Abstract
In order to deepen our knowledge of the natural host response to pathogens our team undertook an in vivo screen of mutagenized 129S1 mice with Salmonella Typhimurium. One mutation affecting Salmonella susceptibility was mapped to a region of 1.3Mb on chromosome 6 that contains 15 protein-coding genes. A missense mutation was identified in the Usp18 (Ubiquitin specific peptidase 18) gene. This mutation results in an increased inflammatory response (IL-6, type 1 IFN) to Salmonella and LPS challenge while paradoxically reducing IFN-γ production during bacterial infection. Increased STAT1 phophorylation correlated with impaired STAT4 phosphorylation resulting in overwhelming IL-6 secretion but reduced IFN-γ production during infection. The reduced IFN-γ levels along with the increased inflammation rationalize the Salmonella Typhimurium susceptibility in terms of increased bacterial load in target organs and cytokine-induced septic shock and death.
Introduction
Salmonella enterica species includes a number of closely related serovars capable of causing serious infections in humans and animals. Typhoid fever caused by Salmonella enterica serovar Typhi is a major public health concern in many developing countries claiming millions of lives annually. This intracellular Gram-negative bacterium follows a fecal-oral infection route and establishes systemic infection in the host. The outcome of the infection will vary from mild to severe, with some infected people remaining healthy carriers, suggesting an important host genetic contribution to the outcome of this disease. In humans, non typhoid Salmonella such as Salmonella enterica serovar Typhimurium (Salmonella Typhimurium) and Salmonella Enteritidis infections usually present as self limiting gastroenteritis, although a certain percentage of these infections may become invasive and result in septicemia. Accumulated data in humans suggest that predisposition to pediatric primary infection with Salmonella involves functional polymorphisms within genes in the IL-12-dependent, IFN-γ-mediated immunity (1–3).
In mice, the study of natural variation in the host response to infection has led to the identification of genes that have a critical impact during infections (4). Oral infection of mice with Salmonella Typhimurium causes a typhoid-like disease where the bacteria invade the M cells of the intestine, gain access to the mesenteric lymph nodes and establish a systemic disease with major sites of replication in the spleen and liver (5). Great phenotypic diversity of the host response to Salmonella Typhimurium is observed among different inbred mouse strains due to the presence of specific mutations that are inherited as monogenic traits (Slc11a1G169D in C57BL/6J and BALB/cJ; Tlr4P712H in C3H/HeJ or PklrI90N in AcB61 mice) (6–8) or complex patterns (9).
Infectious disease models using inbred and recombinant inbred/congenic mice are inherently limited by the finite natural genetic variation present in these strains. To circumvent this problem, functional genomics of infectious disease susceptibility has been approached by our group using random mutagenesis with the chemical mutagen, N-ethyl-N-nitrosourea (ENU). Induction of novel mutations, most of which are inherited in a recessive manner, are initially screened by experimental challenge of mice from a three generation breeding scheme with Salmonella Typhimurium (10). Similar strategies using chemically defined microbial structures and viruses have been successful in identifying novel targets in the mouse genome (11–13) and translating them to human primary immune deficiencies (14). This is the first report of the identification of a novel genetic determinant of susceptibility to a prevalent bacterial disease by direct challenge of the ENU-mutagenized mice with the infectious agent.
In the current report, we describe the identification of a Salmonella-susceptible ENU-mutant named Ity9 (Immunity to Typhimurium locus 9) that carries a non functional allele at the gene encoding USP18 (Usp18Ity9). USP18 has been shown to have dual independent functions as an ISG15 protease (15) and as a negative regulator of the type 1 IFN signaling by binding to the IFN (alpha and beta) receptor 2 (IFNAR2) (16, 17). Consistent with these functions, USP18 was reported to play a role in host defense as a negative regulator of antiviral activities (18–20).
Mice presenting a point mutation in Usp18 exhibit innate susceptibility to lethal Salmonella Typhimurium infection as measured by decreased survival and increased bacterial load in spleen and liver. Usp18Ity9/Usp18Ity9 mutants are more susceptible to LPS challenge and display an increased inflammatory response during Salmonella infection that is associated with increased type 1 IFN signaling through the activation of STAT1 and results in increased expression of IL-6 and Type 1 IFN regulated genes. Contrasting with these enhanced activation effects, the Usp18Ity9/Usp18Ity9 mutant mice have impaired STAT4 phosphorylation and IFN-γ production during Salmonella infection. Our findings suggest that Salmonella susceptibility in Usp18Ity9/Usp18Ity9 mutant mice can be explained by the observed increased in bacterial load in target organs as a consequence of lower IFN-γ production, and by the heightened inflammatory response triggered by STAT1 hyperactivation.
Materials and Methods
Mice
All animal experiments were performed under conditions specified by the Canadian Council on Animal Care, and the animal use protocol was approved by the McGill University Animal Care Committee. 129S1, DBA/2J, BALB/cJ and C.129S2-Stat4tm1Gru/J were purchased from The Jackson Laboratory. The generation of Usp18−/Usp18− mice has been described previously (21). The Usp18− allele was transferred to a FVB/J background by repeated backcrossing.
ENU mutagenesis
G0 129S1 males were mutagenized by the University of Toronto CMHD group led by Dr. Janet Rossant. The 129S1 males received 150 mg/kg of ENU intraperitonally once at 8–12 weeks of age. Infertility of the treated mice following the treatment was confirmed, as well as the recovery of fertility 8 to 12 weeks following the injection.
Genotyping
The genome scan was conducted using a 768 SNPs panel (Mutation Mapping and Developmental Analysis Project, Brigham and Women's Hospital, Harvard Medical School, Boston, USA) (22). Additional genotyping was performed by microsatellite analysis analyzed on high resolution agarose gels or by SNP sequencing. Genotyping of the L361F mutation in Usp18 was performed using custom TaqMan SNP genotyping assay (Applied Biosystems, Carlsbad CA). DNA samples were purchased from The Jackson Laboratory. Wild-derived species Mus caroli/EiJ originates from Thailand and Mus spretus/EiJ originates from Puerto Real, Cadix Province, Spain.
In Vivo Salmonella Infections
Mice were infected i.v. with 1000 CFUs of Salmonella Typhimurium isolate Keller as previously described (23). Briefly bacteria were grown in TSB to an OD of 0.09, cooled to 4°C and plated overnight on TSA. The day after, the infectious dose was adjusted to 5000 CFUs per mL and 0.2mL was injected in the caudal vein of 6–8 weeks old mice of both sexes. For CFUs determination the mice were euthanized with CO2 at the given day post-infection and the spleen and liver were aseptically removed, weighed and homogenized with a Polytron (Kinematica, Bohemia NY). The resulting homogenate was diluted in saline and plated on TSA to determine the organ bacterial load.
LPS and Poly I:C Challenge
Mice were injected i.p. with the given amount of LPS K235 (Sigma #L2143, Oakville ON) once or Poly I:C (Sigma #P0913) daily for 4 days diluted in 0.5mL of saline and monitored for 5 days. Mice were monitored twice daily and euthanized when moribund in accordance with the McGill ethic committee.
Splenocytes
Spleens were aseptically removed from uninfected or Salmonella Typhimurium infected mice and transferred in PBS. They were briefly ground between the frosted area of two microscopes slides. The cells were then dispersed by passages through 21 and 25 gauge needles to obtain a single cell suspension. This suspension was layered over LympholiteM (Cedarlane, Burlington ON) and centrifuged at room temperature, 1460 G, 20 min. The buffy coat was collected, diluted in RPMI and pelleted at 485 G for 5 minutes before treatment with RBC lysis solution (InVitrogen, Burlington ON). After a final centrifugation the cells were resuspended and their concentration adjusted to 2×106 cells/mL. The splenocytes were then either left untreated for 4h to measure IFN-γ production or stimulated overnight with 1uM of CpG DNA (Alpha DNA), 10ug/ml of Zymosan (InvivoGen # TLRL-ZYN), 10ug/ml of LPS055:B5 (Sigma #L6529), 10ug/ml of LTA from S. aureus (Sigma #L2515) or 5ug/ml of imiquimod (InvivoGen #TLRL-IMQ) to measure IL-6 using a sandwich ELISA. For protein extraction the splenocytes were left untreated for 4h and then stimulated for 60 or 90 minutes with 100 U/mL of IFN-β (Sigma #I9032). For the kinetic of IFN-γ production, explanted splenocytes were left untreated for 16h and then stimulated with IFN-β (100 U/ml) or IL-12 (10 ng/mL) for periods of time varying between 4h to 24h.
Bone Marrow Derived Macrophages (BMDM)
The femurs were collected and kept on ice in HBSS. Both ends were cut and the bone marrow extracted by flushing the femurs with 2mL of RPMI using a 26G needle. Single cell suspension was obtained by passing the media though a 27G needle. Red blood cells were lysed for 5 minutes using a RBC lysis solution. The remaining cells were centrifuged 5min at 485 G and resuspended in 10 mL of complete media (RPMI, 10% FCS, 20% L929 supernatant), left to adhere overnight in adherent Primaria petri (BD, Missisauga ON). The cell suspension was then transferred to non-adherent tissue flask and maintained in culture for 5 days with addition of L929 supernatant at day 2 and 4 prior to cell counting and plating.
Protein Extracts
Protein extracts were prepared using the CellLyticM reagent (Sigma #C2978) according to the manufacturer protocol. Proteins were quantified with the Non-Interfering protein assay kit (EMD, Gibbstown NJ).
Western Blots
Western blots were performed with the Novex system (InVitrogen) using 4–12% Bis-Tris gels according to the manufacturer protocols. The PVDF membranes were blotted sequentially (when applicable) with pSTAT4(Tyr693) (Santa Cruz Biotechnology, Santa Cruz CA), pSTAT1(Tyr701) (Cell Signaling, Danvers CA), STAT4 (Cell Signaling), STAT1 (Cell Signaling) and Actin (Sigma) or GAPDH (Cell Signaling) antibodies, the membranes being stripped with Restore buffer (Fisher, Nepean ON) in-between. The blots were revealed using Immobilon (Millipore, Billerica MA) and Hyper ECL films (GE healthcare, Missisauga ON).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 4 (GraphPad Software, La Jolla CA).
Results
A mutation in Usp18 is responsible for the susceptibility of Ity9 mutant mouse to Salmonella Typhimurium infection
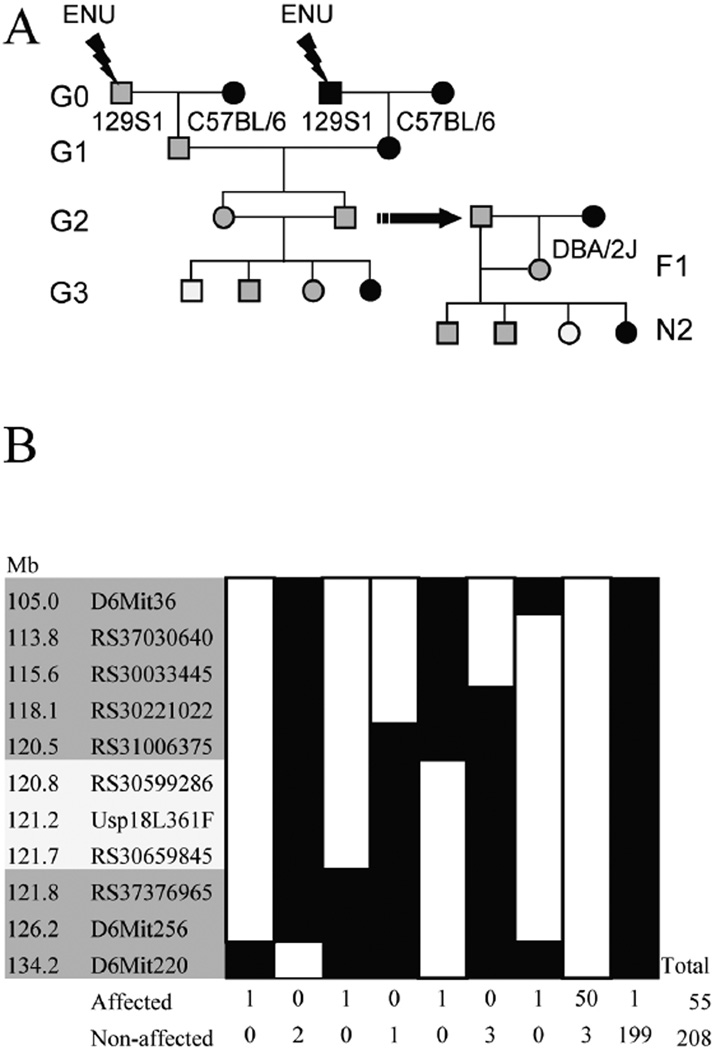
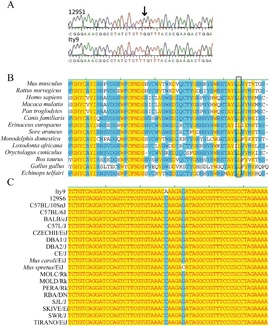
The breeding scheme used to identify the Ity9 pedigree (Figure 1a) involves generation 1 (G1) mice produced by two independently mutagenized generation 0 (G0) males. This strategy was used in order to increase the total number of mutations that could be screened in each G3 and to maintain screening of the X chromosome (10). 129S1/SvImJ (129S1) mice were selected for mutagenesis and subsequently bred with C57BL/6J females to generate the G1 progeny. For each G1 pedigree, four G2 brother-sister pairs were mated to produce G3. Using this breeding scheme, the C57BL/6J Slc11a1Asp169 Salmonella susceptibility allele was introduced into the G2 population. Animals from the G2 generation were genotyped for Slc11a1 and mice carrying the wild-type resistant allele (Slc11a1Gly169) were selected for further G3 breeding to prevent phenotypic variation caused by Slc11a1. Following phenotyping of G3 mice the G2 male carrying the susceptibility allele was outcrossed to a DBA/2J female (Slc11a1Gly169) and the N2 animals were screened to confirm the heritability of the phenotype. Initial mapping of the mutation to chromosome 6 was established using six affected mice (Supplementary Figure 1). Fine mapping with a total of 263 N2 progeny (Figure 1b) delimited the mutation to a 1.3Mb interval on chromosome 6. This region of the chromosome comprises 13 annotated genes, 2 predicted genes and 1 noncoding RNA (Ensembl build 54.37g). All the coding sequences and exon/intron boundaries in this region were sequenced and only one guanine to thymine transversion in the gene Usp18 was found (Figure 2a). This missense mutation causes a leucine to phenylalanine amino acid change at position 361 of USP18, a highly conserved amino acid through the amniotes (Figure 2b). To confirm that the SNP for this codon is exclusive to our ENU mutant strain, a broad range of classical inbred and wild-derived Mus subspecies were sequenced (Figure 2c). This region of the protein is involved in the binding of USP18 to the IFNAR2 receptor (17) suggesting that the Usp18Ity9 mutation may interfere with the activation of type 1 IFN signaling.
Figure 1. ENU recessive screen breeding strategy and mapping of the Ity9 mutant pedigree to chromosome 6.
(a) Schematic representation of the breeding scheme used to identify the mutant family by screening the G3 mice and to confirm the heritability of the phenotype using the N2 mice. Females and males are respectively represented by circles and by square while gray filling denote the presence of heterozygous mutant allele and white filling homozygous mutant alleles. (b) Fine mapping of the Ity9 locus to a 1.3Mb region on chromosome 6 (build 37.1). White boxes represent 129S1 alleles and black boxes represent heterozygous or DBA2/J alleles. Recombinants in this region were selected for progeny testing to characterize their susceptibility to Salmonella infection by survival analysis. Families demonstrating an early death phenotype (prior to day 8) were classified as affected.
Figure 2. Identification of a mutation in Usp18 underlying the Ity9 phenotype.
(a) A guanine to thymine transversion causing a leucine to a phenylalanine missense mutation at the position 361 of USP18 was identified in the mutant pedigree. (b) The alignment of USP18 orthologs showing the conservation of the mutated amino acid in a broad range of eukaryotes. Identical amino acids are highlighted in yellow while conserved amino acids are highlighted in blue (c) Alignment of the segment of the Usp18 sequence of inbred and wild derived mice compared to the Usp18Ity9/Usp18Ity9 mutated strain. The G-C transversion in Mus spretus causes a leucine to valine amino acid change. Identical nucleotides are highlighted in yellow while conserved nucleotides are highlighted in blue. The sequence alignments were done with AlignX (InVitrogen).
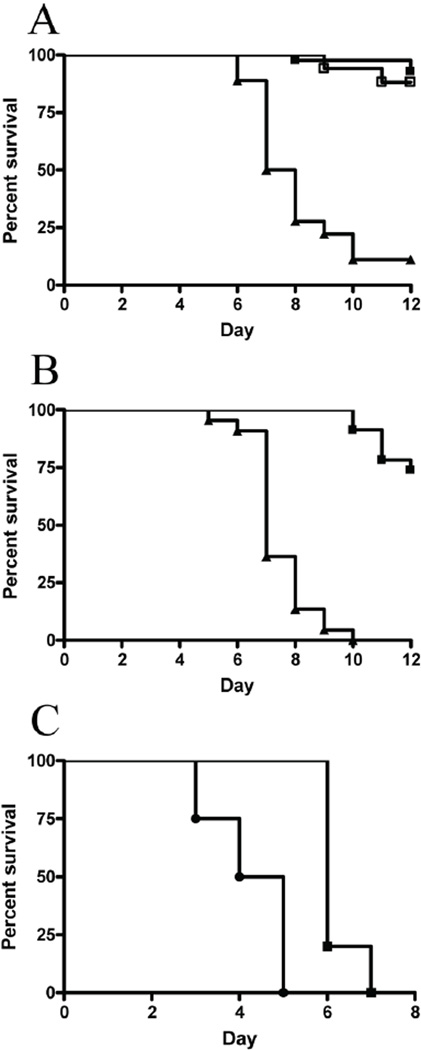
To confirm that Usp18 is indeed responsible for the Ity9 phenotype we carried out allelic complementation assays in Usp18Ity9/Usp18− compound mutants. Usp18Ity9/Usp18+ mice on a DBA/2J background were crossed to Usp18−/Usp18− mice on an FVB/J background to generate F1 progeny. Of note, in this breeding scheme, all resulting F1 animals carried one copy of the wild type Slc11a1 gene precluding the impact of Slc11a1 on the analysis the disease phenotype. Susceptibility to infection was measured by survival analysis in Usp18Ity9/Usp18− and Usp18+/Usp18− heterozygous mice (Figure 3a and 3b). Usp18Ity9/Usp18− mice present the same degree of susceptibility to Salmonella infection (Figure 3b) than Usp18Ity9/Usp18Ity9 mice (Figure 3a). The median survival time for Usp18Ity9/Usp18Ity9 does not significantly differ from the one observed in Usp18Ity9/Usp18− compound heterozygotes (7.0 days versus 7.5 days, p=.1415). These results show the lack of allelic complementation between Usp18Ity9 and Usp18− and confirm that Usp18 is indeed the gene underlying Ity9. We did also evaluate survival to infection in Usp18−/Usp18− mice and compared their degree of susceptibility to FVB/J controls (Figure 3c). Usp18−/Usp18− mice are significantly more susceptible to infection than FVB/J mice. The mean survival time (MST) is 4.3 ±1.0 in Usp18−/Usp18− and 6.2±0.5 in Usp18+/Usp18+ FVB mice (p=0.004). Both groups of mice presented shorter mean survival time compared to Usp18Ity9/Usp18Ity9 and Usp18Ity9/Usp18− because of the presence of a mutant allele at Slc11a1Asp169.
Figure 3. Usp18Ity9 allele confers Salmonella Typhimurium Susceptibility.
(a) Survival curves of N2 animals issued from affected F1 daughter produced in the original screen littermates infected with 1000 CFUs IV according to their Usp18 genotype. Usp18Ity9/Usp18Ity9 are represented by closed triangle (n=18) while Usp18Ity9/Usp18+ (n=42) and Usp18+/Usp18+ (n=17) are represented by closed and open boxes, respectively. Results are representative of 5 independent experiments. (b) Survival curves of F1 mice issued from a cross between Usp18Ity9/Usp18+ and Usp18−/Usp18− mice. Usp18Ity9/Usp18− are represented by closed triangles (n=22) while Usp18+/Usp18− are represented by closed boxes. Usp18Ity9/Usp18Ity9 and compound heterozygous Usp18Ity9/Usp18− mice are as susceptible to infection, validating the candidacy of Usp18 as the gene underlying Ity9. Results are representative of 2 independent experiments. (c) Survival curves of Usp18−/Usp18− FVB congenic mice (circles) and FVB mice (squares) confirming the susceptibility of USP18 deficient mice to Salmonella infection. Log-Rank (Mantel-Cox) P=0.004.
Usp18Ity9/Usp18Ity9 mice present increased bacterial loads in target organs that are associated with decreased IFNγ production
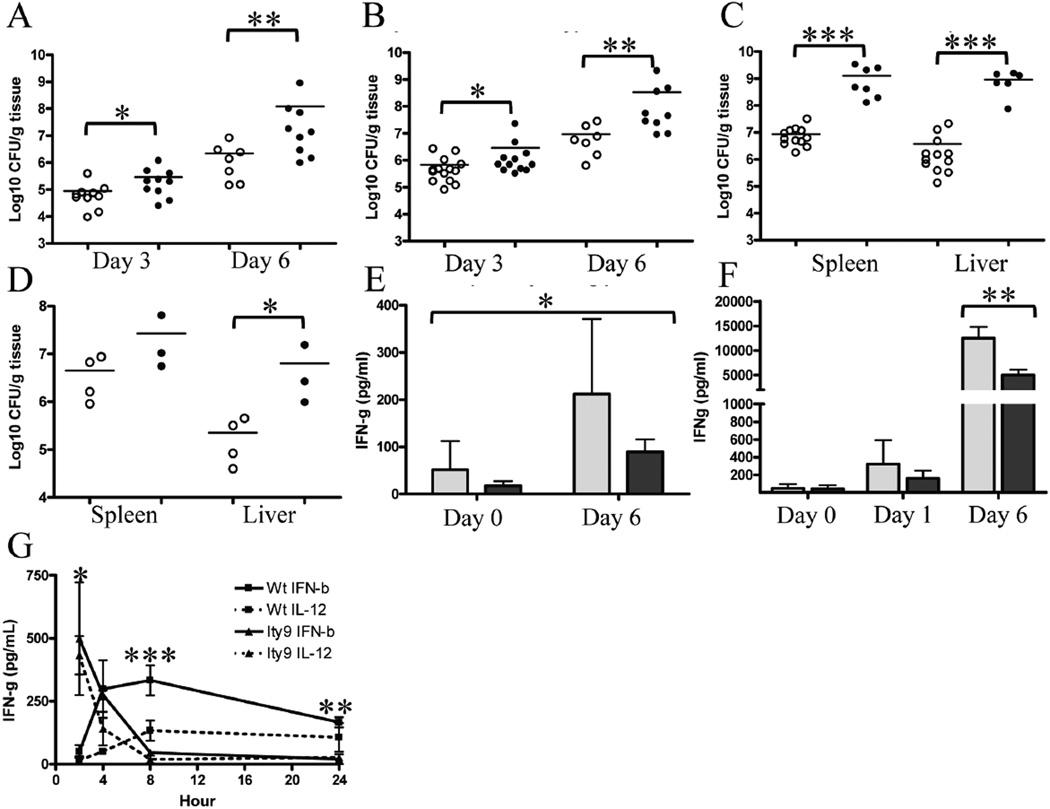
The higher susceptibility of the Usp18Ity9/Usp18Ity9 mice in terms of survival (Figure 3a) correlates with a higher bacterial burden in the spleen (Figure 4a) and liver (Figure 4b) of infected mice at 3 and 6 days post-infection. At day 6, when susceptible mice first become moribund, homozygous mutant animals have a bacterial load that is 36-fold higher in the spleen and 55-fold higher in the liver compared to their heterozygous littermates. The complementation assay in Usp18Ity9/Usp18− compound mutant confirms that the USP18L361F mutation is responsible for the Ity9 increased bacterial load in the spleen and liver of mice 6 days post-infection (Figure 4c). Higher bacterial load after Salmonella infection was also observed in the spleen and liver of Usp18−/Usp18− mice with levels that are 28 and 6 fold higher than in FVB/J controls in the liver and spleen respectively (Figure 4d). These results indicate that mice homozygous for the Ity9 mutation have markedly impaired control of Salmonella replication at two major sites during systemic infection, likely contributing to the disease phenotype.
Figure 4. Usp18Ity9/Usp18Ity9 mice have an increased bacterial load as a consequence of impaired IFN-γ production.
Spleen (a) and liver (b) CFUs 3 and 6 days post-infection with 1000 CFUs of Salmonella Typhimurium IV. Usp18Ity9/Usp18+ (open circles) and Usp18Ity9/Usp18Ity9 (closed circles). One tailed Mann-Whitney test was performed for the spleen (a) *P=0.0186 **P=0.0017 and liver (b) *P=0.0216 and **P=0.0082. (c) Spleen and liver CFUs 6 days post-infection with 1000 CFUs of Salmonella Typhimurium IV. Usp18+/Usp18− (open circles) and Usp18Ity9/Usp18− (closed circles). Two tailed T-Test P=0.0002 (spleen) and P=0.0004 (liver). Of note two Usp18Ity9/Usp18− mice were found dead at day 6 post-infection, excluding them from the CFU analysis. (d) Spleen and liver CFUs 3 days post-infection with 1000 CFUs of Salmonella Typhimurium IV. Usp18−/Usp18− (closed circles) and FVB (open circles). Mann Whitney P=0.0286 (liver). (e) IFN-γ production from explanted splenocytes from Salmonella Typhimurium infected mice incubated for 4h ex vivo in Usp18Ity9/Usp18+ (light grey) and Usp18Ity9/Usp18Ity9 (dark grey) littermates. 2 way ANOVA P=0.0211 N=6/group. (f) Serum IFN-γ concentration of uninfected mice or mice infected with Salmonella Typhimurium at day 1 and day 6 post-infection in Usp18Ity9/Usp18+ (light grey) and Usp18Ity9/Usp18Ity9 (dark grey) littermates. Two-tailed Mann Whitney D6 P=0.0087, N=6/group. (g) 24h Kinetic of IFN-γ production from explanted splenocytes stimulated with IFN-β or IL-12. 2 way ANOVA (interaction) P=0.0003 N=3/group, independent 2 way ANOVA according to the genotype for each time point * 2h P=0.0149, *** 8h P=0.0007, ** 24h P=0.0074. These results are representative of results obtained in 3 (a,b,e,f), 2 (c,g) or 1 (d) independent experiments.
A constant feature of the Usp18Ity9 mutation is seen with respect to IFN-γ production during infection with Salmonella Typhimurium (Figure 4e and 4f). We have observed and reported by the past, that in Salmonella–susceptible mice, increased production of IFNg parallels increased bacterial load (23, 24). In Usp18Ity9/Usp18Ity9 mice the situation is different, despite an increased in bacterial load in spleen and liver, circulating IFN-γ levels are 2.5 times lower in Usp18Ity9/Usp18Ity9 mice 6 days post infection compared to wild-type littermates (Figure 4f). In addition, explanted splenocytes from infected mutant mice produced less IFN-γ compared to control littermates (Figure 4e). To evaluate the regulation of IFN-γ secretion by Usp18Ity9, in vitro stimulation of splenocytes with IL-12 or IFN-β was performed (Figure 4g). In the Usp18Ity9/Usp18Ity9 splenocytes, peak IFN-γ production was observed 2h after IL-12 or IFN-β stimulation and no detectable levels of IFN-γ were present by 8h; in contrast, among controls the peak IFN-γ production was observed 4–8h post-stimulation and was sustained for up to 24h. These observations indicate that both the IL-12 and IFN-β dependent pathways leading to IFN-γ production are affected by the Ity9 mutation. Ifng transcription correlates well with protein production as Ifng mRNA was diminished in the mutant splenocytes compared to control mice infected with Salmonella Typhimurium for 6 days (Suppl. Figure 2a and Suppl. Fig 3a). Low levels of IFN-γ could explain higher bacterial growth in spleen and liver of Salmonella-infected Usp18Ity9/Usp18Ity9 mice.
Usp18Ity9/Usp18Ity9 mice present increased responsiveness to PAMPs and Salmonella-induced septic shock
To further investigate the pathogenesis of decreased resistance to infection in Usp18Ity9/Usp18Ity9 mice, we looked at the hematologic manifestations of Salmonella infection and the host inflammatory response to Pathogen-Associated Molecular Patterns (PAMPs) in vivo and ex vivo. We have shown previously that mice of different genetic backgrounds infected with Salmonella Typhimurium present a progressive anemia and a lymphopenia during infection (8, 23). During the course of Salmonella infection in Usp18Ity9/Usp18Ity9 mice, we observed a mild anemia in combination with a moderate lymphopenia and a marked thrombocytopenia. The lymphopenia was not observed in wild-type animals and the thrombocytopenia was not as severe (supplementary Table I). The administration of PolyI:C, a TLR3 agonist, in Usp18Ity9/Usp18Ity9 mice had also a dramatic impact on circulating lymphocytes and platelets reflecting an increased activation of the immune system.
Usp18Ity9/Usp18Ity9 mice showed an increased susceptibility to lethal in vivo challenge with LPS, a major PAMP of Salmonella as shown by decreased survival after LPS administration in vivo (Figure 5 a,b,c). This observation suggests an exacerbation of the inflammatory response induced by type 1 IFN in response to TLR activation by LPS or Salmonella. In fact, Salmonella-induced expression of IFN-β mRNA in Usp18Ity9/Usp18Ity9 macrophages was increased compared to wild-type littermates (Suppl. Figure 2b and Suppl. Figure 3d) supporting a role for TLR-dependent IFN-β induced shock in our model.
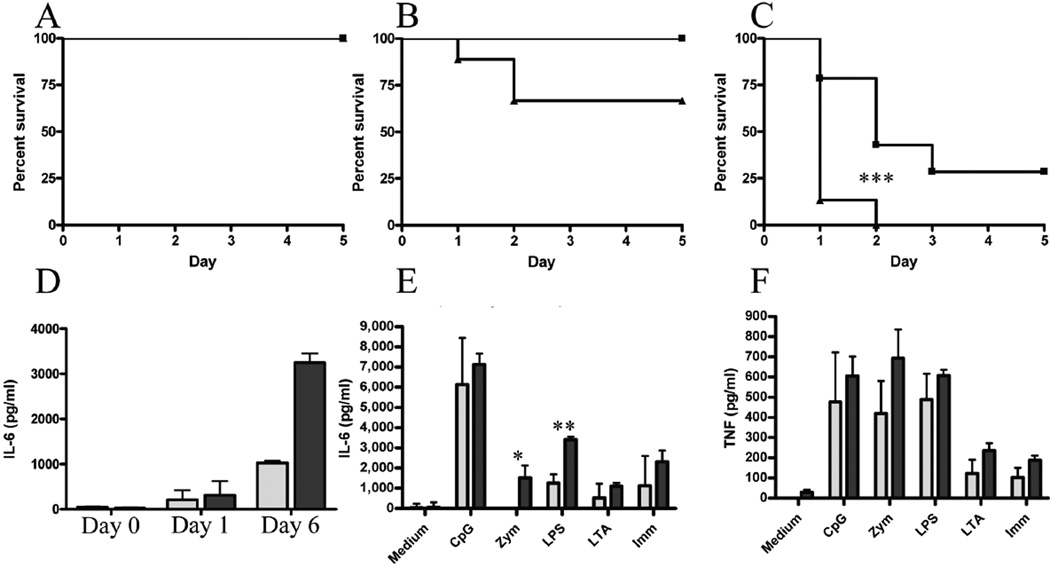
Figure 5. Usp18Ity9/Usp18Ity9 mice are more susceptible to LPS challenge.
Survival of Usp18Ity9/Usp18Ity9 (closed triangles) and control littermates Usp18Ity9/Usp18+ (closed boxes) challenged with 10 µg (a), 100 µg (b) or 500 µg of LPS IV. Survival curves comparison for the 500 µg challenge P=0.0002 (***). (d) Serum IL-6 concentration in Usp18Ity9/Usp18+ (light grey) and Usp18Ity9/Usp18Ity9 (dark grey) mice infected with Salmonella Typhimurium at day 0, day 1 and day 6 post-infection. 2 way ANOVA P<0.0001 N=3/group. IL-6 (e) and TNF (f) production of explanted splenocytes from Usp18Ity9/Usp18+ (light grey) and Usp18Ity9/Usp18Ity9 (dark grey) littermates stimulated overnight with an array of PAMPs. 2 way ANOVA (e) P=0.0023 (f) P=0.0084 N=3/group. 2 tailed T-Test were also performed for each treatment indendently (e) P=0.05 (*) P=0.002 (**). These results are representative of results obtained in 3 (a–d) or 2 (e,f) independent experiments.
Early during Salmonella infection, different immune cell types including macrophages, neutrophils, and lymphocytes induce cytokines (TNF and IL-6 for example) that mediate inflammation and are involved in the pathogenesis of septic shock (5). A cardinal feature of Salmonella infection in Usp18Ity9/Usp18Ity9 mice is the presence of high IL-6 levels in circulation and in infected tissues and cells. This is observed in vivo in the serum of Usp18Ity9/Usp18Ity9 mice following Salmonella Typhimurium infection (Figure 5d). Compared to control cells, Usp18Ity9/Usp18Ity9 splenocytes also secreted a significantly higher level of IL-6 following in vitro stimulation with LPS and zymosan (Figure 5e), although no statistically significant differences in the production of TNF were detected (Figure 5f). High levels of IL-6 production together with high expression of Ifnb and additional interferon-regulated genes (Cxcl10, Irf7), in splenocytes (Suppl. Figure 2a and Suppl. Figure 3b) and Il15, Isg15, and Mx1 in BMDM (Suppl. Figure 2b and Suppl. Figure 3c) during Salmonella infection most likely contribute to the development of septic shock in the Usp18Ity9/Usp18Ity9 mice during infection. The heightened response to LPS can synergize with the increased bacterial load to lower the survival of the Usp18Ity9/Usp18Ity9 mice during Salmonella infection.
STAT1 induction and STAT4 repression by type 1 IFN in response to Salmonella infection in Usp18Ity9/Usp18Ity9 mice
With the aim of dissecting the signaling modifications caused by the Usp18Ity9 mutation that could explain the opposite impact of the mutation on IFN-γ and IL-6 production, the expression and phosphorylation of STAT1 and STAT4 were evaluated in BMDM (Figure 6a) and splenocytes (Figure 6b and 6c). STAT1 is known to be phosphorylated after type 1 IFN binding to the IFNAR receptor and its phosphorylation is increased in Usp18−/Usp18− mice (25). In both cell types, basal STAT1 and phosphorylated STAT1 (pSTAT1) levels were higher in the Usp18Ity9/Usp18Ity9 mice compared to the control littermates (Figure 6). STAT1 was highly expressed in splenocytes during Salmonella Infection (Figure 6b) and in BMDM 24h after stimulation with IFN-β (data not shown). Hyperactivation of the JAK/STAT pathway in the Usp18Ity9/Usp18Ity9 mice is consistent with high IL-6 production and the higher susceptibility of the mice to cytokine-mediated septic shock.
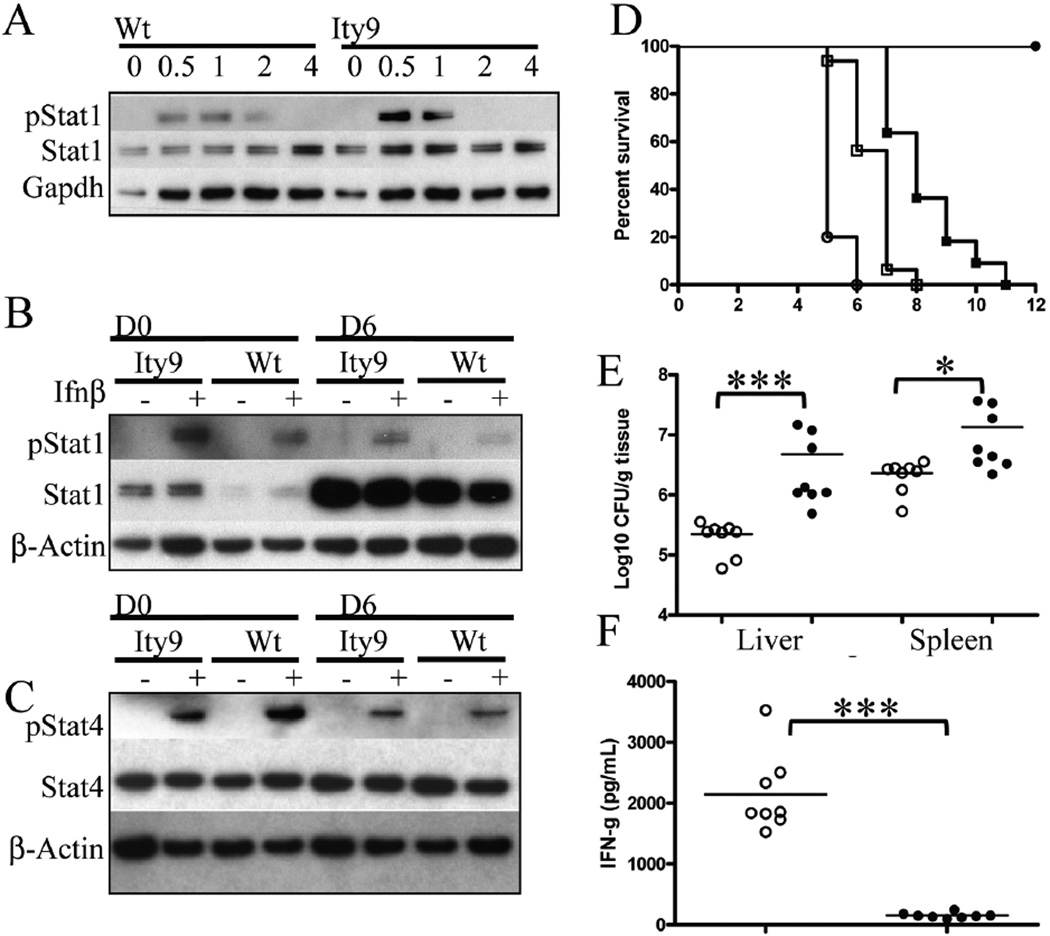
Figure 6. Critical role of Stat4 in the host response to Salmonella Typhimurium infection in vivo.
(a,b) Western blot of protein extracts from (a) BMDM stimulated with 100 U/mL of IFN-β for 0.5, 1, 2 and 4 h probed with anti-phosphoSTAT1 (pSTAT1), anti-STAT1 and anti-GAPDH. Explanted splenocytes from mice uninfected (D0) or infected (D6) with a 1000 CFUs of Salmonella Typhimurium IV for 6 days and either left unstimulated or stimulated with 100U/mL of Ifn-β probed with (b) anti-pSTAT1, anti-STAT1, anti-β-ACTIN and (c) anti-phosphoSTAT4 (pSTAT4), STAT4 and anti-β-ACTIN. These results are representative of results obtained with 6 independent mice. (d) Survival curves of Balb/cJ (empty circles), Stat4tm1Gru/Stat4tm1Gru (empty squares), Stat4tm1Gru/Stat4wt (filled squares) and 129S1 (filled circles) mice infected with 1000 CFUs IV of Salmonella. Log-Rank (Mantel-Cox) for Balb/cJ and Stat4tm1Gru/Stat4tm1Gru P=0.0006, Stat4tm1Gru/Stat4tm1Gru and Stat4tm1Gru/Stat4wt P=0.0004, Stat4tm1Gru/Stat4wt and 129S1 P=0.0004. MSTs are 5.2±0.4 days for BALB/cJ, 6.6±0.7 days for Stat4tm1Gru/ Stat4tm1Gru and 8.2±1.2 days for Stat4tm1Gru/Stat4wt. (e) Bacterial burden in the liver and spleen of Stat4tm1Gru/Stat4tm1Gru (filled circles) and Stat4tm1Gru/Stat4wt (empty circles) mice infected with 1000 CFUs IV of Salmonella 4 days post-infection. Mann-Whitney Two-tailed *** P=0.0002 and * P=0.0262. (f) Serum IFN-γ concentration of Stat4tm1Gru/Stat4tm1Gru (filled circles) and Stat4tm1Gru/Stat4wt (empty circles) mice infected with Salmonella Typhimurium at day 4 post-infection. *** Unpaired t test Two=−tailed with Welch's correction P<0.0001.
STAT4 is important for IFN-γ induction and type 1 IFNs have been reported to activate STAT4. During viral infection a high STAT1 level negatively correlates with type 1 IFN induced phosphorylation of STAT4 and the resulting production of IFN-γ (26). In our model, basal STAT4 levels were not different in the splenocytes of Usp18Ity9/Usp18Ity9 mice and control littermates. During infection, pSTAT4 levels decreased at D6 in both the mutant and control mice, however, lower levels of pSTAT4 were consistently observed in Usp18Ity9/Usp18Ity9 mice when the day 0 (D0) splenocytes were stimulated with IFN-β (Figure 6c). The ratios of pStat4/Stat4 after quantification of the bands and adjustment for loading were significantly lower in Usp18Ity9/Usp18Ity9 compared to littermate control by a factor of 26% both prior infection and at day 6 post infection. These observations are consistent with the competition between STAT1 and STAT4 at the IFNAR level for phosphorylation. Since STAT4 is central to IFN-γ production both through the IL-12 and IFN-β pathways, lower pSTAT4 levels contributes to the decreased IFN-γ production observed in the Usp18Ity9/Usp18Ity9 mice.
To further demonstrate the importance of STAT4 during infection, we did challenge Stat4 knockout mice (http://jaxmice.jax.org/strain/002826.htm) with Salmonella Typhimurium (Figure 6d). The Stat4 gene is located on chromosome 1 approximately 20 mb proximal to Slc11a1. Stat4tm1Gru mice were transferred onto a BALB/cJ background, a strain that normally carry a mutant Slc11a1 allele, however during the creation of the congenic strain, the 129S2 wild-type allele of Slc11a1 was transferred with the Stat4 null allele (data not shown). To create appropriate controls for this particular experiment, we crossed C.129S2-Stat4tm1Gru/J to BALB/cJ mice to create F1 progeny that were backcrossed to C.129S2-Stat4tm1Gru/J. All progeny issued from the backcross carried at least one copy of the wild-type allele at Slc11a1 and were either Stat4−/− or Stat4wt/−. We showed that mice deficient for Stat4 are significantly more susceptible to infection than mice carrying one (Stat4wt/−) (P=0.0004) or two (129S1) (P<0.0001) wild type allele at Stat4. These results confirm the importance of STAT4 during acute systemic model of Salmonella Typhimurium infection.
Discussion
The past few years have validated the use of chemical mutagenesis in the mouse as a powerful functional genomics tool to produce genetic variants for immunologic/host defense functions and to study diverse physiologic and pathologic conditions (27, 28). This strategy is particularly attractive since it does not require assumptions about the nature of the immunological defect and approaches more closely the genetic analysis of natural variation in populations and the possibility to translate the research findings into improved understanding of the molecular basis of susceptibility to infectious diseases in humans. Here we present the identification of a novel Salmonella-susceptibility gene using a direct primary recessive screen with an important bacterial human pathogen.
We report in the current paper the identification of the molecular basis of Ity9 as being the ubiquitin-specific peptidase USP18. The evidence leading to this conclusion are the following: the mutation identified in USP18 (L361F) was the sole coding change within the critical genomic interval; L361 is highly conserved in all mouse strains tested and other animal species; and finally complementation assays in vivo using Usp18Ity9/Usp18− compound mutants have shown unambiguously that Usp18 is indeed the gene underlying the locus Ity9. USP18, also known as UBP43, belongs to the deubiquinating protease family. USP18 is expressed in the thymus and macrophages of adult mice, as well as in hematopoietic cell lines, and its expression can be modulated by IFN and LPS. The protein has a de-ISGylation activity specific for the ubiquitin-like protein ISG15 and is involved in the negative regulation of type 1 IFN signaling by interacting with the IFNRA2 receptor and limiting JAK-STAT1 activation. Characterization of USP18 confirmed a critical role for this protease in the down-regulation of the JAK-STAT pathway by preventing the interaction between JAK1 and the IFNAR2 (17). Some genes involved in the JAK-STAT pathway (Tyk2, Jak3, STAT1) have been shown in mouse models of infection and in rare human immunodeficiencies to play a critical role in the host response to bacterial, viral and parasitic infections (4).
Overall, the data presented here is consistent with the fact that the mutation identified in Usp18 interferes with the type I IFN signaling pathway, resulting in increased inflammatory response triggered by STAT1 hyperactivation that is responsible for the susceptibility of Usp18Ity9/Usp18Ity9 mice to LPS-induced septic shock. Hyperactivation of STAT1 has also an impact on type 1 IFN-mediated STAT4 phosphorylation for IFN-γ production in mutant mice resulting in increased bacterial load in target organs and increased susceptibility to Salmonella infection. We did not observe any functional difference in phagocytic capabilities, bacterial killing or pyropoptosis of bone marrow derived macrophages from Usp18Ity9/Usp18Ity9 mice (data not shown), excluding a direct effect of the mutation on macrophage function and highlighting the importance of analyzing the impacts of this mutation at the whole organism level.
Type 1 IFNs are traditionally viewed and have been best described as antiviral cytokines and have been shown to play a major role in LPS-induced shock as revealed by the observation that mice deficient in the production of type 1 IFN as a consequence of a disruption of either TYK2, a JAK protein tyrosine kinase family member engaged at the IFNAR1 receptor, or the adaptor protein TRIF are resistant to the toxic effect of LPS (29, 30). IFN-β production by bacterial stimulation can occur through at least 2 recognition pathways, that are either TLR-dependent or TLR-independent (reviewed in (31)). TLR3, 4, 7, and 9 are involved in the production of type 1 IFN through ligand recognition (dsRNA for TLR3, LPS for TLR4, ssRNA for TLR7 and unmethylated CpG-DNA for TLR9) and recruitment of the adaptor proteins MyD88 (TLR4, TLR7, TLR9) and/or Trif (TLR4, TLR3). In the case of Salmonella recognition, TLR4 stimulation is the most likely candidate pathway leading to the induction of IFN-β. Secreted IFN-β then signals through the IFNAR receptor and stimulates the ISGF3-mediated production of several IFN inducible genes, a pathway that is hyperactivated in Usp18Ity9/Usp18Ity9 mice. As a consequence, high IL-6 levels detected in the circulation and in the infected tissues of Usp18Ity9/Usp18Ity9 mice most likely contributes to the development of septic shock in these mice (32), a cellular response that may be amplified by increased bacterial load in spleen and liver.
IFN-γ, also known as type 2 IFN, is a key cytokine modulating host defenses during Salmonella infections. Both the injection of IFN-γ (33) or antibodies against IFN-γ (34) prior to infection affects the outcome of the infection respectively by enhancing or reducing resistance of the mice to the bacterial challenge in terms of bacterial replication. During Salmonella infection, NK cells are important IFN-γ producers and their depletion impaired survival to infection as a consequence of increased bacterial load in spleen and liver (35, 36) and impaired survival to infection (37). IFN-γ is also a key cytokine in human Salmonella infections as defects in the IL-12/IFN-γ pathway increase susceptibility toward Salmonella and mycobacterial intracellular pathogens (1, 2). The IFN-γ production from cultured splenocytes stimulated with heat killed Salmonella was later shown to be at least partly dependent on IFN-β-induced STAT4 phosphorylation (38). In fact, the ratio of STAT4 and STAT1 is crucial for IFN-γ production by splenic NK cells during viral (LCMV) infection (26). STAT4 was shown to be highly expressed in resting NK cells, which allows them to produce IFN-γ early during infection. Later during the course of LCMV infection, STAT1 expression is increased in NK cells and STAT4 phosphorylation is inhibited (26). In our model, an analogous increase in STAT1 expression is seen in the splenocytes during the course of infection that may account for the reduced levels of STAT4 phosphorylation, and subsequent IFN-γ deficiency leading to the observed increase in bacterial burden. More importantly, we have shown for the first time using Stat4-deficient mice that STAT4 is an important player in the host response to infection with Salmonella Typhimurium.
Usp18Ity9/Usp18Ity9 mice share several phenotypic similarities with Usp18-deficient mice. Usp18−/Usp18− and Usp18Ity9/Usp18Ity9 mice are more sensitive to LPS-induced shock in vivo as measured by percentage of mortality (current study and (39). In the current model of infection, Usp18Ity9/Usp18Ity9 and Usp18−/Usp18− showed increased susceptibility to infection with Salmonella Typhimurium in terms of decreased survival and increased bacterial load in spleen and liver. These observations are different from a previous report where Usp18−/Usp18− mice were shown to exhibit lower bacterial load in spleen and liver compared to wild-type mice after Salmonella infection although the survival curves between the two groups of mice were not different (39).
The fact that USP18 has been associated in different studies with either decreased or increased susceptibility to Salmonella infection reflects the importance of the genetic background of the mice, the route and the dose of infection in the development of clinical disease. In previous studies, Usp18− allele was transferred to a mixed C57BL6/129S6 background, where the mutation in the gene Slc11a1 (Asp169), a major determinant of Salmonella susceptibility segregated. It was shown previously by us (8, 40, 41) and others (42) that the allele present at Slc11a1 (Gly169 versus Asp169) is of most importance during the evaluation of Salmonella susceptibility in mutant mice. In addition, the model of infection was using the i.p. route (IV in the current study) with a small inoculum (40 CFUs versus 1000 CFUs). During mild infection, it is conceivable that enhanced Type I IFN signaling in the absence of Usp18 could be transiently beneficial with respect to bacterial growth. In addition, the impact of the route of infection during Salmonella infection has been previously shown to affect disease outcome by mobilizing specific inflammatory response (43, 44).
Collectively, the current results indicate that in our model, mutations within Usp18 causes susceptibility to Salmonella infection. Susceptibility of Usp18Ity9/Usp18Ity9 mice to infection was also observed using an oral infection model (Richer and Malo, unpublished), confirming the detrimental role of USP18 dysfunction during Salmonella infection. The comparable profiles of survival and Salmonella replication in Usp18Ity9/Usp18Ity9, Usp18−/Usp18− and compound heterozygous (Usp18Ity9/Usp18−) suggest strongly that Usp18F361 allele at Ity9 is a null allele, identifying a new residue essential for the function of the protein.
In conclusion, these studies establish a critical role of USP18 in host resistance to infection with Salmonella and illustrate the complex interplay between Type 1 and Type 2 IFN in antimicrobial defense mechanisms, providing better insight into the mechanisms involved in Type 1 IFN signaling during Salmonella infection. In humans, only a few genetic factors known to influence the predisposition to pediatric primary infection with Salmonella have been identified and involve polymorphisms within the IL-12/-23/IFN-γ-mediated axis (reviewed in (3)). The patients were defective either in IFN-γ production (as observed in our model) or in responding to IFN-γ, due to mutations in the genes encoding for IFNGR1, IFNGR2, IL12B, IL12RB1 or STAT1 (2, 45–47). Here we validate the use of ENU mutagenesis to identify novel important genes in host susceptibility to Salmonella infection. These discoveries will be of utmost importance to elucidate novel disease mechanisms, in particular the involvement of Type 1 IFN signaling and USP18, in human studies.
Supplementary Material
Acknowledgements
This project was initiated with the help of Janet Rossant whom we wish to thanks for providing the G0 mice. We are grateful for the technical assistance of Nadia Prud.’homme, Danica Albert, Line Larivière, Isabelle Angers and Rosalie Wilkinson.
This work was supported by National Institutes of Health Grant HL091549 (D.E.Z.), the New Emerging Team program of the Canadian Institutes of Health Research (S.M.V., S.T.Q. and D.M.) and the Research Institute of the McGill University Health Centre (S.M.V., S.T.Q. and D.M.). ER was a recipient of a Fonds de la recherche en santé du Québec fellowship. SV and SQ hold Canada Research Chairs. DM is a McGill Dawson Scholar.
Abbreviations used in this paper
- BMDM
bone marrow-derived macrophages
- ENU
N-ethyl-N-nitrosourea
- IFNAR2
IFN (alpha and beta) receptor 2
- IFNGR
interferon gamma receptor
- IL12RB1
interleukin 12 receptor, beta 1
- Irf7
interferon regulatory factor 7
- Isg15
ISG15 ubiquitin-like modifier
- Ity9
Immunity to Typhimurium, locus 9
- Mx1
myxovirus (influenza virus) resistance 1
- PAMP
Pathogen-Associated Molecular Patterns
- Pklr
pyruvate kinase liver and red blood cell
- Slc11a1
solute carrier family 11 (proton-coupled divalent metal ion transporters), member 1
- Trif
toll-like receptor adaptor molecule 1
- Tyk2
tyrosine kinase 2
- Usp18
ubiquitin specific peptidase 18
References
- 1.de Jong R, Altare F, Haagen IA, Elferink DG, Boer T, van Breda Vriesman PJ, Kabel PJ, Draaisma JM, van Dissel JT, Kroon FP, Casanova JL, Ottenhoff TH. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science (New York, N.Y. 1998;280:1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 2.Jouanguy E, Doffinger R, Dupuis S, Pallier A, Altare F, Casanova JL. IL-12 and IFN-gamma in host defense against mycobacteria and salmonella in mice and men. Current opinion in immunology. 1999;11:346–351. doi: 10.1016/s0952-7915(99)80055-7. [DOI] [PubMed] [Google Scholar]
- 3.Bustamante J, Boisson-Dupuis S, Jouanguy E, Picard C, Puel A, Abel L, Casanova JL. Novel primary immunodeficiencies revealed by the investigation of paediatric infectious diseases. Current opinion in immunology. 2008;20:39–48. doi: 10.1016/j.coi.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Vidal SM, Malo D, Marquis JF, Gros P. Forward Genetic Dissection of Immunity to Infection in the Mouse. Annu Rev Immunol. 2007 doi: 10.1146/annurev.immunol.26.021607.090304. [DOI] [PubMed] [Google Scholar]
- 5.Maskell D. Salmonella infections. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 6.Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) The Journal of experimental medicine. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vidal S, Tremblay ML, Govoni G, Gauthier S, Sebastiani G, Malo D, Skamene E, Olivier M, Jothy S, Gros P. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. The Journal of experimental medicine. 1995;182:655–666. doi: 10.1084/jem.182.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy MF, Riendeau N, Bedard C, Helie P, Min-Oo G, Turcotte K, Gros P, Canonne-Hergaux F, Malo D. Pyruvate kinase deficiency confers susceptibility to Salmonella typhimurium infection in mice. The Journal of experimental medicine. 2007;204:2949–2961. doi: 10.1084/jem.20062606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sancho-Shimizu V, Malo D. Sequencing, expression, and functional analyses support the candidacy of Ncf2 in susceptibility to Salmonella typhimurium infection in wild-derived mice. J Immunol. 2006;176:6954–6961. doi: 10.4049/jimmunol.176.11.6954. [DOI] [PubMed] [Google Scholar]
- 10.Richer E, Qureshi ST, Vidal SM, Malo D. Chemical mutagenesis: a new strategy against the global threat of infectious diseases. Mamm Genome. 2008;19:309–317. doi: 10.1007/s00335-008-9114-0. [DOI] [PubMed] [Google Scholar]
- 11.Tabeta K, Hoebe K, Janssen EM, Du X, Georgel P, Crozat K, Mudd S, Mann N, Sovath S, Goode J, Shamel L, Herskovits AA, Portnoy DA, Cooke M, Tarantino LM, Wiltshire T, Steinberg BE, Grinstein S, Beutler B. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nature immunology. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 12.Croker B, Crozat K, Berger M, Xia Y, Sovath S, Schaffer L, Eleftherianos I, Imler JL, Beutler B. ATP-sensitive potassium channels mediate survival during infection in mammals and insects. Nature genetics. 2007;39:1453–1460. doi: 10.1038/ng.2007.25. [DOI] [PubMed] [Google Scholar]
- 13.Crozat K, Hoebe K, Ugolini S, Hong NA, Janssen E, Rutschmann S, Mudd S, Sovath S, Vivier E, Beutler B. Jinx, an MCMV susceptibility phenotype caused by disruption of Unc13d: a mouse model of type 3 familial hemophagocytic lymphohistiocytosis. The Journal of experimental medicine. 2007;204:853–863. doi: 10.1084/jem.20062447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casrouge A, Zhang SY, Eidenschenk C, Jouanguy E, Puel A, Yang K, Alcais A, Picard C, Mahfoufi N, Nicolas N, Lorenzo L, Plancoulaine S, Senechal B, Geissmann F, Tabeta K, Hoebe K, Du X, Miller RL, Heron B, Mignot C, de Villemeur TB, Lebon P, Dulac O, Rozenberg F, Beutler B, Tardieu M, Abel L, Casanova JL. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science (New York, N.Y. 2006;314:308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- 15.Malakhov MP, Malakhova OA, Kim KI, Ritchie KJ, Zhang DE. UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J. Biol. Chem. 2002;277:9976–9981. doi: 10.1074/jbc.M109078200. [DOI] [PubMed] [Google Scholar]
- 16.Kim KI, Yan M, Malakhova O, Luo JK, Shen MF, Zou WG, de la Torre JC, Zhang DE. Ube1L and protein ISGylation are not essential for alpha/beta interferon signaling. Molecular and cellular biology. 2006;26:472–479. doi: 10.1128/MCB.26.2.472-479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malakhova OA, Kim KI, Luo JK, Zou W, Kumar KG, Fuchs SY, Shuai K, Zhang DE. UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. The EMBO journal. 2006;25:2358–2367. doi: 10.1038/sj.emboj.7601149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JH, Luo JK, Zhang DE. The Level of Hepatitis B Virus Replication Is Not Affected by Protein ISG15 Modification but Is Reduced by Inhibition of UBP43 (USP18) Expression. J. Immunol. 2008;181:6467–6472. doi: 10.4049/jimmunol.181.9.6467. [DOI] [PubMed] [Google Scholar]
- 19.Randall G, Chen L, Panis M, Fischer AK, Lindenbach BD, Sun J, Heathcote J, Rice CM, Edwards AM, McGilvray ID. Silencing of USP18 potentiates the antiviral activity of interferon against hepatitis C virus infection. Gastroenterology. 2006;131:1584–1591. doi: 10.1053/j.gastro.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 20.Ritchie KJ, Hahn CS, Kim KI, Yan M, Rosario D, Li L, de la Torre JC, Zhang DE. Role of ISG15 protease UBP43 (USP18) in innate immunity to viral infection. Nat. Med. 2004;10:1374–1378. doi: 10.1038/nm1133. [DOI] [PubMed] [Google Scholar]
- 21.Ritchie KJ, Malakhov MP, Hetherington CJ, Zhou L, Little MT, Malakhova OA, Sipe JC, Orkin SH, Zhang DE. Dysregulation of protein modification by ISG15 results in brain cell injury. Genes & development. 2002;16:2207–2212. doi: 10.1101/gad.1010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moran JL, Bolton AD, Tran PV, Brown A, Dwyer ND, Manning DK, Bjork BC, Li C, Montgomery K, Siepka SM, Vitaterna MH, Takahashi JS, Wiltshire T, Kwiatkowski DJ, Kucherlapati R, Beier DR. Utilization of a whole genome SNP panel for efficient genetic mapping in the mouse. Genome research. 2006;16:436–440. doi: 10.1101/gr.4563306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy MF, Riendeau N, Loredo-Osti JC, Malo D. Complexity in the host response to Salmonella Typhimurium infection in AcB and BcA recombinant congenic strains. Genes and immunity. 2006;7:655–666. doi: 10.1038/sj.gene.6364344. [DOI] [PubMed] [Google Scholar]
- 24.Sebastiani G, Krzywkowski P, Dudal S, Yu M, Paquette J, Malo D, Gervais F, Tremblay P. Mapping genetic modulators of amyloid plaque deposition in TgCRND8 transgenic mice. Human molecular genetics. 2006;15:2313–2323. doi: 10.1093/hmg/ddl157. [DOI] [PubMed] [Google Scholar]
- 25.Malakhova OA, Yan M, Malakhov MP, Yuan Y, Ritchie KJ, Kim KI, Peterson LF, Shuai K, Zhang DE. Protein ISGylation modulates the JAK-STAT signaling pathway. Genes & development. 2003;17:455–460. doi: 10.1101/gad.1056303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyagi T, Gil MP, Wang X, Louten J, Chu WM, Biron CA. High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. The Journal of experimental medicine. 2007;204:2383–2396. doi: 10.1084/jem.20070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- 28.Cook MC, Vinuesa CG, Goodnow CC. ENU-mutagenesis: insight into immune function and pathology. Current opinion in immunology. 2006;18:627–633. doi: 10.1016/j.coi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Karaghiosoff M, Steinborn R, Kovarik P, Kriegshauser G, Baccarini M, Donabauer B, Reichart U, Kolbe T, Bogdan C, Leanderson T, Levy D, Decker T, Muller M. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nature immunology. 2003;4:471–477. doi: 10.1038/ni910. [DOI] [PubMed] [Google Scholar]
- 30.Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 31.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 32.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumura H, Onozuka K, Terada Y, Nakano Y, Nakano M. Effect of murine recombinant interferon-gamma in the protection of mice against Salmonella. International journal of immunopharmacology. 1990;12:49–56. doi: 10.1016/0192-0561(90)90067-w. [DOI] [PubMed] [Google Scholar]
- 34.Nauciel C, Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992;60:450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griggs ND, Smith RA. Adoptive transfer of natural killer cell activity in B6D2F1 mice challenged with Salmonella typhimurium. Cellular immunology. 1991;135:88–94. doi: 10.1016/0008-8749(91)90256-b. [DOI] [PubMed] [Google Scholar]
- 36.Schwacha MG, Meissler JJ, Jr, Eisenstein TK. Salmonella typhimurium infection in mice induces nitric oxide-mediated immunosuppression through a natural killer cell-dependent pathway. Infect Immun. 1998;66:5862–5866. doi: 10.1128/iai.66.12.5862-5866.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schafer R, Eisenstein TK. Natural killer cells mediate protection induced by a Salmonella aroA mutant. Infect Immun. 1992;60:791–797. doi: 10.1128/iai.60.3.791-797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freudenberg MA, Merlin T, Kalis C, Chvatchko Y, Stubig H, Galanos C. Cutting edge: a murine, IL-12-independent pathway of IFN-gamma induction by gram-negative bacteria based on STAT4 activation by Type I IFN and IL-18 signaling. J Immunol. 2002;169:1665–1668. doi: 10.4049/jimmunol.169.4.1665. [DOI] [PubMed] [Google Scholar]
- 39.Kim KI, Malakhova OA, Hoebe K, Yan M, Beutler B, Zhang DE. Enhanced antibacterial potential in UBP43-deficient mice against Salmonella typhimurium infection by up-regulating type I IFN signaling. J Immunol. 2005;175:847–854. doi: 10.4049/jimmunol.175.2.847. [DOI] [PubMed] [Google Scholar]
- 40.Bihl F, Salez L, Beaubier M, Torres D, Lariviere L, Laroche L, Benedetto A, Martel D, Lapointe JM, Ryffel B, Malo D. Overexpression of Toll-like receptor 4 amplifies the host response to lipopolysaccharide and provides a survival advantage in transgenic mice. J Immunol. 2003;170:6141–6150. doi: 10.4049/jimmunol.170.12.6141. [DOI] [PubMed] [Google Scholar]
- 41.Turcotte K, Gauthier S, Malo D, Tam M, Stevenson MM, Gros P. Icsbp1/IRF-8 is required for innate and adaptive immune responses against intracellular pathogens. J Immunol. 2007;179:2467–2476. doi: 10.4049/jimmunol.179.4.2467. [DOI] [PubMed] [Google Scholar]
- 42.Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, Coyle AJ, Flavell RA, Galan JE. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. The Journal of experimental medicine. 2006;203:1407–1412. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monack DM, Hersh D, Ghori N, Bouley D, Zychlinsky A, Falkow S. Salmonella exploits caspase-1 to colonize Peyer's patches in a murine typhoid model. The Journal of experimental medicine. 2000;192:249–258. doi: 10.1084/jem.192.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature. 2003;422:522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 45.Dorman SE, Holland SM. Interferon-gamma and interleukin-12 pathway defects and human disease. Cytokine & growth factor reviews. 2000;11:321–333. doi: 10.1016/s1359-6101(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 46.Jouanguy E, Lamhamedi-Cherradi S, Altare F, Fondaneche MC, Tuerlinckx D, Blanche S, Emile JF, Gaillard JL, Schreiber R, Levin M, Fischer A, Hivroz C, Casanova JL. Partial interferon-gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guerin infection and a sibling with clinical tuberculosis. The Journal of clinical investigation. 1997;100:2658–2664. doi: 10.1172/JCI119810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jouanguy E, Lamhamedi-Cherradi S, Lammas D, Dorman SE, Fondaneche MC, Dupuis S, Doffinger R, Altare F, Girdlestone J, Emile JF, Ducoulombier H, Edgar D, Clarke J, Oxelius VA, Brai M, Novelli V, Heyne K, Fischer A, Holland SM, Kumararatne DS, Schreiber RD, Casanova JL. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nature genetics. 1999;21:370–378. doi: 10.1038/7701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.