabetic participants had a 0.99 unit lower SPPB score than non-diabetic participants (p < 0.01). Model 2 additionally adjusted for NCV, resulting in a minimal change in the effect of diabetes status on SPPB. Adjusting for neuropathy score in Model 3 resulted in a reduction in the association of diabetes with SPPB score, from − 0.99 units in Model 1 to − 0.80 units. Further adjustment for cognition and comorbidities in Model 4 resulted in an additional reduction of the diabetes-SPPB relationship by an additional 0.13 SPPB units (− 0.80 to − 0.67); however diabetic participants’ SPPB scores remained statistically lower than non-diabetic participants’ scores.
Similarly, the association between diabetes status and usual gait speed is shown in Table 3. Model 1 adjusted for age, sex, education, and BMI, resulting in diabetic participants having a 0.1 m/s slower usual gait speed than non-diabetic participants. There was a 10% reduction in the magnitude of the diabetes-gait speed association in Models 2, 3, and 4.
Conclusions
Participants with diabetes had significantly lower SPPB and slower gait speed than non-diabetic participants. It is important to understand the meaningfulness of the magnitudes of differences observed in this study. A difference of 1 point in SPPB, as seen between diabetic and non-diabetic participants in Model 1 (Table 3), was previously found by Perera et al. to be associated with a 1.79 (1.12-2.86 CI) hazard of mortality within 5 years 18. A difference of 0.1 m/s in usual gait speed, as seen between diabetic and non-diabetic participants in Models 1-4 (Table 3), was associated with a 2.03 (1.31-3.16 CI) hazard of mortality within 5 years 18. After adjusting for comorbidities and neuropathy, there was still a meaningful difference between diabetic participants and non-diabetic participants in SPPB score (0.67) and usual gait speed (.10 m/s), as Perera et al. previously reported a difference in the SPPB of 0.5 points constitutes a small meaningful difference and a difference in gait speed of .10 m/s constitutes a substantial meaningful difference 19.
While differences in demographics and comorbidities were observed between the IFG and non-diabetic groups, no significant differences in lower extremity function were seen between the IFG and non-diabetic groups (Table 3). Therefore, diabetic older adults should be followed more closely for lower extremity dysfunction that IFG older adults. Differences in prevalence of comorbidities were observed between the IFG and diabetic group for CHD, CHF, and stroke (Table 1). These findings seem to imply that diabetes may result in physiological changes which in turn result in decreased lower extremity function; however, the cardiovascular comorbidities associated with an elevated blood glucose level may also contribute to the poorer lower extremity function observed among diabetic participants. Thus these findings highlight the need to further understand the mechanistic and physiological impact of diabetes to elucidate the pathway that results in diabetic older adults exhibiting poorer physical function than non-diabetic older adults.
Slower NCV was significantly associated with decreased SPPB scores, but was not associated with usual gait speed adjusting for age, sex, education, BMI, smoking status, and diabetes status, indicating that nerve function may impact the balance and lower-extremity strength components of the SPPB and not the walking component (Table 3, Models 2 and 3). The effect of diabetes on SPPB was reduced by 8% when adjusted for NCV but was reduced by 19% when adjusted for peripheral neuropathy score. The final model, including NCV, peripheral neuropathy score, and comorbidities, resulted in 33% reduction in the strength of the association of diabetes with lower extremity function. These findings indicate that peripheral nerve function partially mediates the relationship between diabetes and physical function, and that the neuropathy score may better reflect peripheral nerve function than NCV alone. The effect of diabetes on SPPB was even further reduced by 13% when cognitive function, depressive symptoms, and medical conditions were adjusted for, indicating that peripheral nerve function is not the sole pathway through which diabetes is associated with physical function. Longitudinal research is needed to better understand the multiple pathways through which diabetes adversely affects physical function over time.
The results from the current study are consistent with previous published results from other studies. Diabetes was found to be associated with decreased lower extremity function, and peripheral nerve dysfunction was a significant contributing factor to this association, in the Women’s Health and Aging Study as well as the Health, Aging, and Body Composition Study9,11,20,21. A limitation of the current study and previous studies is the cross-sectional nature of the analyses, eliminating the determination of causality. Additionally, this study sample is not representative of older adults in the United States. As was previously stated, peripheral nerve function is not the sole pathway through which diabetes is associated with physical function. Additional pathways, including diabetic autonomic neuropathy, sarcopenia, and others, were not assessed in this study.
A major strength of this study is the use of two objective measures of physical function, the SPPB as well as usual gait speed, which resulted in an indication of the physical function components impacted by peripheral nerve function. An additional strength of this study is the use of a new method for assessing peripheral nerve function, the neuropathy score, which combined multiple assessments of peripheral nerve function, and resulted in a greater mediation effect than NCV alone. Also, this analysis compared IFG older adults to non-diabetic adults as well, finding that they did not differ in functional performance.
In conclusion, diabetes is associated with poorer physical function and this relationship is mediated by peripheral nerve function. While it is known that diabetes results in accelerated loss of muscle function and muscle protein synthesis, and peripheral nerve dysfunction can result in decreased muscle mass and strength, longitudinal research is needed to assess and quantify these relationships as explanatory factors of poorer physical function among diabetic versus non-diabetic older adults 12,22-25. Research to assess the physical function of IFG older adults is also necessary in order to determine if their physical function over time mirrors that of diabetic older adults.
Acknowledgements
The InCHIANTI study was supported as a targeted project (ICS 110.1[tnp]RS97.71) by the Italian Ministry of Health and, in part, by the Intramural Research Program of the National Institute on Aging, NIH (contracts N01-AG-916413, N01-AG-821336, and N01-AG-5-0002)
Footnotes
There are no potential conflicts of interest to report.
References
- 1.Centers for Disease Control and Prevention . National diabetes fact sheet: General information and national estimates on diabetes in the united states, 2007. 2008. [Google Scholar]
- 2.Dobretsov M. Early diabetic neuropathy: Triggers and mechanisms. shi jie chang wei bing xue za zhi. 2007;13(2):175. doi: 10.3748/wjg.v13.i2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Head K. Peripheral neuropathy: Pathogenic mechanisms and alternative therapies. Alternative medicine review. 2006;11(4):294–329. [PubMed] [Google Scholar]
- 4.Deshpande AD. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88(11):1254. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Candrilli SD, Davis KL, Kan HJ, Lucero MA, Rousculp MD. Prevalence and the associated burden of illness of symptoms of diabetic peripheral neuropathy and diabetic retinopathy. J Diabetes Complications. 2007;21(5):306–314. doi: 10.1016/j.jdiacomp.2006.08.002. doi: DOI: 10.1016/j.jdiacomp.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Gregg EW. Prevalence of lower-extremity disease in the US adult population= 40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care. 2004;27(7):1591. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 7.Pirart J. Diabetes mellitus and its degenerative complications: A prospective study of 4,400 patients observed between 1947 and 1973 (author’s transl) Diabète métabolisme. 1977;3(2):97–107. [PubMed] [Google Scholar]
- 8.Adler AI. Risk factors for diabetic peripheral sensory neuropathy. results of the seattle prospective diabetic foot study. Diabetes Care. 1997;20(7):1162. doi: 10.2337/diacare.20.7.1162. [DOI] [PubMed] [Google Scholar]
- 9.Volpato S, Blaum C, Resnick H, et al. Comorbidities and impairments explaining the association between diabetes and lower extremity disability: The women’s health and aging study. Diabetes Care. 2002;25(4):678–683. doi: 10.2337/diacare.25.4.678. [DOI] [PubMed] [Google Scholar]
- 10.Inzitari M, Carlo A, Baldereschi M, et al. Risk and predictors of motor-performance decline in a normally functioning population-based sample of elderly subjects: The italian longitudinal study on aging. J Am Geriatr Soc. 2006;54(2):318–324. doi: 10.1111/j.1532-5415.2005.00584.x. doi: 10.1111/j.1532-5415.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- 11.Resnick HE. Independent effects of peripheral nerve dysfunction on lower-extremity physical function in old age: The women’s health and aging study. Diabetes Care. 2000;23(11):1642. doi: 10.2337/diacare.23.11.1642. [DOI] [PubMed] [Google Scholar]
- 12.Resnick HE, Stansberry KB, Harris TB, et al. Diabetes, peripheral neuropathy, and old age disability. Muscle Nerve. 2002;25(1):43–50. doi: 10.1002/mus.1217. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association [Accessed 10/3/2012];How to tell if you have diabetes or prediabetes. 2012 http://www.diabetes.org/diabetes-basics/prevention/pre-diabetes/diagnosis.html.
- 14.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 15.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48(12):1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 16.Lauretani F, Bandinelli S, Strotmeyer ES, et al. Erythropoietin and polyneuropathy in older persons. Mech Ageing Dev. 2008;129(6):299–303. doi: 10.1016/j.mad.2008.02.006. doi: 10.1016/j.mad.2008.02.006; 10.1016/j.mad.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.K/DOQI clinical practice guidelines for chronic kidney disease. Evaluation, classification, and stratification. American Journal of Kidney Diseases. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 18.Perera S. Magnitude and patterns of decline in health and function in 1 year affect subsequent 5-year survival. The journals of gerontology.Series A, Biological sciences and medical sciences. 2005;60(7):894. doi: 10.1093/gerona/60.7.894. [DOI] [PubMed] [Google Scholar]
- 19.Perera S. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 20.Resnick HE, Vinik AI, Heimovitz HK, Brancati FL, Guralnik JM. Age 85+ years accelerates large-fiber peripheral nerve dysfunction and diabetes contributes even in the oldest-old: The women’s health and aging study. J Gerontol A Biol Sci Med Sci. 2001;56(1):M25–31. doi: 10.1093/gerona/56.1.m25. [DOI] [PubMed] [Google Scholar]
- 21.Strotmeyer E, de Rekeneire N, Schwartz A, et al. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and black adults: The health, aging, and body composition (health ABC) study. Diabetes Care. 2008;31(9):1767–1772. doi: 10.2337/dc08-0433. doi: 10.2337/dc08-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes. 2006;55(6):1813–1818. doi: 10.2337/db05-1183. doi: 10.2337/db05-1183. [DOI] [PubMed] [Google Scholar]
- 23.Park SW, Goodpaster BH, Lee JS, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32(11):1993–1997. doi: 10.2337/dc09-0264. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morley JE. Diabetes, sarcopenia, and frailty. Clin Geriatr Med. 2008;24(3):455. doi: 10.1016/j.cger.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Strotmeyer ES, de Rekeneire N, Schwartz AV, et al. Sensory and motor peripheral nerve function and lower-extremity quadriceps strength: The health, aging and body composition study. J Am Geriatr Soc. 2009;57(11):2004–2010. doi: 10.1111/j.1532-5415.2009.02487.x. doi: 10.1111/j.1532-5415.2009.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]