Abstract
Cortical fast spiking (FS) interneurons possess autaptic, synaptic, and electrical synapses that serve to mediate a fast, coordinated response to their postsynaptic targets. While FS interneurons are known to participate in numerous and diverse actions, functional subgroupings within this multi-functional interneuron class remain to be identified. In the present study, we examined parvalbumin positive FS interneurons in layer 4 of the primary somatosensory (barrel) cortex - a brain region well-known for specialized inhibitory function. Here we show that FS interneurons fall into two broad categories identified by the onset of the first action potential in a depolarizing train as: “Delayed Firing FS interneurons (FSD) and Early Onset Firing FS interneurons (FSE). Subtle variations in action potential firing reveal 6 subtypes within these two categories: delayed non-accommodating (FSD-NAC), delayed stuttering (FSD-STUT), early onset stuttering (FSE-STUT), early onset-late spiking (FSE-LS), early onset early-spiking (FSE-ES), and early onset accommodating (FSE-AC). Using biophysical criteria previously employed to distinguish neuronal cell types, the FSD and FSE categories exhibit several shared biophysical and synaptic properties that coincide with the notion of specificity of inhibitory function within the cortical FS interneuron class.
Keywords: interneuron, inhibitory neurotransmission, basket cells
Introduction
The diversity exhibited by cortical interneurons is considered a requisite for specialized function in inhibitory synaptic transmission (Ascoli et al., 2008). Nomenclature for cortical interneurons is based on descriptive characteristics of morphology, biochemistry and action potential firing patterns; however, no singular classification technique is fail-safe as there are often overlapping features. Fast spiking (FS) interneurons are characterized by high frequency action potential firing, the expression of the calcium binding protein parvalbumin and fast inhibitory GABAA receptor-mediated synaptic and autaptic currents (Kawaguchi and Kubota, 1997, Gupta et al., 2000, Bacci et al., 2003a, Bacci et al., 2003b, Li et al., 2009). The FS interneuron class is known for forming inhibitory synapses on either the axon initial segment (e.g., chandelier cells) or somatic (e.g., basket cells) regions of their target neurons (Kawaguchi and Kubota, 1998, Wang et al., 2002). These intrinsic and synaptic specializations enable FS interneurons to form complex feedforward and feedback circuits (Thomson and Bannister, 2003, Staiger et al., 2009) to regulate multiple key functions such as rhythmic activity and sensory evoked responses (Porter et al., 2001, Sohal et al., 2009). Given the numerous and complex functional attributes of FS interneurons they nevertheless remain grouped together in a singular functional category with no clearly defined identification of diversity of function within this interneuron class.
While interneuron classification appears arbitrary, most agree that using multiple criteria in brain regions with diverse and increased populations of interneurons is a means towards understanding specialized circuitry (Beierlein et al., 2003). Layer 4 of the rodent barrel cortex represents a key platform to examine functional heterogeneity because of the high density and diverse population of FS interneurons (Karagiannis et al., 2009, Staiger et al., 2009). Neurons located within the multicellular barrel structures in layer 4 receive thalamic afferents carrying information from the mystacial vibrissae (Woolsey and Van der Loos, 1970). FS interneurons are postsynaptic to whisker-driven thalamic afferents and are highly sensitive to a diverse range of whisker movements (White and Rock, 1981, Swadlow, 2003). In vitro studies show that activation of thalamocortical fibers results in robust (Cruikshank et al., 2007) and sometimes variable thalamic-evoked responses indicating selective activation of layer 4 FS interneurons (Agmon and Connors, 1992, Porter et al., 2001). Emerging evidence indicates that action potential onset may be an identifier of subdivisions within the FS interneuron class (Goldberg et al., 2008, Karagiannis et al., 2009). In the present study, we propose a categorization into two functional populations of FS interneurons identified by action potential onset, and supported by statistical differences in biophysical and synaptic properties. These data suggest a new functional subgrouping and the existence of specialized FS interneuron circuitry germane to barrel cortex function.
Experimental Procedures
Preparation of slices for electrophysiology
All animal use procedures were carried out in strict accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Georgetown University and Children's National Medical Center. For all experiments, adult (P25-40) mice (C57BL/6, Jackson Laboratories) of either sex were used. Animals were deeply anaesthetized with brief exposure to carbon dioxide (CO2) and decapitated. Brains were removed, blocked, and placed in an ice-cold and oxygenated high sucrose slicing solution for 2-3 minutes (in mM): 234 sucrose, 11 glucose, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4*H2O, 10 MgSO4 and 0.5 CaCl2; gassed with 95% O2 / 5% CO2. Slices including the somatosensory cortex were cut in either the tangential or thalamocortical configuration as previously described (Agmon and Connors, 1991, Fleidervish et al., 1998, Li et al., 2009). Tangential slices were employed in order to isolate the columnar and cross-columnar cellular connections within layer 4. Slices were generated by placing the brain ventral side down on a constructed angle indicator and sliced with a sterilized razor blade at simultaneous 10° and 30° from the midline cuts. The blocked brain was glued (cut side down) on a vibratome stage and immersed in cold sucrose-slicing solution. Once the position of the pial surface of the brain was identified, the first two slices at 50μm and 250μm were discarded and a third slice at 270-300μm was collected. Thalamocortical brain slices were prepared by making simultaneous 10° horizontal and 35° from the midline, with the brain placed ventral side down in a constructed angle indicator. The blocked brain was glued cut side down on a vibratome stage (Leica) and immersed in cold sucrose-slicing solution. Once the position of the pial surface of the brain was identified, the first 2400μm were cut and discarded and three consecutive slices of 300μm were collected. Tangential and thalamocortical slices were incubated in oxygen saturated artificial cerebral spinal fluid (aCSF) containing the following (in mM): 126 NaCl, 26 NaHCO3, 10 glucose, 2.5 KCl, 1.25 NaH2PO4*H2O, 2 MgCl2*6H2O, and 2 CaCl2*2H2O; pH 7.4. All slices incubated at 32°C for at least one hour prior to recording. Slices were placed in a recording chamber and visualized with a fixed staged, upright microscope (Nikon, E600 FN) equipped with a 4x objective and a 60x insulated objective, infrared (IR) illumination, Nomarski optics, and an IR-sensitive video camera (COHU).
Electrophysiological recordings
Whole-cell patch-clamp recordings from layer 4 FS interneurons in barrel cortex were performed, unless otherwise noted, at room temperature with continuous perfusion (2ml/minute) of aCSF. Glass pipettes (non-filament, Garner Glass Company) were pulled (Model P-97, Sutter Instruments) to obtain electrodes with resistances between 2.5-3.5 MΩ when filled with intracellular solution. Two intracellular solutions were used in this study. A high chloride concentration solution was used to enhance GABAA receptor-mediated inhibitory currents (in mM): 70 K-gluconate, 70 KCl, 2 NaCl, 10 HEPES, 4 EGTA, 2 Na2-ATP, 0.5 Na2-GTP (Ecl -16 mV). For thalamic activation of fast glutamatergic currents a solution containing physiological levels of chloride was used (in mM): 130 K-gluconate, 10 KCl, 2 MgCl2, 10 HEPES, 10 EGTA, 2 Na2-ATP, 0.5 Na2-GTP. All electrophysiological recordings were performed in the whole cell configuration. A gigaohm seal was formed between the cell and glass pipette and a solenoid controlled vacuum transducer was used to apply brief suction pulses (120psi at 20-50ms) to break into the cell. All recordings were performed in either current clamp or voltage clamp mode (Multiclamp 700A, Molecular Devices) and digitized (DigiData 1322, Molecular Devices) for fast acquisition of raw traces and offline analysis (PClamp 9, Molecular Devices). All cortical FS interneurons were characterized in current clamp mode using a series of hyperpolarizing and depolarizing current injections in order to measure action potential firing patterns and properties for additional characterization such as: rheobase current, action potential threshold, resting membrane potential, input resistance, saturation frequency, accommodation ratio, action potential duration at half-width, action potential amplitude, afterhyperpolarization potential (AHP), sag (or Ih current), rise time and time constant. Some of the characterized FS interneurons were selected to study their firing patterns at physiological temperatures (32°C). Spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded in voltage clamp mode (VHold = -60mV) in the presence of the glutamate receptor blockers, 6,7-Dinitroquinoxaline-2,3-dione (DNQX, 20 μM final, Tocris) and DL-2-Amino-5-phosphonopentanoic acid (DL-AP5, 100 μM final, Tocris). GABAA receptor mediated autaptic inhibitory postsynaptic currents (autIPSCs) were obtained in voltage clamp mode using a brief (0.5 ms) depolarization step from -70mV to +10 mV to elicit a spike followed by an inward GABAA receptor mediated IPSC (identified by blocking with the competitive GABAA receptor antagonist SR 95531 hydrobromide [Gabazine, Tocris]).
For thalamic activation of glutamatergic currents, a 25μm concentric bipolar stimulating electrode (FHC) was positioned such that it contacted intact fibers projecting from the ventrobasal complex of the thalamus. Recordings of excitatory postsynaptic currents (EPSCs) were made from layer 4 FS interneurons in barrels receiving input from the targeted fibers in the whole-cell configuration (VHold = -60mV). After determining cell type, depolarizing current pulses were delivered to ascending thalamocortical fibers at a rate of once every 15 seconds (Isoflex, A.M.P.I.; CPI, Carl Pisaturo, Stanford University). In order to measure thalamic evoked EPSC characteristics, a minimal stimulation protocol was employed, such that the external stimulus amplitude was adjusted to generate an EPSC at a failure rate of approximately 50%. The amplitude of the required current pulse varied by cell between 8.8 μA and 150 μA. Stimulus duration was 0.1 ms in all cases.
Histology
To further investigate cell morphology and biochemical characterization of each recorded cell, biocytin (1%, Thermo Scientific) was added to the intracellular solution and injected into the cell with 4 to 5 depolarizing current pulses of 1nA amplitude. Screen images were captured in order to record the position of each cell in the barrel pattern. Slices were transferred from the recording chamber and fixed overnight in 4% paraformaldehyde in 0.1 M phosphate buffer solution (PBS, pH 7.4). Slices only used for morphological analysis were rinsed twice in 0.1M PBS, then incubated in Fluorescein conjugated Avidin-D (1:200, Vector Laboratory) in 0.1M PBS containing 10% normal goat serum, 2% BSA and 0.5% Triton X-100 for one hour. After three rinses of 0.1M PBS, 10 minutes each, slices were mounted and cover slipped with Vectashield mounting medium. Slices used to verify parvalbumin expression of recorded cells were cryoprotected in 30% sucrose in 0.1M PBS for at least 30 minutes, then re-sectioned at 50 μm on a sliding microtome (Leica), collected in 0.1M PBS, incubated in 0.6% H2O2 for 30 minutes, and transferred into 50% ethanol for 10 minutes twice. After two rinses of 0.1M PBS, sections were then incubated for one hour in Texas-red conjugated Avidin-D (1:200, Vector Laboratory) in 0.1M PBS containing 10% normal goat serum, 2% BSA and 0.5% Triton X-100. Slices were then transferred into primary antibody mouse anti-parvalbumin (1:4,000 in 0.1M PBS, Chemicon) and incubated at 4°C overnight. The following day, sections were rinsed twice with 0.1M PBS for 20 minutes each and incubated in fluorescein conjugated secondary antibody (1:200 in 0.1M PBS, Vector Laboratory) for 60 minutes at room temperature. After two 15 minutes washes, sections were mounted and cover slipped. Images were analyzed and captured under confocal microscopy (Olympus Fluoview).
Statistical analysis
All measurements of intrinsic and synaptic properties were analyzed off-line using Clampfit software (v. 9.2 Molecular Devices). We followed the methodology for obtaining electrophysiological parameters for active and passive membrane properties as previously described (Ma et al., 2006) such as: input resistance, resting membrane potential (Vrest), rheobase, action potential threshold, action potential half width, action potential amplitude, action potential accommodation ratio, action potential saturation frequency, rise time, sag and time constant. Biophysical parameters of sIPSCs were analyzed from baseline-subtracted averaged inhibitory events (that decay to baseline and are not on the rising phase of a previous event) with the offset forced to zero. The time decay of averaged sIPSCs was fit based on the double exponential function:
These fits were used to determine a weighted time constant:
Results were presented as mean values +/- standard error (SE). Unless otherwise noted, an independent Student's t-test was used to compare biophysical data derived from different subtypes of FS interneuron populations.
Results
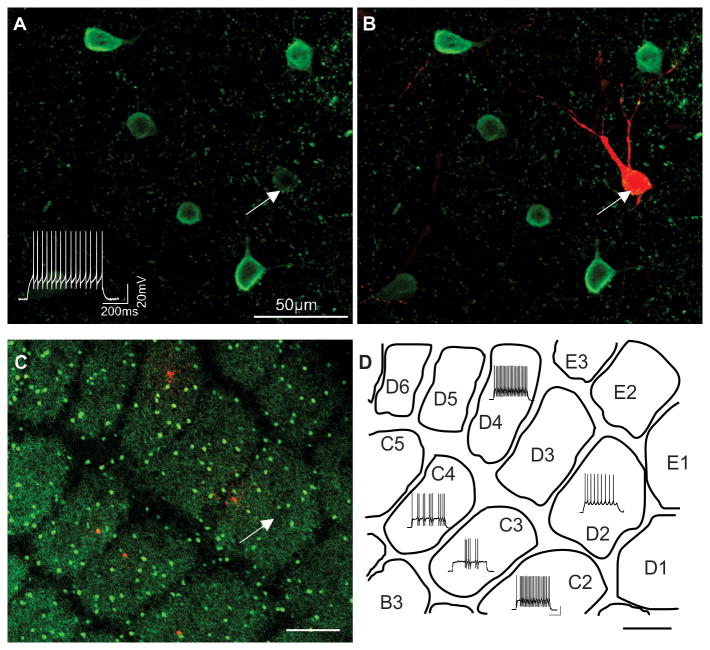
In the present study, a total of 252 FS interneurons with somata located in the barrel structures of layer 4 primary somatosensory cortex were recorded from acute slices in both the tangential and thalamocortical orientations. All FS interneurons examined in this study exhibited a high saturating frequency in excess of 150 Hz. This holds true for all high frequency firing layer 4 interneurons with the exception of the somatostatin positive “X94” interneuron [referenced in (Ma et al., 2006)]. The most consistent feature of high frequency firing FS interneurons is the expression of the calcium binding protein parvalbumin (Cauli et al., 1997, Kawaguchi and Kubota, 1997). Therefore, representative biocytin filled neurons exhibiting various firing patterns yet high firing frequency in some cases biocytin was added to the intracellular pipette forwere selected for post-hoc morphological analysis and immunocytochemical identification of parvalbumin expression (Fig. 1A-C). All physiologically identified FS interneurons selected for immunocytochemistry were confirmed as parvalbumin immunopositive (26 out of 26) within the barrel structures (Fig. 1A,B). In tangential slices, parvalbumin positive interneurons were abundant across the posteromedial barrel field yet showed no preference for the intervening septa, wall or hollow subregions of the barrel structures (Fig. 1C, D).
Fig. 1. Distribution of parvalbumin expressing fast spiking interneurons in mouse barrel cortex.
High magnification confocal images of fluorescently labeled parvalbumin immunopositive interneurons (A,B). (B) Selected interneuron filled with biocytin and processed with Texas Red reveals co-localization of parvalbumin expression (white arrows in A, B). Depolarizing current injection evokes high frequency firing of this cell prior to histology (lower left in A). (C) Low magnification confocal image of a tangential slice through layer 4 of the primary somatosensory cortex illustrates distribution of parvalbumin immunopositive interneurons (GFP-labeled cells) across the barrels of the postero-medial barrel subfield. Five selected cells were processed with biocytin and labeled with Texas Red. (D) The firing patterns of Texas Red biocytin labeled cells in (C) superimposed onto a schematic drawing of the barrel pattern (C). Scale bar (C, D) = 200μm.
Layer 4 FS interneurons are physiologically categorized in two distinct functional groups
All FS interneurons were physiologically characterized in current clamp mode by injecting increasing amplitudes of depolarizing current to produce distinct action potential firing patterns. Figure 2 illustrates FS interneuron responses at increasing levels of injected current. Although all FS interneurons fired action potentials at high frequencies with sub-saturating current injection, we observed many subtle differences in firing patterns at threshold current levels. This allowed for an initial organization into two broadly grouped categories according to their action potential onset time: “Delayed Firing” (FSD, n = 107) and “Early Onset Firing” (FSE, n = 145). For example, FSD interneurons exhibited a prominent delay in the appearance of the first action potential (Fig. 2Ai, Bi). At current amplitudes just above threshold, interneurons within each class were further sub-divided based on variations in firing patterns (Fig. 2Aii, Bii). We grouped FSD interneurons into two subtypes: Delayed Non-Accommodating (FSD-NAC) and Delayed Stuttering (FSD-STUT). At threshold, FSD-NAC interneurons (n = 63) showed an initial delay (Fig. 2Ai), which persisted with increased current injections but was then followed by a continuous, non-accommodating firing pattern (Fig. 2Aii). With increasing current injections, action potentials in FSD-NAC interneurons convert to a continuous sweep (Fig. 2Aiii) and with little accommodation (Fig. 1Aiv, Table 1). FSD-STUT interneurons (n = 44, Fig. 2Bi-Biv) also exhibited a delay in the appearance of the first action potential; however, increased current injection elicited a typical stuttering pattern (Fig. 2Bii). Similar to FSD-NAC interneurons, at increasing current steps, FSD-STUT interneurons show a continuous train of action potentials (Fig. 2Biii). In contrast, we classified all the FSE interneurons on the presence of an action potential at the beginning of the sweep for all levels of depolarizing current injection (Fig. 2C-F). This group was further sub-divided into four subtypes based on subtleties of firing with increased current levels from threshold into: Early Onset-Stuttering (FSE-STUT), Early Onset-Late Spiking (FSE-LS), Early Onset-Early Spiking (FSE-ES) and Early Onset-Accommodating (FSE-AC). For FSE-STUT interneurons (n = 82, Fig. 2Ci-Ciii) the threshold response began with an action potential with no delay. With increased current, action potentials appeared in a stuttering pattern (Fig. 2Cii) and then displayed continuous firing of action potentials under further increased current injections (Fig. 2Ciii). FSE-LS interneurons (n = 24) fired one or multiple action potentials at the beginning of the threshold sweep (Fig. 2Di), followed by a pause then a train of multiple action potentials until the end of the sweep (Fig. 2Dii). As FSE-LS interneurons became more depolarized with greater current injection, they fired at higher frequency and eventually formed a constant sweep of action potentials (Fig. 2Diii). At threshold current levels, FSE-ES interneurons (n = 24, Fig. 2Ei) fired one or multiple action potentials at the onset followed by no action potential firing for the remainder of the sweep. This pause of repetitive action potential firing occurred at increased current injections as well (Fig. 2Eii). When these interneurons became more depolarized, the action potentials in the beginning of the sweep extend and the pause of firing becomes shorter until a full sweep of action potentials eventually ensues (Fig. 2Eiii). FSE-AC interneurons (n =15) exhibited a single action potential at threshold (Fig. 2Fi). With increased current injections, FSE-AC interneurons showed different firing frequency within a sweep (Fig. 2Fii). For example, the inter-spike-interval (ISI) among the first three to five action potentials were shorter than the rest of the ISIs, such as the 10th ISI: ISI-10, (p < 0.0005, n = 15) and the last ISI: ISI-L (p < 0.0001, n = 15) (Fig. 2Fiv).
Fig. 2. Actional potential onset determines two categories of FS interneurons.
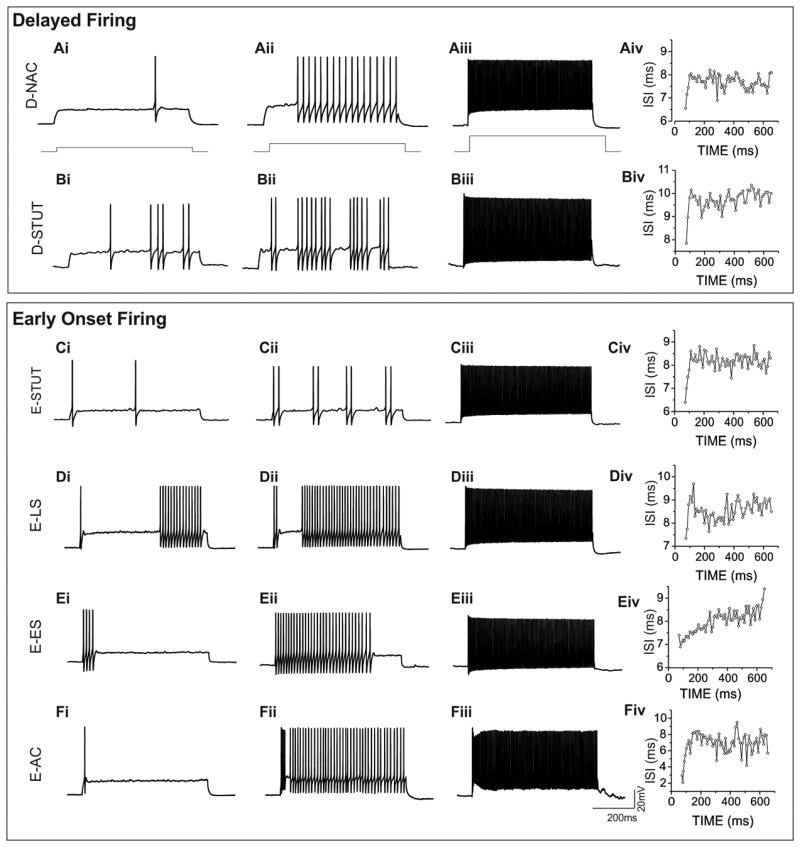
The appearance of the first action potential at threshold separates layer 4 FS interneurons into delayed and early onset firing subgroups. FSD-NAC (Ai) and FSD-STUT (Bi) interneurons exhibit a prominent delay but with increased current injections, a continuous non-accommodating firing pattern for FSD-NAC (Aii) compared to stuttering pattern of FSD-STUT (Bii). Both subtypes demonstrate a tonic firing state at supra-threshold current levels (Aiii and Biii). All early onset firing FS interneurons fire an action potential at the beginning of the trace (Ci-Fi). FSE-STUT interneurons stutter (Cii) then form a continuous firing pattern with higher current injection (Ciii). FSE-LS interneurons have a pause before more action potentials appear later in the trace (Dii) and as depolarizing current increases, the delay shortens (Dii) and becomes continuous (Diii). Action potential firing in FSE-ES interneurons occur at the beginning of the sweep (Ei) and increase across the trace as more current is injected into the cell (Eii) until finally a continuous tonic firing pattern emerges with more depolarizing current (Eiii). FSE-AC interneurons fire a single action potential at the beginning of the threshold sweep (Fi). With increased current depolarization, action potential frequency is higher at the beginning of the trace then at the end (Fii). Inter-spike intervals (ISI) taken near 100Hz firing frequency of each representative FS subtype are shown (A-Fiv). FSE-AC and FSE-ES demonstrate a high degree of fluctuation of ISI at the beginning and end of the action potential firing sweeps.
Table 1. Active and passive membrane properties of layer 4 FS interneurons.
| FSD (n=83) | FSE (n = 88) | p value | |
|---|---|---|---|
| Saturation Frequency (Hz) | 192.5 ± 3.32 | 181.5 ± 3.02 | <0.00001 |
| AP accommodation ratio | 0.921 ± 0.016 | 0.797 ± 0.016 | <0.00001 |
| AP Half-width (ms) | 0.629 ± 0.010 | 0.626 ± 0.008 | 0.781 |
| Rise time (10-90%) (ms) | 0.363 ± 0.011 | 0.378 ± 0.009 | 0.351 |
| AP threshold (mV) | -40.1 ± 0.57 | -44.9 ± 0.45 | <0.00001 |
| AP amplitudes (mV) | 65.4 ± 1.35 | 71.9 ± 1.53 | 0.0005 |
| AHP (mV) | 16.1 ± 0.56 | 16.6 ± 0.63 | 0.602 |
| Rheobase (pA) | 150.6 ± 8.78 | 213.2 ± 9.74 | <0.00001 |
| Vrest (mV) | -61.9 ± 0.42 | -59.7 ± 0.8 | 0.348 |
| Sag (mV) | 5.61± 0.37 | 4.82 ± 0.39 | 0.068 |
| Input Resistance (MΩ) | 156.3 ± 7.32 | 119.7 ± 4.16 | 0.0001# |
| Time Constant (mS) | 11.84 ± 0.69 (n = 34) | 9.01± 0.34 (n = 64) | 0.00008 |
Significance (in bold) was determined by unpaired student's t-test, or Mann-Whitney test if data are not normally distributed (#). AP, action potential; Vrest, resting membrane potential; n = cell number.
Previous publications report FS interneuron firing patterns at various current intensities and temperatures. In order to determine if the delayed or early onset characteristic changes with increasing temperatures or current intensity, we recorded FS interneuron firing patterns under variable conditions (Fig. 3). Recordings from FSD interneurons (n = 15) maintain delayed onset firing when current was increased at small increments (Fig. 3 Ai) and exhibit similar firing patterns when the interneurons were activated repetitively under same current intensity (Fig. 3Aii). This phenomenon holds true when the temperature was increased to 32°C (Fig.3Aiii). In a like manner, FSE interneurons (n = 6) also maintain early onset firing characteristics as reported at room temperature (Fig.3Bi,Bii) and when the temperature was increased to 32°C (Fig.3Biii).
Fig. 3. Action potential firing patterns for FSD and FSE interneurons are consistent at varying temperatures.
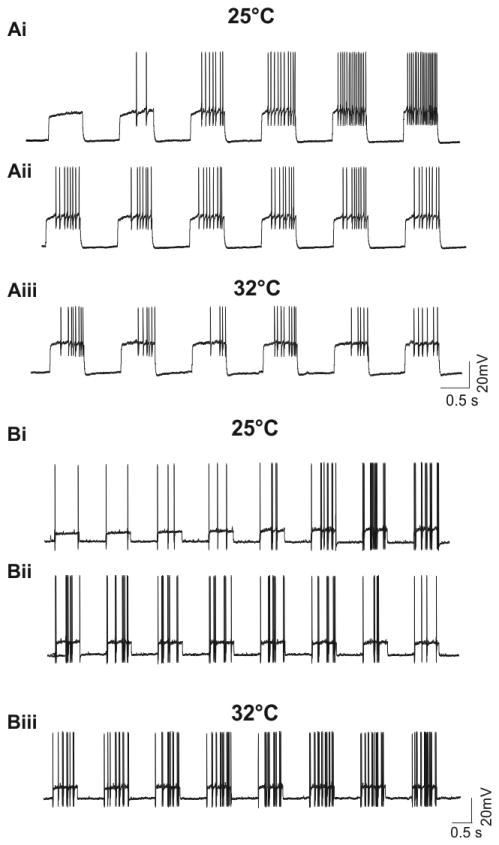
(Ai) Representative current clamp traces from an FSD interneuron in response to serial current injections at 10 pA increments (current steps from left to right: starting at 180pA to 230pA). Action potential firing patterns recorded from the same FSD interneuron as in (Ai) in response to constant current (200pA) injections at room temperature (Aii) and 32°C (Aiii) (n = 15). (Bi) Representative current clamp traces from an FSE interneuron in response to serial current injections under room temperature (current steps from left to right: 80pA to 150pA,10pA increments) . Action potential firing patterns recorded from the same FSE interneuron as in (Bi) at room temperature (under serial of 130pA current injections) (Bii) and 32°C (under serial of 100pA current injections) (Biii) (n = 6).
Morphological characterization of layer 4 FS interneurons
Cortical FS interneurons are morphologically characterized into different cell types based on axonal arborizations as: large basket cells, small basket cells, nest basket cells and chandelier cells (Kawaguchi and Kubota, 1998, Wang et al., 2002). In select recordings of physiologically characterized layer 4 FSD and FSE interneurons, we included biocytin in the intracellular pipette to evaluate morphological details (n=60, Fig. 4). Consistent with a previous report, we did not detect FS interneurons with the chandelier cell morphology in layer 4 of the barrel cortex (Staiger et al., 2009). All FSD and FSE interneurons with somata located in the layer 4 barrel structures were basket cells (large basket cells; small basket cells; and nest basket cells). We found that most subtypes of FSD and FSE interneurons showed no definitive trend in somata distribution that favored the wall or hollow sub-regions of the posteromedial barrel field. The one exception was the FSE-LS interneurons, which were exclusively located in the barrel hollow. Of all the recovered cells, there were morphological subtleties with regard to dendritic projections and axonal arborizations for each interneuron subtype (Fig. 4). The FSD-NAC interneurons were all basket cells (n=5) that exclusively extend both dendrites and axons vertically (inter-laminar) into the lower portion of layer 3 with minor projections into layer 5 (Fig. 4A). In contrast FSD-STUT interneurons (Fig. 4B) were more diverse with cells that have projections confined to a barrel (2 of 9) and others that extended both vertically across layers and horizontally into neighboring barrels (7 of 9 ) exhibiting the morphology of large basket cells (Wang et al., 2002). Most of the FSE-STUT interneurons exhibited vertically (inter-laminar) oriented projections (n=9 of 31) or both inter-laminar and inter-columnar (20 of 31, Fig. 4C), with only the exception of an intrabarrel projection (1 of 31) and an intercolumnar projection (1 of 31). All FSE-LS interneurons were found in the hollow but with projections that were restricted to the barrel hollow (2 of 5 interneurons) and inter-columnar and inter-laminar (3 of 5) (Fig. 4D). All FSE-ES interneurons have restricted dendritic and axonal projections within the parent barrel (n=7). In some cases, we observed dendrites that extend to the wall and reverse direction into the barrel hollow (Fig. 4E). Similar to FSE-ES interneurons, all projections of the FSE-AC interneurons were restricted to the parent barrel with shortened and thick dendrites and dense axon arborizations that remained within the parent barrel. In most cases, the dense plexus surrounds neighboring neurons within the barrel and show characteristic features of nest basket cells (Wang et al., 2002) (Fig. 4F).
Figure 4. Morphological heterogeneity of FS interneurons.
(A) Two biocytin filled FSD-NAC interneurons in neighboring barrels (barrel outlines are depicted as white boxes in all panels) showing axon projections extending vertically into the lower portion of layer 2/3, with minor projections to layer 5 (white arrow). (B) Dendritic projections of FSD-STUT interneurons remain with the barrel walls and in some cases projections reverse direction and turn back into the barrel hollow (white arrow). Note, the axon mainly stays within the parent barrel with minor horizontal projections to neighboring barrels. (C) Two FSE-STUT interneurons in neighboring barrels reveal dendritic and axonal projections extending up into layer 2/3. The axons of these interneurons extend vertically up through layer 2/3 and cover the region above the parent barrel and across the cortical column into the area above the adjacent barrel. (D) An FSE-LS interneuron shown with axonal projections extending to adjacent barrels on either side. (E) An FSE-ES interneuron illustrating projections of both axons and dendrites remaining inside the parent barrel. Note, a major dendrite is shown curving back into the hollow of the parent barrel (white arrow). (F) Illustration of an FSE-AC interneuron showing intrabarrel arborization. Note the axon plexus wraps around cell bodies (white arrows) indicative of the nest basket cell morphology. All scale bars = 100μm.
Biophysical properties identify FSD and FSE interneuron subgroups
Previous studies of cortical interneurons rely heavily on biophysical properties for classification and terminology (Markram et al., 2004, Ascoli et al., 2008). In the present study we observe FS interneurons in layer 4 can be separated into two broad categories based on the appearance of the first action potential in a depolarizing train (Fig. 2). In order to determine if these two groups could be classified with biophysical parameters, we measured some biophysical properties and compared the values between FSD and FSE interneurons (Table 1; Fig. 5). In seven of twelve parameters we found significant differences that separate the two subgroups. For example, FSD interneurons exhibited higher values for saturation frequency, accommodation ratio and time constant (Table 1). However, there were no observable differences in action potential half-width, action potential rise time and after hyperpolarization potential (Table 1). Both groups also exhibited similar values for sag (or Ih current) at hyperpolarizing current injections (Table 1). With regard to passive membrane properties FSD interneurons showed a higher input resistance than FSE cells but similar resting membrane potentials (Table 1). Additionally, FSD interneurons had lower rheobase current values (the current required to elicit a threshold response), higher action potential threshold and smaller action potential amplitude (Table 1). It is worth noting that even though the averaged accommodation ratio from all recorded FS interneurons (with high chloride intracellular solution) was above 0.8 (accommodation ratio = 0.85 ± 0.012, n = 171), we did observe a higher accommodation ratio in FSD cells than in FSE cells. An especially lower accommodation ratio was observed in the FSE-AC subtype (accommodation ratio = 0.607± 0.024, n = 15). It is possible that the low accommodation ratio of FSE-AC interneurons would affect the whole FSE population to a statistically significant lower value when compared to FSD interneurons. However, when the accommodation ratios were compared between FSD and FSE with the FSE-AC subtype excluded, significant differences still stand true between these two populations (FSE without FSE-AC: accommodation ratio = 0.832 ± 0.015, n = 73, p = 0.00011). In order to illustrate the complexity of the distribution of individual cells we plotted values for accommodation ratio and rheobase (Fig. 5 A,B). In summary, based on the significance on these seven key biophysical parameters, our study provides evidence for biophysical separation of layer 4 FS interneurons into two functional categories.
Fig. 5. Distributions of selected biophysical properties in FSD and FSE interneurons.
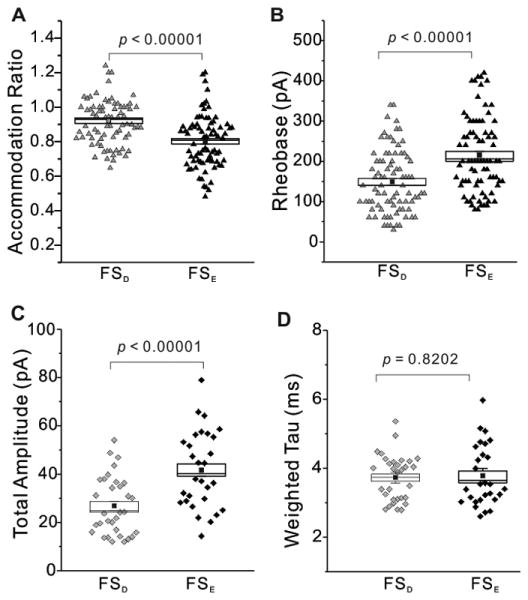
Plotted raw data from FSD (grey) and FSE (black) interneurons showing significant differences from data in Tables 1 and 2 of action potential accommodation ratio (A, triangles), rheobase (B, triangles), sIPSC total amplitude (C, diamonds). Non-significant differences of sIPSC weighted tau (D, diamonds) are shown for comparison. Standard error (open rectangle), statistical mean (small filled square) and p values are shown.
Synaptic and autaptic properties of layer 4 FS interneurons
FS interneurons are also differentiated from other cortical interneurons based on synaptic (Bacci et al., 2003b, Li et al., 2009) and autaptic (Tamas et al., 1997, Bacci et al., 2003a) GABAA receptor-mediated inhibitory currents. Therefore, we investigated several components of synaptic and autaptic neurotransmission in FSD and FSE interneurons. sIPSCs were recorded in voltage clamp mode in order to measure synaptic decay, rise time, amplitude, frequency and charge (Figs. 5 & 6; Table 2). All inhibitory events from individual FS interneurons were averaged and fit to two exponentials to illustrate fast and slow components of sIPSC decay in layer 4 FS interneurons. The major synaptic difference between FSD and FSE interneurons was observed as a reduction in sIPSC amplitude (Fig. 5C, Fig. 6A,B;) and charge in FSD interneurons (Table 2). However, the weighted time constant (τD,W), and fast and slow components of sIPSC decay from both FSD and FSE interneurons revealed no significant differences in decay rate (Table 2; Fig. 5D). Therefore, just as high action potential firing frequency is a characteristic of the FS interneurons, it appears as though fast synaptic decay is also preserved.
Fig. 6. Large amplitude synaptic and autaptic IPSCs in FSE interneurons.
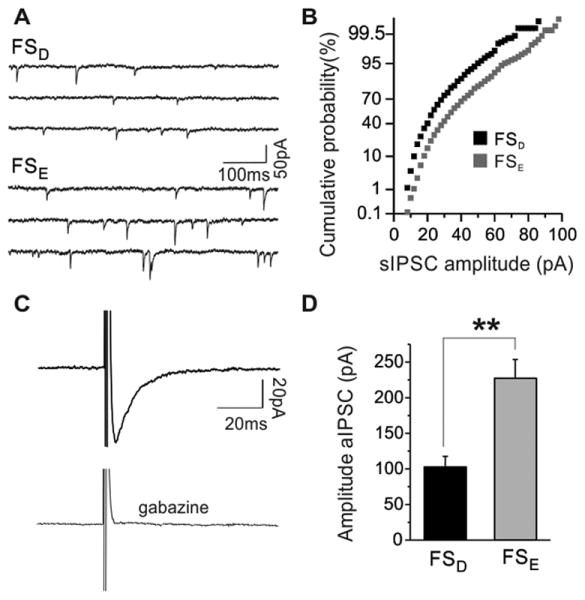
(A) representative voltage-clamp recording showing raw traces of sIPSCs from FSD (upper trace) and FSE (bottom trace) interneurons. (B) Cumulative probability histogram of sIPSC amplitude distribution from the FSD (black) and FSE (gray) shown in (A) demonstrate the relative distributions of events within a recording period. (C) Averaged autIPSCs traces recorded from an FS interneuron elicited by a brief (0.5ms) depolarizing ramp from -70mV to 10mV. The depolarization elicited a fast inward sodium current (truncated), followed by a slow inward GABAA receptor mediated current that is blocked by gabazine (5μM) (below). (D) pooled data of the first autIPSC amplitude reveal significant differences between FSD and FSE interneurons. ** = p < 0.01.
Table 2. Synaptic Properties of layer 4 FS interneurons.
| FSD (n = 30) | FSE (n = 34) | p value | |
|---|---|---|---|
| Frequency (Hz) | 7.07 ± 0.53 | 8.50 ± 0.82 | 0.1424 |
| Amplitude 1 (fast A1, pA) | 21.38 ± 1.64 | 32.32 ± 2.12 | <0.0001 |
| Tau 1 (ms) | 2.49 ± 0.09 | 2.54 ± 0.08 | 0.6319 |
| Amplitude 2 (slow A2, pA) | 5.58 ± 0.56 | 9.36 ± 1.15 | 0.0040 |
| Tau 2 (ms) | 9.50 ± 0.58 | 8.93 ± 0.47 | 0.4452 |
| Weighted Tau, τD,W (ms) | 3.73 ± 0.10 | 3.77 ± 0.14 | 0.8202 |
| Total Amplitude (pA) | 26.81 ± 1.90 | 41.68 ± 2.54 | <0.00001 |
| Charge (pC/s) | 716.8 ± 86.1 | 1210.4 ± 118.9 | 0.0012 |
| Rise time (ms) | 0.81 ± 0.036 | 0.75 ± 0.033 | 0.2632 |
Significance (in bold) was determined by unpaired student's t-test. n = cell number.
In addition to inhibitory inputs from other GABAergic interneurons, FS interneurons self-innervate with autaptic connections from collaterals from the parent axon (Tamas et al., 1997, Bacci et al., 2003a). Autapses are characteristic features of FS interneurons and function to facilitate precise action potential timing and promote synchronized cortical network oscillations (Bacci and Huguenard, 2006). Therefore differences in the size and timing of autaptic responses may indicate separate ways in which FSD and FSE interneurons modulate their own output. In the present study, autaptic IPSCs (autIPSCs) were generated from FS interneurons with brief (0.5ms) depolarizing ramps from a holding potential of -70mV to 10mV (Fig. 6C). In order to maximize autIPSC detection, a high chloride pipette solution was employed. Under these conditions, we observed a fast inward Na+ current, followed by a slower GABAA receptor mediated current that can be blocked with 5μM gabazine (Fig. 6C) (Bacci et al., 2003a). While all subtypes of layer 4 FS interneurons exhibit autIPSCs, they appeared more frequently in FSE interneurons (40 of 44) than in FSD interneurons (21 of 31). In addition to a higher occurrence of functional autapses, the autIPSCs recorded from FSE interneurons were over double in size (FSE: 227.1 ± 26.5 pA, n = 40 versus FSD: 102.9 ± 14.9 pA, n = 21, p < 0.01) (Fig. 6D).
Circuit properties of layer 4 FS interneurons
FS interneurons in layer 4 mediate feedforward inhibition onto layer 4 excitatory neurons (Porter et al., 2001, Beierlein et al., 2003, Swadlow, 2003, Cruikshank et al., 2007). Therefore glutamatergic responses can be evoked in FS interneurons from thalamic stimulation (Agmon and Connors, 1992, Porter et al., 2001). In order to study the nature of thalamic input onto FSD and FSE interneurons in layer 4 barrel cortex, thalamic-evoked excitatory postsynaptic currents (EPSCs) were investigated in a thalamocortical slice preparation using a minimal stimulation protocol (Fig. 7A,B, see methods). In most cases fast AMPA receptor-mediated monosynaptic thalamic-evoked EPSCs were recorded from both FSD and FSE interneurons. However, some FSE interneurons (5 of 15) exhibited delayed recurrent excitatory EPSCs (Fig. 7B). These recurrent EPSCs (which are observed with minimal stimulation) could not be removed by applying DNQX (0.1uM) (data not shown), suggesting that NMDA receptor mediated currents are contributing to the recruitment of layer 4 excitatory cells in response thalamic activity (Hull et al., 2009). Both FSD and FSE interneurons required similar current values to initiate a threshold response (FSD = 59.3 ± 15.5 μA, n=9; FSE = 48.5 ± 5.4 μA, n=15, p=0.459, Fig. 7C) despite having very different intrinsic properties, such as the lower input resistance in FSE interneurons. Comparisons of the monosynaptic responses between the two categories revealed significantly increased EPSC amplitude for FSE interneurons (FSD= 99.79 ± 13.84 pA n= 9; FSE = 145.04 ± 12.37pA, n=15, p < 0.05, Fig. 7D). The larger response in FSE interneurons may be indicative of postsynaptic differences (Cruikshank et al., 2007, Hull et al., 2009) and as such, may be an indicator of distinct excitatory microcircuits between the two FS interneuron categories.
Fig. 7. Thalamocortical evoked responses are larger in FSE interneurons.
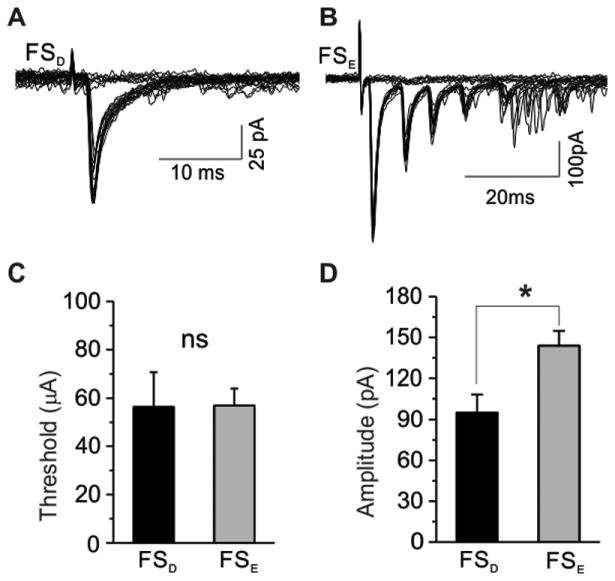
Representative traces of EPSCs evoked with minimal stimulus to acheive 50 percent failure ratio are shown in both FSD (A) and FSE (B) interneurons. Note, recurrent excitatory EPSCs (B) following the monosynaptic TC evoked response were only observed in FSE interneurons. (C) Mean values of pooled data show no significant difference in the threshold current required to evoke a thalamacortical response (p = 0.459). (D) Mean values of pooled data reveal increased evoked EPSC amplitudes in FSE interneurons. * = p < 0.05.
Discussion
It is often proposed that cortical interneuron diversity implies a specialization of inhibitory function (Amitai and Connors, 1995, Beierlein et al., 2003). Therefore, identification of divergent circuitry requires both intrinsic cellular and synaptic differentiation in regions with highly diverse interneuron populations. This is especially true when morphological sub-groupings are problematic (Markram et al., 2004). The results of the present study represent the first grouping of a morphologically heterogeneous population of cortical FS interneurons into two functional categories in layer 4 of the mouse barrel cortex. Using functional criteria previously employed to identify interneuron populations, we found FSD and FSE interneurons differed in 7 key biophysical properties (saturation frequency, rheobase current, input resistance, time constant, action potential accommodation, action potential threshold and action potential amplitude), in synaptic properties (sIPSCs or autIPSCs) and in their response to the stimulation of thalamic afferents. These cumulative differences suggest that FSE interneurons possess intrinsic and synaptic specializations that allow them to more reliably mediate feedforward inhibitory neurotransmission than their FSD counterparts and as such represent a variation of function within the FS interneuron class.
FSD and FSE as functional categories
Intrinsic and synaptic physiological criteria are often employed to distinguish neuronal identification (McCormick et al., 1985, Kawaguchi and Kubota, 1997, Gupta et al., 2000, Wang et al., 2002, Bacci et al., 2003b, Beierlein et al., 2003, Wang et al., 2004, Xu et al., 2006). These criteria are often used to separate inhibitory interneurons from excitatory neurons as well as to classify differences between interneuron subpopulations. In the present study, we employed similar approaches to determine if different subcategories of FS interneurons exist in layer 4 of the primary somatosensory cortex - a region with known functional diversity of FS interneurons. Axon terminals from FS interneurons are positioned to deliver powerful inhibition over the summated synaptic potential as well as dictate action potential firing of the target cell; and as such contribute vital roles in various cortical activities through distinct functional circuits such as: feedforward inhibition of sensory evoked responses, plasticity of sensory map reorganization and the control of synchronized firing of excitatory microcircuits at distinct frequencies (Wang et al., 2002, Beierlein et al., 2003, Fagiolini et al., 2004, Maffei et al., 2006, Cardin et al., 2009, Sohal et al., 2009). Therefore, speculation on diversity of function should not just govern all interneurons (Markram et al., 2004) but may likely extend to within the FS interneuron class, especially with respect to sensory responsiveness and the selective participation during periods of heightened coordinated activity or UP states (Puig et al., 2008, Moore et al., 2010). Previous work has shown that high variable electrophysiological properties result from the combined activity of different membrane ion channels (Llinas, 1988) and cell morphology (Mainen et al., 1996). Among those ion channels, the voltage gated potassium channel Kv1 family plays an important role in action potential initiation at near threshold potentials and also serves to suppress action potential firing (Coetzee et al., 1999, Golomb et al., 2007). Additionally, Kvβ1 is an auxiliary subunit that forms complexes with the Kv1 family of ion channels leading to fast inactivation of evoked currents in heterozygous expression systems (Pongs et al., 1999). In a recent study, it was suggested that Kv1.1 channels at the axon initial segment dampen near-threshold excitability and thus likely causes the delayed firing behaviour at threshold potentials in certain types of layer 2/3 FS interneurons (Goldberg et al., 2008).
In the present study, we observe quantitative differences in key intrinsic, synaptic and circuit properties between layer 4 FSD and FSE interneurons that indicate a basis for distinct microcircuitry in barrel cortex. As a prominent mediator of feedforward inhibition, FS interneurons are important in performing spatial and temporal control of thalamocortical sensory information (Porter et al., 2001, Beierlein et al., 2003, Cruikshank et al., 2007). Multiple properties contribute to the responsiveness of layer 4 interneurons including intrinsic and synaptic properties that allow for reliable activation from thalamic fibers. FSD interneurons have higher input resistance and a longer time constant values than FSE interneurons. Based on intrinsic properties alone, this may indicate a higher responsiveness in FSD interneurons. However, with similar threshold current pulses, FSE interneurons exhibit significantly larger thalamic-evoked AMPA-mediated EPSCs suggesting increased excitatory synaptic connectivity. The differences in magnitude of thalamic-evoked EPSCs in FSE interneurons may indicate increased thalamocortical innervation (White and Keller, 1987, Staiger et al., 1996). Furthermore, it is worthy to note that we recorded thalamic-evoked responses with recurrent EPSCs that are insensitive to low concentration of AMPA receptor antagonist DNQX from 5 out of 15 FSE cells. This suggests that these particular cells are subject to NMDA-mediated recurrent polysynaptic excitation from intrinsic excitatory cells (Hull et al., 2009). Therefore, FSE interneurons may be more suitable for eliciting a more reliable feedforward inhibitory response in layer 4 excitatory neurons (Hull et al., 2009).
Synaptic and autaptic inhibitory specificity
In addition to biochemical and membrane properties, neuronal populations are also differentiated by GABAA receptor-mediated inhibitory synaptic neurotransmission (Huntsman and Huguenard, 2000, Bacci et al., 2003b, Li et al., 2009). Differences in IPSC decay kinetics and amplitude across different brain regions and during periods of development are reflective of differential GABAA receptor subunit expression and distribution (Huntsman and Huguenard, 2000). Cortical FS interneurons are characterized with fast decaying α1-containing GABAA receptor mediated sIPSCs (Bacci et al., 2003b, Li et al., 2009). Comparisons of FSD and FSE interneurons, revealed no differences in synaptic decay kinetics. We did however observe significantly larger sIPSC amplitudes and overall charge in FSE interneurons. Larger sIPSCs may reflect subtleties in activity and innervation from presynaptic interneurons such that FSE interneurons may have higher receptor density at synaptic sites. Since there are no significant differences observed in sIPSC rise time kinetics, frequency and decay, this synaptic difference may stem from higher receptor density and not likely include presynaptic release mechanisms, somatic location or different composition of the postsynaptic receptor subtypes. Therefore, the amplitude difference may be the only factor contributing to differences in charge properties in FSE interneurons.
Another characteristic feature of FS interneurons is the presence of autaptic connections (Tamas et al., 1997, Bacci et al., 2003a). Autaptic neurotransmission represents a form of robust and transient feedback inhibition that is important for the intrinsic regulation of spike timing in FS interneurons (Bacci and Huguenard, 2006). We observed autIPSCs in all subtypes of FS interneurons as fast, GABAA receptor-mediated events with short, reproducible latencies and similar decay kinetics (Bacci et al., 2003a). However, functional autaptic transmission was much more readily observed in FSE interneurons. Moreover, autIPSCs in FSE interneurons were significantly larger in amplitude. Differences in autIPSC amplitude reveal structural and/or functional similarities within the FSE category that set them apart from FSD interneurons in three non-mutually exclusive details: 1) the higher recovery rate in FSE interneurons may indicate that this category of FS interneurons have shorter autaptic connections and as such, are not excised in the slicing process, 2) the increased amplitude of autIPSCs in FSE interneurons may result from a higher proportion of somatic versus on proximal dendritically located autapses (Tamas et al., 1997) and 3) FSE interneurons may have a higher density of GABAA receptors at the autaptic connection. Therefore, based on the larger amplitude autaptic currents in FSE interneurons, we would speculate that FSE interneurons, especially the FSE-LS, FSE-AC subtypes, are more powerful in facilitating precise spike timing and promoting synchronized cortical network oscillations (Bacci and Huguenard, 2006, Sohal et al., 2009).
Functional implications for plasticity and disease
The proper identification of functionally distinct categories of interneuron subtypes is critically important for understanding why certain subsets of FS interneurons are compromised after sensory deprivation or in the diseased brain. Parvalbumin positive interneurons have been implicated in sensory deprivation-induced plasticity and also in mechanisms of disease. This is observed as reductions in parvalbumin staining in the pathogenesis of disorders such as epilepsy, neurodevelopmental disorders and schizophrenia (Lewis et al., 2001, Powell et al., 2003, Selby et al., 2006, Andrioli et al., 2007, Canty et al., 2009). Therefore, a critical question to ask is whether reductions in parvalbumin staining signify a global loss of FS interneurons or the depletion of specific functional subtypes. The answer is multifaceted because there are several circuit, cellular and intrinsic factors that underlie the propensity of certain cell types to be compromised. While the present study does not specifically investigate this scenario, there is evidence of differential changes of layer 4 parvalbumin positive interneurons following whisker removal. For example, FS interneurons in whisker-deprived barrels undergo biochemical, physiological and morphological changes (Micheva and Beaulieu, 1995, Jiao et al., 2006, McRae et al., 2007, Nowicka et al., 2009, Sun, 2009). Reports of decreased GABA-immunopositive interneuron density and partial down-regulation of perineuronal net expression reveal selective survival of parvalbumin positive interneurons (Micheva and Beaulieu, 1995, Jiao et al., 2006, McRae et al., 2007). Based on the observed biophysical and synaptic differences between FSD and FSE interneurons, it may be possible that the two functionally different populations are reacting differently to changes in afferent input. Additionally, it is clear that the presence of mixed populations of FS interneurons may complicate previous findings of biophysical changes of FS interneurons in deprived barrels (Sun, 2009) because this may reflect comparisons of different FS interneuron subtypes under the two disparate conditions.
Conclusions
As FSD and FSE interneurons exhibit variations in their dendritic and axonal projections within and across barrel boundaries. While electrophysiological parameters provide insight into classification groupings - molecular and morphological descriptions prove to be more problematic as variations are subtle and numerous (Markram et al., 2004). Therefore, it is important to keep track of both physiological and morphological variations as this may lead to identification of alterations that may occur as a result of disease or lesion-induced plasticity.
HIGHLIGHTS.
We physiologically define two subtypes of FS interneurons
Cortical interneuron classification
Increasing information of interneuron classification may indicate disease states
Acknowledgments
This work was supported by the National Institute of Disorders and Stroke (NS053719).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. doi: 10.1016/0306-4522(91)90333-j. [DOI] [PubMed] [Google Scholar]
- Agmon A, Connors BW. Correlation between intrinsic firing patterns and thalamocortical synaptic responses of neurons in mouse barrel cortex. J Neurosci. 1992;12:319–329. doi: 10.1523/JNEUROSCI.12-01-00319.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai Y, Connors BW. Intrinsic physiology and morphology of single neurons in neocortex. In: Jones EG, Diamond IT, editors. Cerebral Cortex. New York: Plenum; 1995. pp. 299–331. [Google Scholar]
- Andrioli A, Alonso-Nanclares L, Arellano JI, DeFelipe J. Quantitative analysis of parvalbumin-immunoreactive cells in the human epileptic hippocampus. Neuroscience. 2007;149:131–143. doi: 10.1016/j.neuroscience.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvarday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Munoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC, Yuste R. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR. Enhancement of spike-timing precision by autaptic transmission in neocortical inhibitory interneurons. Neuron. 2006;49:119–130. doi: 10.1016/j.neuron.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Functional autaptic neurotransmission in fast-spiking interneurons: a novel form of feedback inhibition in the neocortex. J Neurosci. 2003a;23:859–866. doi: 10.1523/JNEUROSCI.23-03-00859.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci A, Rudolph U, Huguenard JR, Prince DA. Major differences in inhibitory synaptic transmission onto two neocortical interneuron subclasses. J Neurosci. 2003b;23:9664–9674. doi: 10.1523/JNEUROSCI.23-29-09664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- Canty AJ, Dietze J, Harvey M, Enomoto H, Milbrandt J, Ibanez CF. Regionalized loss of parvalbumin interneurons in the cerebral cortex of mice with deficits in GFRalpha1 signaling. J Neurosci. 2009;29:10695–10705. doi: 10.1523/JNEUROSCI.2658-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci. 1997;17:3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci. 2007;10:462–468. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Low K, Mohler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- Fleidervish IA, Binshtok AM, Gutnick MJ. Functionally distinct NMDA receptors mediate horizontal connectivity within layer 4 of mouse barrel cortex. Neuron. 1998;21:1055–1065. doi: 10.1016/s0896-6273(00)80623-6. [DOI] [PubMed] [Google Scholar]
- Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron. 2008;58:387–400. doi: 10.1016/j.neuron.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb D, Donner K, Shacham L, Shlosberg D, Amitai Y, Hansel D. Mechanisms of firing patterns in fast-spiking cortical interneurons. PLoS Comput Biol. 2007;3:e156. doi: 10.1371/journal.pcbi.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- Hull C, Isaacson JS, Scanziani M. Postsynaptic mechanisms govern the differential excitation of cortical neurons by thalamic inputs. J Neurosci. 2009;29:9127–9136. doi: 10.1523/JNEUROSCI.5971-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntsman MM, Huguenard JR. Nucleus-specific differences in GABA(A)-receptor- mediated inhibition are enhanced during thalamic development. J Neurophysiol. 2000;83:350–358. doi: 10.1152/jn.2000.83.1.350. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Zhang C, Yanagawa Y, Sun QQ. Major effects of sensory experiences on the neocortical inhibitory circuits. J Neurosci. 2006;26:8691–8701. doi: 10.1523/JNEUROSCI.2478-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis A, Gallopin T, David C, Battaglia D, Geoffroy H, Rossier J, Hillman EM, Staiger JF, Cauli B. Classification of NPY-expressing neocortical interneurons. J Neurosci. 2009;29:3642–3659. doi: 10.1523/JNEUROSCI.0058-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience. 1998;85:677–701. doi: 10.1016/s0306-4522(97)00685-4. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia:evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- Li P, Rudolph U, Huntsman MM. Long-term sensory deprivation selectively rearranges functional inhibitory circuits in mouse barrel cortex. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0900922106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci. 2006;26:5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- Mainen ZF, Carnevale NT, Zador AM, Claiborne BJ, Brown TH. Electrotonic architecture of hippocampal CA1 pyramidal neurons based on three-dimensional reconstructions. J Neurophysiol. 1996;76:1904–1923. doi: 10.1152/jn.1996.76.3.1904. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- McRae PA, Rocco MM, Kelly G, Brumberg JC, Matthews RT. Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex. J Neurosci. 2007;27:5405–5413. doi: 10.1523/JNEUROSCI.5425-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. Neonatal sensory deprivation induces selective changes in the quantitative distribution of GABA-immunoreactive neurons in the rat barrel field cortex. J Comp Neurol. 1995;361:574–584. doi: 10.1002/cne.903610403. [DOI] [PubMed] [Google Scholar]
- Moore CI, Carlen M, Knoblich U, Cardin JA. Neocortical interneurons: from diversity, strength. Cell. 2010;142:189–193. doi: 10.1016/j.cell.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicka D, Soulsby S, Skangiel-Kramska J, Glazewski S. Parvalbumin-containing neurons, perineuronal nets and experience-dependent plasticity in murine barrel cortex. Eur J Neurosci. 2009;30:2053–2063. doi: 10.1111/j.1460-9568.2009.06996.x. [DOI] [PubMed] [Google Scholar]
- Pongs O, Leicher T, Berger M, Roeper J, Bahring R, Wray D, Giese KP, Silva AJ, Storm JF. Functional and molecular aspects of voltage-gated K+ channel beta subunits. Ann N Y Acad Sci. 1999;868:344–355. doi: 10.1111/j.1749-6632.1999.tb11296.x. [DOI] [PubMed] [Google Scholar]
- Porter JT, Johnson CK, Agmon A. Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. J Neurosci. 2001;21:2699–2710. doi: 10.1523/JNEUROSCI.21-08-02699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell EM, Campbell DB, Stanwood GD, Davis C, Noebels JL, Levitt P. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J Neurosci. 2003;23:622–631. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Ushimaru M, Kawaguchi Y. Two distinct activity patterns of fast-spiking nterneurons during neocortical UP states. Proc Natl Acad Sci U S A. 2008;105:8428–8433. doi: 10.1073/pnas.0712219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby L, Zhang C, Sun QQ. Major defects in neocortical GABAergic inhibitory circuits in ice lacking the fragile X mental retardation protein. Neurosci Lett. 2006 doi: 10.1016/j.neulet.2006.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger JF, Zilles K, Freund TF. Innervation of VIP-immunoreactive neurons by the ventroposteromedial thalamic nucleus in the barrel cortex of the rat. J Comp Neurol. 1996;67:194–204. doi: 10.1002/(SICI)1096-9861(19960401)367:2<194::AID-CNE3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Staiger JF, Zuschratter W, Luhmann HJ, Schubert D. Local circuits targeting parvalbumin-containing interneurons in layer IV of rat barrel cortex. Brain Struct Funct. 2009;214:1–13. doi: 10.1007/s00429-009-0225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QQ. Experience-dependent intrinsic plasticity in interneurons of barrel cortex layer IV. J Neurophysiol. 2009;102:2955–2973. doi: 10.1152/jn.00562.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA. Fast-spike interneurons and feedforward inhibition in awake sensory neocortex. Cereb Cortex. 2003;13:25–32. doi: 10.1093/cercor/13.1.25. [DOI] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Somogyi P. Massive autaptic self-innervation of GABAergic neurons n cat visual cortex. J Neurosci. 1997;17:6352–6364. doi: 10.1523/JNEUROSCI.17-16-06352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP. Interlaminar connections in the neocortex. Cereb Cortex. 2003;13:5–14. doi: 10.1093/cercor/13.1.5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Gupta A, Toledo-Rodriguez M, Wu CZ, Markram H. Anatomical, physiological, molecular and circuit properties of nest basket cells in the developing somatosensory cortex. Cereb Cortex. 2002;12:395–410. doi: 10.1093/cercor/12.4.395. [DOI] [PubMed] [Google Scholar]
- Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J Physiol. 2004;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EL, Keller A. Intrinsic circuitry involving the local axon collaterals of corticothalamic projection cells in mouse SmI cortex. J Comp Neurol. 1987;262:13–26. doi: 10.1002/cne.902620103. [DOI] [PubMed] [Google Scholar]
- White EL, Rock MP. A comparison of thalamocortical and other synaptic inputs to dendrites of two non-spiny neurons in a single barrel of mouse SmI cortex. J Comp Neurol. 1981;195:265–277. doi: 10.1002/cne.901950207. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM. Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J Comp Neurol. 2006;499:144–160. doi: 10.1002/cne.21101. [DOI] [PubMed] [Google Scholar]