Summary
Amphibians have a remarkable capacity for limb regeneration. Following a severe injury, there is complete regeneration with restoration of the patterning and cellular architecture of the amputated limb. While studies have focused on the structural anatomical changes during amphibian limb regeneration, the signaling mechanisms that govern cellular dedifferentiation and blastemal progenitors are unknown. Here, we demonstrate the temporal and spatial requirement for hedgehog (Hh) signaling and its hierarchical correlation with respect to Wnt signaling during newt limb regeneration. While the dedifferentiation process of mature lineages does not depend on Hh signaling, the proliferation and the migration of the dedifferentiated cells are dependent on Hh signaling. Temporally controlled chemical inactivation of the Hh pathway indicates that Hh-mediated antero-posterior (AP) specification occurs early during limb regeneration and that Hh is subsequently required for expansion of the blastemal progenitors. Inhibition of Hh signaling results in G0/G1 arrest with a concomitant reduction in S-phase and G2/M population in myogenic progenitors. Furthermore, Hh inhibition leads to reduced Pax7-positive cells and fewer regenerating fibers relative to control tissue. We demonstrate that activation of Wnt signaling rescues the inhibition of Hh pathway mainly by enhancing proliferative signals; possibly mediated through TCF4 activity. Collectively, our results demonstrate coordinated signaling of Hh and Wnt activities in regulating blastemal progenitors and their hierarchical positioning during limb regeneration.
Keywords: regeneration, blastema, signaling pathways, dedifferentiation
Introduction
In contrast to mammals, lower vertebrates such as amphibians and teleost fish have a remarkable capacity to regenerate the entire amputated appendage (Brockers and Kumar, 2002; Poss et al., 2003; Wallace, 1981). In these animals, regeneration progresses through distinct but molecularly and cellularly interlinked stages beginning with wound healing, followed by blastema formation, patterning and differentiation (Akimenko et al., 2003; Gardiner et al., 2002; Stoick-Cooper et al., 2007). Specifically, wound healing and blastema formation are critical stages for complete regeneration (Campbell and Crews, 2008; Globus et al., 1980). Animals lacking blastema are incapable of complete regeneration, indicating the importance of blastema formation during regeneration (Whitehead et al., 2005). Histologically, the blastema is a heterogeneous group of cells that gives rise to the mesodermal tissues, such as cartilage and muscle (Kragl et al., 2009). Currently, the cues and signaling pathways that coordinate the growth and patterning of the different tissue types during regeneration are unknown.
Previous studies have demonstrated that the blastema forms from dedifferentiation of mature cells (Casimir et al., 1988; Lo et al., 1993; Slack, 2006; Tanaka, 2003). Multiple experiments by McGann et al. (2001) have demonstrated that regenerating limb extract could induce dedifferentiation in murine myotubes (McGann et al., 2001). Furthermore, lineage tracing experiments demonstrated that endogenous multinucleated muscle fibers dedifferentiate into mononucleated cells and contribute to the blastema during tail regeneration (Echeverri et al., 2001). Additionally, identification of myogenic Pax7+ stem/progenitor cells in the regenerating limb further supports the notion that stem/progenitor cells are colocalized with dedifferentiated cells in the blastema and contribute to the regenerated lineages (Morrison et al., 2006; Slack, 2006). Moreover, signaling pathways which govern either the dedifferentiation and/or mobilization of resident stem cells are incompletely understood. Recently, Knopf et al. (2011) have showed that FGF signaling does not affect the dedifferentiation process during fin regeneration (Knopf et al., 2011). Whether other signaling factors such as Hh or Wnt ligands play any role in this process is not clear. While the expression of Hh morphogens, Shh and Ihh has been documented during regeneration (Imokawa and Yoshizato, 1997; Tsonis et al., 2004), the mechanistic action remains unclear. Limited studies indicate that the regenerating blastema harbors a Shh expressing region called the zone of polarizing (ZPA) activity as the signaling center for the anterior-posterior (AP) patterning (Imokawa and Yoshizato, 1997). However, it is unclear whether the AP patterning is coupled or uncoupled with the expansion process during regeneration.
Studies focused on limb development and regeneration support the notion that common signaling pathways that include FGF, Hh, Wnt, BMP, etc. may be important for development and regeneration of lineages (Iovine, 2007; Kawakami et al., 2006; Yokoyama et al., 2007; Zeller et al., 2009). While these pathways are important during development, their roles in regeneration are incompletely defined. For example, the correlation between Wnt signaling and FGF signaling indicate that both pathways are essential for regeneration with Wnt activity being upstream of FGF activity (Lin and Slack, 2008; Yokoyama et al., 2007, 2011). These findings suggest a coordinated interaction between signaling pathways are necessary for regeneration but the details regarding interdependence and/or hierarchical relationship between pathways are unknown.
Here, we have dissected the critical role of Hh signaling and its temporal and transient requirement in anterior-posterior patterning during limb regeneration. Our data suggest that Hh signaling has dual and distinct roles in the modulation of the patterning and expansion processes during newt limb regeneration. In addition, we provide evidence for the hierarchical positioning of the Hh- and Wnt signaling pathways; Hh signaling lies upstream of Wnt signaling during the regeneration process. Activation of Wnt pathway rescues Hh inhibition mainly by promoting proliferative signals mediated through TCF4 activity. Collectively, these studies provide an enhanced understanding of the mechanisms that govern limb regeneration.
Materials and Methods
Antibodies and reagents
Mouse monoclonal anti-Pax7, anti-myosin heavy chain (Developmental Studies Hybridoma Bank), anti-Shh (Santa Cruz Biotechnology), anti-PCNA (Novocastra), rat monoclonal anti-BrdU (Abcam) and rabbit polyclonal anti–collagen type IV (Rockland Immunochemicals, Inc.) sera were used as primary antibodies. Alexa-488- (Molecular Probes) and Cy3-conjugated (Jackson ImmunoResearch laboratories) sera were used as secondary antibodies for immunofluorescence studies. Cyclopamine was purchased from LC laboratories and purmorphamine and IWR-1-endo were from Calbiochem, EMB Biosciences Inc. BIO was procured from Tocris. Lipofectamine and Opti-MEM were purchased from Invitrogen and the luciferase assay kit was from Promega.
Animals and surgery
All experiments were performed according to the University of Minnesota IACUC guidelines. Adult red-spotted newts, Notophthalmus viridescens, were obtained from Charles D. Sullivan Co., Inc., maintained in an aquarium at 18-20 °C and fed on red blood worms. For the limb amputation/regeneration studies, animals were anesthetized in 0.1% MS-222 solution for 10 min. For each animal, one forelimb was amputated proximal to the body and the animals were monitored overnight in 0.5% sulfamerazine solution and later placed in the water environment. At specified time periods, animals were sacrificed and tissues were collected.
Inhibitor/activator treatment, imaging and qRT-PCR
After the recovery period from anesthesia, the amputated animals were continuously exposed to either cyclopamine (Hh inhibitor), purmorphamine (Hh agonist), BIO (Wnt activator) or IWR-1-endo (Wnt inhibitor) alone or in combination till 60 days post amputation. The stock solutions of the respective activators or inhibitors were diluted in aquarium water and animals were placed in the respective aquariums containing small molecules or DMSO alone. The aquarium water containing activators and/or inhibitors was changed daily throughout the experimental procedures. For imaging, animals from each treatment group were anesthetized at specified time-points and placed under 0.5X objective (Zeiss, SteREO 2.0) and imaged using AxioVision Rel 4.8 software. Regenerating animals with complete digit structures and pattern (similar to contralateral unamputated limbs) were considered full regenerates, while with less or no digits (spike-like structures) were categorized into partial regenerates. The images were processed using Adobe Photoshop software. For gene expression analysis, qRT-PCR analysis was performed using total RNA. Primers used are listed in the Supplementary Table S1.
Histological and skeletal analysis
For histological analysis, animals were euthanized at specified time periods and tissues were fixed in 4% paraformaldehyde, decalcified, infiltrated and embedded in paraffin blocks. Tissue sections (7 μm) were processed for H & E staining at HistoCore facility at University of Minnesota. Stained sections were imaged using light microscopy (Zeiss, AxioPlan2) with AxioVision Rel 4.8 software. Alcian blue and alizarin red staining for skeletal analysis was performed as described elsewhere (Hanken and Wassersug, 1981).
Transmission electron microscopy (TEM)
Transmission electron microscopy was performed as described (Lentz, 1969). In brief, regenerating tissue was fixed in 2.5% glutaraldehyde-cocodylate buffer for 12h, rinsed and post-fixed in 1% osmium tetroxide (OsO4) followed by washing with distilled water. The tissues were dehydrated in graded series of alcohol (50%-100%) and embedded for sectioning. Ultrathin sections (70 nm) were stained with uranyl acetate and examined using transmission electron microscope (JEOL 1200-EX II TEM). The images obtained were arranged using Adobe Photoshop software.
Immunohistochemistry
Tissues processed for immunohistochemistry were snap frozen in isopentane and cooled to freezing point in liquid nitrogen. Frozen sections (8.0 μm) were thawed and fixed in acetone/methanol (1:1) for 10 min at −20 °C. Sections were blocked with 20% normal goat serum for 1h at room temperature and subsequently incubated with specific primary antibodies at 4 °C overnight. Tissue sections were then incubated with the appropriate secondary antibodies for 1h at room temperature, costained with DAPI (nuclear stain) and mounted using Vectashield mounting medium (Vector laboratories).
Cell culture, FACS analysis and migration assay
C2C12 mouse myoblasts were maintained at subconfluent densities in DMEM supplemented with 10% (v/v) FBS at 37 °C. Cells were treated with cyclopamine (2-10 μg/ml) alone or in combination with BIO (0.1-1.0 μM) for varied time periods. At specified time points, cells were trypsinized, fixed in 80% ethanol and stained with propidium iodide (PI) for cell cycle analysis. For the migration assay, C2C12 myoblasts were grown to 80-90% confluence and scratched using a P1000 pipet tip. Following the scratch, the cells were incubated with media containing either DMSO or cyclopamine at the specified concentrations. Migrating C2C12 cells and wound closure images were collected using phase contrast microscopy (Zeiss) and analyzed using Image J software. These assays were performed in triplicate and independently analyzed.
Luciferase assay
Transcriptional assays utilizing human 1.7 kb CCND1 promoter reporter construct, was performed as described earlier (Alvarez-Medina et al., 2009). TOPflash reporter system was used to measure Tcf/LEF-dependent transcriptional activity. C2C12 myoblasts were transfected with 1 μg of reporter construct along with pRL-TK luciferase (50 ng) using Lipofectamine 2000. pRL-TK expressing Renilla luciferase was used as a control for transfection efficiency. Transfected cells were treated with cyclopamine alone or in combination with BIO and harvested after 24 h of incubation. Luciferase activity was measured using the Dual-Luciferase Reporter Assay system (Promega) and expressed in relative light units normalized to Renilla luciferase activity.
Statistical analysis
Data represent the average of at least three replicates and shown as s.e.m. Student’s t-test (unpaired and two tailed variants) was used to determine the statistical significance of individual datasets using Excel software. Differences are considered significant with p < 0.05, very significant; p < 0.01 and highly significant; p < 0.005.
Results
Hedgehog signaling is critical for early stages of limb regeneration
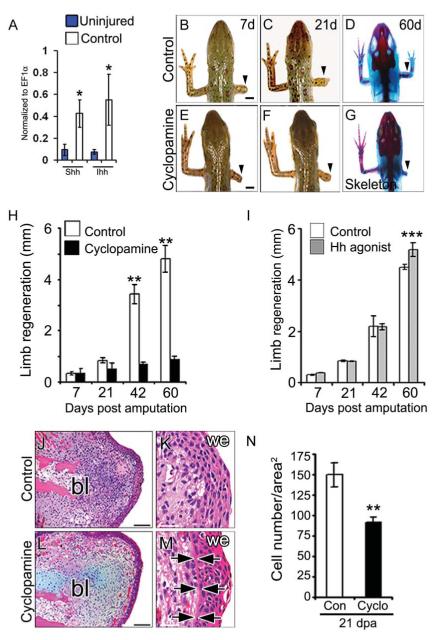
Expression of hedgehog morphogens including Sonic hedgehog (Shh) and Indian hedgehog (Ihh) and their spatial gradient have been described in multiple regenerating tissues (Endo et al., 1997; Imokawa and Yoshizato, 1997; Tsonis et al., 2004; Schnapp et al., 2005). Our qRT-PCR analysis indicates induced expression of both Ihh and Shh transcripts in the regenerating tissue as compared to uninjured tissue (Fig. 1A). In order to determine the requirement of hedgehog (Hh) signaling during limb regeneration, we perturbed Hh pathway using the chemical inhibitor, cyclopamine, which blocks hedgehog signaling by antagonizing the hedgehog receptor Smoothened (Smo) (Chen et al., 2002). The effect of cyclopamine on the inhibition of Hh pathway is evident by the reduced expression of Ptch1 [a direct downstream target of Hh signaling (Goodrich et al., 1996)] in the regenerating tissue (Supplementary Fig. S1A).
Fig. 1. Hh signaling is essential for limb regeneration.
(A) qRT-PCR analysis of Shh and Ihh transcripts expression in the regenerating tissue at 21 days post-amputation (21d). Note the increased abundance of Hh transcripts in the regenerates relative to the uninjured tissue (n=3; *, p < 0.01). (B-G) Whole mount view and skeletal analysis of regenerating limbs in control (B-D) and cyclopamine treated (E-G) animals at specified time periods following amputation. Arrowheads indicate the site of amputation. (H, I) Quantification of the average length of the regenerating limb from control (white bar), cyclopamine (black bar) and Hh agonist (grey bar) treated regenerates. Data points represent average length of the regenerates (n=14 animals; **, p<0.005; ***, p<0.05). (J M) Histological analysis of the 21d regenerating limb from control and cyclopamine treated animals at lower magnification (J, L) and at higher magnification (K, M) showing a basement membrane (arrowheads) between the wound epithelium and the underlying mesenchyme in the cyclopamine treated tissues. Scale bars: 2mm (B-G), 200 μm (J, L), 50 μm (K, M). (N) Quantitative analysis of the cell number per square area from 21d regenerating tissue. Data points include cell number from three independent tissues at three different levels (**, p<0.005). Error bars indicate s.e.m. bl, blastema; we, wound epithelium.
We find that the untreated newts could successfully regenerate the amputated limb with complete digit structure and patterning within a 60 day period (Fig. 1B-D, H; Table 1). Bone and cartilage staining reveals the presence of complete skeletal elements with digits, similar to the contralateral unamputated limb (Fig. 1D). In contrast, continuous inhibition of Hh signaling using cyclopamine (2 μg/ml) led to complete perturbation of regeneration resulting in stump formation following limb amputation in the newt (Fig. 1EG, H; Table 1). Continuous inhibition of Hh signaling is necessary, as intermittent treatment (1h/day) with cyclopamine (10-40 μg/ml) resulted in limb growth similar to the vehicle treated animals (data not shown). To ascertain the role of Hh signaling during limb regeneration, we treated the amputee with Hh agonist (10 μM) to monitor whether it has any effect on the regenerative ability. We observed that activation of Hh signaling resulted in slightly enhanced limb regeneration with complete digit formation at 60 days post-amputation (dpa) (Fig. 1I, Supplementary Fig. S1B-G), thereby indicates that continued Hh activity is required for limb regeneration.
Table 1.
Temporal requirement of Shh signaling during limb regeneration
| Regeneration status | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| No Reg. | Reg. Bud | Partial | Full | n | % of cases regenerating |
|
| Control | 0 | 16 | 0 | 16 | 16 | 100 |
| Cyclo. | ||||||
| 0-60d | 16 | 18 | 2 | 0 | 18 | 11.1 |
| 0-7d | 0 | 12 | 0 | 12 | 12 | 100 |
| 0-14d | 0 | 10 | 10 (spike) | 0 | 10 | 100 |
| 0-21d | 9 | 12 | 3 | 0 | 12 | 25 |
| 7-14d | 0 | 12 | 2 | 10 | 12 | 100 |
Next, we examined distinct regenerative stages and their dependence on Hh signaling. Limb regeneration progresses through characteristic stages including wound healing, dedifferentiation, blastema formation and redifferentiation (Akimenko et al., 2003; Gardiner et al., 2002; Stoick-Cooper et al., 2007). Each of these stages occurs during a defined period following amputation (Campbell and Crews, 2008). Upon amputation, the epidermal cells migrate to cover the wound surface; subsequently, proliferate to form a multilayered apical epidermal cap (AEC), which is necessary for blastema formation and regeneration (Campbell and Crews, 2008; Globus et al., 1980). Our histochemical analysis indicates that the early phase of epidermal migration and AEC formation is similar in both control and cyclopamine treated tissues. Interestingly, a distinct thick basement membrane was observed adjacent to the proximal epidermis in the cyclopamine treated samples compared to the control samples (Fig. 1J-M, arrows). We observed that the cellular density at the blastema region (bl) was low and the cell number per square area was reduced from 149±12 to 92±6 cells (p<0.01) in the cyclopamine treated newts relative to the controls (Fig. 1N). These results indicated that inhibition of Hh signaling modulates the blastemal cells and adjacent epidermis with no effect on the AEC formation.
Previous studies using in situ hybridization techniques indicated that the Shh gene is transcribed early during regeneration (Endo et al., 1997; Imokawa and Yoshizato, 1997). Using immunohistochemical techniques, we observed that Shh was expressed by 7 dpa (days post amputation) in the posterior region of the regenerating limb, whereas low or no expression was seen in the uninjured tissue (Supplementary Fig. S1H, S1I-K, I’-K’). Given the expression of Ihh and Shh morphogens during regeneration, we investigated whether Hh signals were essential at early stages of limb regeneration. Interestingly, inhibition of Hh signals with cyclopamine from 0 to 21 days, followed by no treatment resulted in significant perturbation of regeneration at 60 dpa (Supplementary Fig. S2A-G; Table 1). These cyclopamine treated newts have been monitored up to 120 dpa with no further growth, indicating that the effect is not due to a delay in regeneration. Collectively, our data suggest that Hh signaling is required at the early stages of limb regeneration and blocking Hh signaling leads to irreversible perturbation of regeneration in the newts.
Hh signaling regulates blastema patterning at the initial stages of regeneration
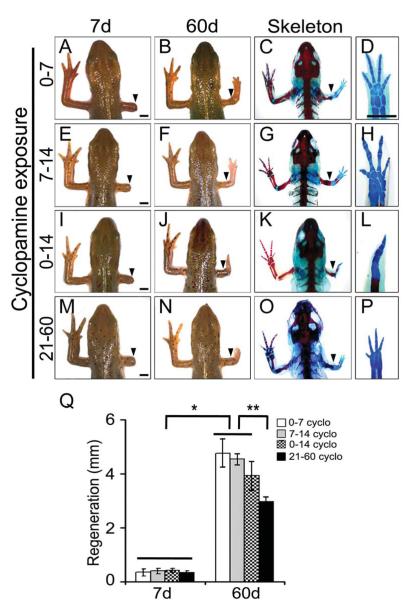
To further define the functional role of Hh signalling during regeneration, we inactivated the Hh signaling using cyclopamine for defined periods followed by no treatment. Our data indicate that inhibition of Hh signaling between 0 dpa to 7 dpa (0-7d) had no effect on the regenerative process and results in complete digit formation (Fig. 2A-D, Q; Table 1). Similarly, inhibition of Hh signaling between 7 dpa to 14 dpa (7-14d) did not have any defects in digit formation and regeneration (Fig. 2E-H, Q; Table 1). In contrast, inhibition of Hh signaling from 0 dpa to 14 dpa (0-14d) resulted in spike-like structures with complete loss of posterior elements (Fig. 2I-L, Q; Table 1). In these animals (0-14d), the overall growth of the regenerated limb was similar to 0-7d or 7-14d treated animals. These results demonstrate an early requirement of Hh signalling for AP patterning and even a brief period of Hh activity is sufficient to ensure proper patterning. To further evaluate the dependency of AP patterning on Hh signaling for 0-14d, we allowed the animals to regenerate for 14 days without treatment, followed by continuous treatment with cyclopamine from 14 dpa to 21 dpa (14-21d). In another group, the amputees were allowed to regenerate for 21 days without treatment, followed by continuous cyclopamine treatment from 21 dpa to 60 dpa (21-60d) and analyzed at 60 days. Cyclopamine treatment either for 14-21d or 21-60d resulted in retarded regeneration; however there was complete digit patterning and skeletal elements (Fig. 2M-P, Q). Overall, these results indicate that Hh is required transiently (0-14d) for AP patterning at early time periods and at later time periods it is mainly required for the expansion of the regenerating tissue.
Fig. 2. Temporal inhibition of Hh signaling affects AP patterning during limb regeneration.
(A-L) Morphological and skeletal analysis from the regenerates treated with cyclopamine from 0-7 days (A-D), 7-14 days (E-H) and 0-14 days (I-L) followed by no treatment. (M-P) represents the regenerates treated with cyclopamine from 21-60 days. Arrowheads indicate the point of amputation. (Q) Quantification of the average length of the regenerating limb from the different regenerates (n=10-12 animals; *, p<0.01; **, p<0.005). Error bars indicate s.e.m. Scale bars: 2 mm.
Hh signaling does not affect the dedifferentiation process
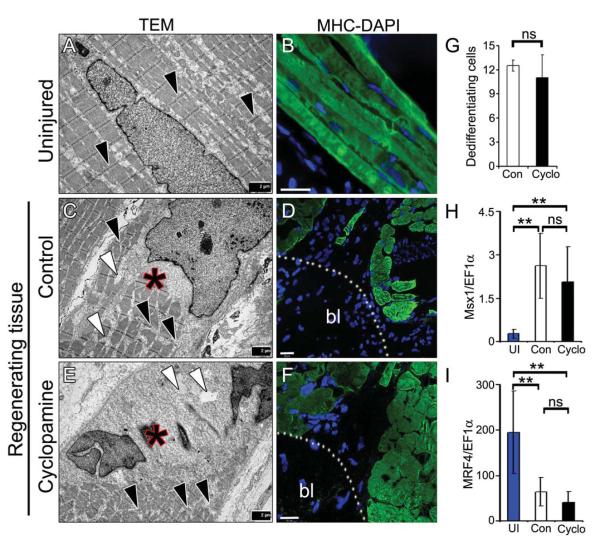
Having established that Hh signaling is essential during early stages of regeneration, we examined its role during dedifferentiation. Multiple studies indicate that limb regeneration depends on blastema formation, which arises from dedifferentiation of the mature differentiated cells (Casimir et al., 1988; Echeverri et al., 2001; Knopf et al., 2011; Slack, 2006). We argued whether inhibition of Hh signaling affects the dedifferentiation process, which could be attributed to the lack of regenerative ability. To address the dedifferentiation process, we analyzed the regenerating tissue using transmission electron microscopy (TEM). In the uninjured limb, myofibers showed an ordered arrangement of sarcomeric filaments and mitochondria with distinct Z-lines (Fig. 3A, black arrowheads). In contrast, the amputated limb had large inter- and intra-cellular spaces (Fig. 3C, white arrowheads; Supplementary Fig. S3A, C) with disorganized sarcomeres and ill-defined Z-lines (Fig. 3C, black arrowheads; Supplementary Fig. S3A, C). Additionally, we observed altered nuclear architecture with distinct electron dense structures relative to the uninjured tissue, which is indicative of a reorganized nucleus (Fig. 3A, C, E). Importantly, we could not find any differences during the dedifferentiation stage between the control and cyclopamine treated regenerates (Fig. 3E; Supplementary Fig. S3B, D) indicating that Hh signalling is not involved in the dedifferentiation process. Quantitative analysis of the structurally and morphologically altered cells in the control and cyclopamine treated tissues indicated no significant differences in the number of dedifferentiated cells (Fig. 3G). In support of our electron microscopic studies, we investigated the effect of Hh inhibition on the expression of various markers following amputation. Our qRT-PCR analysis indicated increased expression of Msx1 (dedifferentiation marker) and decreased expression of MRF4 (differentiation marker) mRNA levels in the regenerating tissue relative to uninjured tissue (Fig. 3H, I). The extent of expression of these transcripts was similar in both control and cyclopamine treated tissue indicating that Hh inhibition does not affect the dedifferentiation process. Importantly, immunohistochemical analysis indicated lack of myosin heavy chain (MHC) staining near the amputation site [blastema region (bl)], however the expression of MHC was maintained at the remote area (Fig. 3D, F) in both control and cyclopamine treated tissues. Furthermore, we detected fragmented myofibers and mononucleated myocytes with intercellular spaces in the control and cyclopamine treated tissues by H & E staining and immunohistochemistry [Supplementary Fig. S3E, F, H, I (white arrows in H, I)]. Similar to areas of MHC-positive structures with no nuclei in the blastema region, we found structures with membrane bound cellular components without nuclei in our TEM studies (Supplementary Fig. S3G, J), that further support the occurrence of the dedifferentiation process. The reduced expression of MHC at the blastema region was observed at as early as 7 dpa suggesting that mature myocytes down regulate differentiation markers and dedifferentiate to yield proliferating progenitors. Together, these results indicate that limb amputation results in cellular dedifferentiation of mature cells and inhibition of Hh signaling have no effect on the dedifferentiation process.
Fig. 3. Inhibition of Hh signaling does not affect the dedifferentiation process.
(A, C, E) TEM images from uninjured (A), control (C) and cyclopamine (E) treated regenerating tissues. Note the loss of Z-lines (black arrowheads) with large intracellular spaces (white arrowheads) in the regenerates. Asterisks indicate a cell undergoing dedifferentiation, evident by partly stained sacromeric structures with altered nuclear architecture in control (C) and cyclopamine (E) treated regenerates. (B, D, F) Myosin heavy chain (MHC) staining (green) from uninjured (B), control (D) and cyclopamine (F) treated regenerating tissues. DAPI (blue) stain the nuclei. Fragmented myofibers stained positive for MHC in the remote area with loss of MHC staining in the blastema (bl) region. Scale bars: 2 μm (A, C, E), 50 μm (B, D, F). (G) Quantification of the dedifferentiated cells (structurally and morphologically altered muscle cells) in the control and cyclopamine treated tissues (n=3; p=0.588). (H, I) qRT-PCR analysis of Msx1 and MRF4 transcripts expression from the regenerating limb tissue at 21d. Note the increased abundance of Msx1 transcript, and reduced expression of MRF4 in the regenerates relative to uninjured group (n=3; **, p<0.005). Error bars indicate s.e.m.
Hh signalling controls blastemal cell proliferation
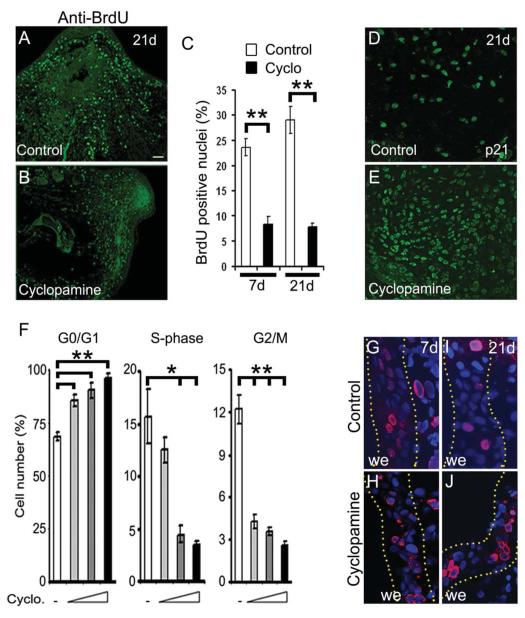
To further characterize the perturbed regeneration associated with Hh inhibition, we examined programmed cell death and cellular proliferation following limb amputation. TUNEL staining for the control and cyclopamine treated samples were indistinguishable at 21d, indicating that apoptosis did not account for the reduced cell number and smaller blastema size (data not shown). To monitor the cellular proliferation, a BrdU labelling assay was performed in the regenerating tissues. In response to injury, we found increased cellular proliferation (BrdU-positive nuclei) primarily concentrated at the amputated region in the control tissue at 7 and 21d (Fig. 4A, C; Supplementary Fig. S4A, B). In contrast, Hh inhibition led to reduced cellular proliferation from 22 to 9% (p<0.005) at 7d and 30 to 7% (p<0.005) at 21d, respectively (Fig. 4B, C; Supplementary Fig. S4C, D). Similarly, the number of PCNA positive cells were reduced in cyclopamine treated tissues (data not shown), suggesting that Hh signalling has an important role in blastemal cell proliferation.
Fig. 4. Hh signaling is required for proliferation of blastemal cells.
(A, B) Longitudinal sections of regenerating limb tissue at 21d, stained with a BrdU antibody (green) showing reduced cellular proliferation in cyclopamine treated (B) relative to the control (A) animals. (C) Quantitative analysis of the BrdU-positive nuclei in control (white bar) and cyclopamine treated (black bar) tissues at 7d and 21d of regeneration (n=4; **, p<0.005). (D, E) Immunohistochemical analysis of the regenerating tissues using anti-p21 antibody reveals increased abundance of p21-positive nuclei (green) in cyclopamine treated (E) tissue relative to the control (D) tissue. (F) Cell cycle analysis of the myoblast cells upon Hh inhibition showed significant increase in G0/G1 arrested cells with a concomitant decrease in the S- and G2/M-phase cells in a dose dependent manner (n=5; *, p<0.01; **, p<0.005). (G-J) Immunohistochemical analysis using anti-BrdU sera in the epidermal region of the regenerating tissues at 7d and 21d. Dotted yellow lines indicate regions of wound epithelium (we). DAPI (blue) stain the nuclei. Error bars indicate s.e.m. Scale bar: 200 μm.
To decipher the requirement of Hh pathway in the activation of proliferative signals, we performed transcriptional assays utilizing 1.7 kb CCND1-promoter-luciferase (CyclinD1 promoter luciferase) reporter constructs. Consistent with the reduced proliferation, luciferase activities were markedly reduced upon cyclopamine treatment as compared to untreated samples (Supplementary Fig. S4E). Furthermore, analysis of p21 (CDK inhibitor) expression in the regenerating tissue indicated increased number of p21-positive cells upon Hh inhibition relative to control tissue (Fig. 4D, E). FACS analysis for cell cycle kinetics showed increased percentage of G0/G1 arrested cells in Hh inhibited condition as compared to untreated cells. The percentage of G0/G1 arrested cells increased upon increasing concentration of cyclopamine and with concomitant decrease in S- and G2/M- phase populations (Fig. 4F); overall indicating that Hh inhibition results in cell cycle arrest. Interestingly, inhibition of Hh signalling showed no effect on the proliferation of epidermal cells (Fig. 4G-J). Importantly, none of the MHC-positive cells stained positive for BrdU (Supplementary Fig. S4F-K), in both control and cyclopamine treated tissues supporting the notion that mature cells dedifferentiate and proliferate to achieve regeneration. Collectively, our data indicate that Hh signalling has an essential role in modulating blastemal cell-cycle progression during regeneration.
Hh signalling modulates activation and migration of muscle precursors
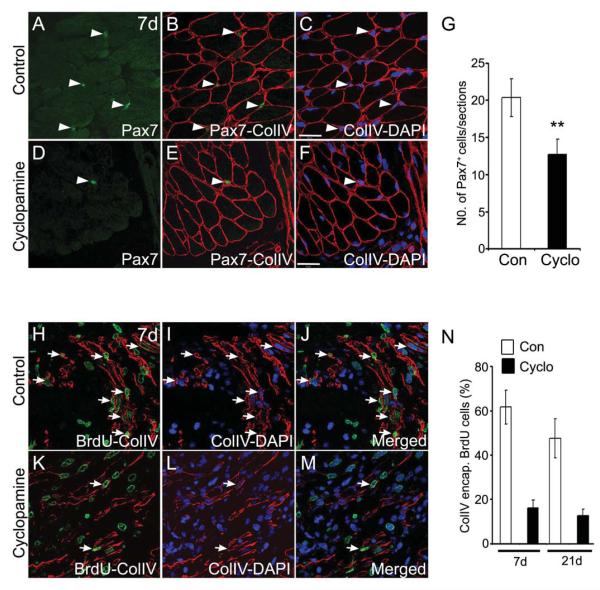
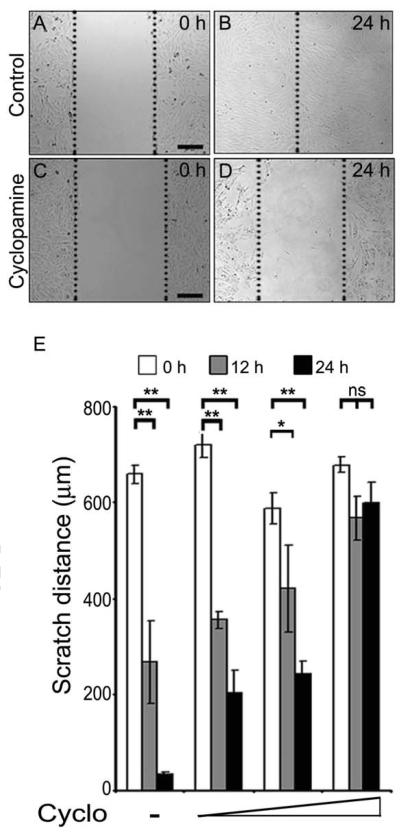
The origin of the blastemal cells is unknown. Recently, Morrison et al. have demonstrated that similar to mammalian skeletal muscle regeneration, Pax7+ muscle satellite cells can contribute to the regenerating limb tissue in newts (Morrison et al., 2006). In response to injury, mammalian skeletal muscle regeneration involves activation and proliferation of satellite cells (Pax7+) and incorporation into nascent or pre-existing myofibers (Charge and Rudnicki, 2004; Collins et al., 2005). To test this possibility, we examined the effect of cyclopamine treatment on Pax7+ cells in the regenerating limb. Inhibition of Hh signalling resulted in significantly fewer Pax7+ labelled nuclei as compared to controls (Fig. 5A-G). To further investigate the activation of muscle satellite cells, we performed co-staining using collagen type IV (colIV) and anti-BrdU antibodies to monitor the regenerating fibers. In the regenerating tissue, we found a large number of BrdU-positive nuclei encapsulated within colIV positive regenerating myofibers (intra-laminar) along with extralaminar BrdU-positive nuclei (Fig. 5H-J, N). Treatment with cyclopamine resulted in a reduced number of newly forming myofibers (decreased number of colIV encapsulated BrdU-positive cells), indicative of impaired differentiation (Fig. 5K-N). We undertook studies to examine whether the reduction of regenerating myofibers is due to impaired migration of the activated satellite cells in Hh inhibited tissues. In these studies, we utilized C2C12 myoblast cells, which are a cell line that is derived from satellite cells (myogenic progenitors) (Yaffe and Saxel, 1977). The cell migration assay, using C2C12 myoblasts,indicated that inhibition of Hh signalling results in significantly retarded migration of the myoblasts (Fig. 6A-D) in a dose dependent manner (Fig. 6E). Overall, our results indicated that blastema formation occurs by the synergistic events of activation and migration of the muscle precursors and Hh signalling plays an important role in both of these processes.
Fig. 5. Pax7+ cell populations and regenerating fibers are reduced upon Hh inhibition.
(A-F) Immunohistochemical analysis for Pax7+ cells (green) and collagen type IV [colIV (red)] basal lamina from control (A-C) and cyclopamine (D-F) treated regenerating tissue at 7d. (C, F) shows nuclei [DAPI (blue)] merged with colIV+ lamina. Arrowheads indicate the position of the Pax7+ cells. (G) Quantification of the Pax7+ cells in the regenerating limbs from control and cyclopamine treated tissues. Note the reduced number of Pax7+ cells in the cyclopamine treated tissue (n=4; **, p<0.005). (H-M) Longitudinal sections of regenerating limb tissue from control (H-J) and cyclopamine (K M) treated animals at 7d, stained with anti-BrdU (green) and anti-colIV (red) antibody. Nuclei are stained with DAPI (blue). (J, M) show the combined images of BrdU (green), colIV (red) and DAPI (blue) channels. Regenerating fibers are indicated by arrows. (N) Quantification of the percentage of colIV-encapsulated-BrdU+ cells (intralaminar) at 7d and 21d regenerating tissue. Error bars indicate s.e.m. Scale bar: 50 μm.
Fig. 6. Hh signaling is required for cellular migration.
(A-D) Representative photomicrographs of migrating C2C12 muscle myoblasts from untreated (A, B) and cyclopamine treated (C-D) cultures at 0 h and 24 h time points. (E) Quantification of the scratch distance at 0 h (white bars), 12 h (grey bars) and 24 h (black bars) in untreated and treated C2C12 cells. Data points represent the average scratch distance from all experiments (n=4; *, p<0.01; **, p<0.005). Error bars indicate s.e.m. Scale bars: 200 μm.
Hh signaling acts upstream of Wnt signaling
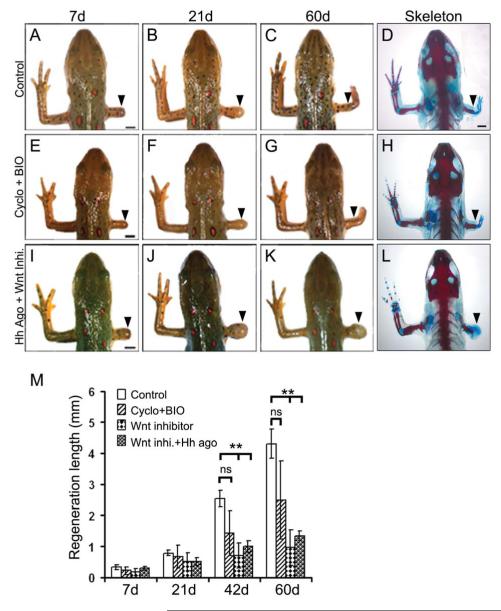
A number of studies have previously characterized the role of Wnt signalling in blastemal proliferation and tissue patterning in regeneration (Caubit et al., 1997; Poss et al., 2000). We established the parameters wherein activation or inhibition of μ-catenin dependent Wnt signaling resulted in enhanced or perturbed limb regeneration (Supplementary Fig. S5A-F) respectively. In order to examine the hierarchical positioning between Hh and Wnt pathways during limb regeneration, we used an activator-inhibitor approach, where we chemically activated one pathway while the other was inhibited. Treatment of the amputated newts with cyclopamine in combination with BIO (Wnt activator) resulted in complete regeneration of the skeletal pattern while the animals treated with cyclopamine alone failed to regenerate (Fig. 7E-H, 1E-G; Table 2), suggesting that Wnt pathway is activated after Hh signaling. To further confirm the hierarchical positioning, we treated the amputated newts with Hh agonist alone or in combination with Wnt inhibitor. After 60 days of treatment, we observed that inhibition of Wnt signaling completely blocked the regenerative ability irrespective of the presence/absence of Hh agonist (Fig. 7I-L; Table 2). Whole mount analysis revealed that animals treated with Wnt inhibitor alone or in combination with Hh agonist, had similar bulb-like structure at the amputation plane with impaired regeneration. Taken together, these results indicated that Wnt/β-catenin signaling acts downstream of Hh signaling during limb regeneration.
Fig. 7. Wnt signaling functions downstream of Hh signaling during limb regeneration.
(A-L) Whole mount and skeletal micrographs of the regenerates at defined time periods from control (A-D), cyclopamine+BIO (E-H), and Hh agonist+Wnt inhibitor (I L) treated animals. Arrowheads indicate the points of amputation. Note that activating Wnt signaling overrides the effect of Hh inhibition. (M) Quantification of the regenerating limbs from control, cyclopamine+BIO, Wnt inhibitor and Hh agonist+Wnt inhibitor treated animals at specified time points. Data points represent average lengths of the regenerates (n=12 animals; **, p<0.005). Error bars indicate s.e.m. Scale bars: 2 mm.
Table 2.
Effect of activators and inhibitors of Hh and Wnt signaling during regeneration
| Regeneration status | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| No Reg. | Reg. Bud | Partial | Full | n | % of cases regenerating |
|
| Control | 0 | 14 | 0 | 14 | 14 | 100 |
| Cyclopamine | 15 | 16 | 1 | 0 | 16 | 6.2 |
| Wnt Act. (BIO) | 0 | 14 | 0 | 14 | 14 | 100 |
| Cyclo+BIO | 0 | 12 | 2 | 10 | 12 | 100 |
| Hh Agonist | 0 | 12 | 1 | 11 | 12 | 100 |
| Wnt Inhibitor | 12 | 12 | 0 | 0 | 12 | 0.0 |
| Hh Ago+Wnt Inhi | 12 | 12 | 0 | 0 | 12 | 0.0 |
Activating Wnt signaling overrides Hh inhibition
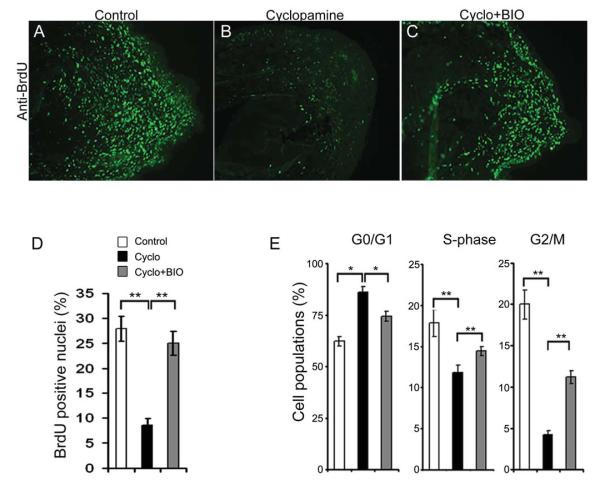
We examined the cellular nature and molecular mechanism by which Hh and Wnt signaling cooperate with each other to promote regeneration. Histological analysis revealed that the animals treated with cyclopamine and BIO together had cone-shaped blastema with 5-6 cell layer AEC, with no thickened basement membrane (Supplementary Fig. S5G-L), hence indicates regenerative potential. We reasoned that activating Wnt signaling could override the Hh inhibition in promoting proliferative signals for regeneration. Our BrdU labeling assay showed that activation of Wnt signaling could restore the proliferation from 8 to 25% (p<0.005) imposed by Hh inhibition (Fig. 8A-D). This is further supported by increased percentage of cells in the S- and G2/M- phase in cyclopamine and BIO treated cells as compared to cells treated with cyclopamine alone (Fig. 8E). The number of cells in the S- and G2/M phase increased with increasing concentration of BIO with a concomitant decrease in G0/G1 arrested cells (data not shown). Importantly, BrdU-positive nuclei encapsulated within colIV-positive regenerating myofibers in cyclopamine and BIO treated tissues were similar to the control tissues. In contrast, the numbers of regenerating myofibers with BrdU-positive nuclei were reduced in tissue treated with Wnt inhibitor alone or in combination with Hh agonist (data not shown). Collectively, these results suggested that Wnt signaling acts downstream of Hh signaling during limb regeneration and regulates myogenesis.
Fig. 8. Activation of canonical Wnt pathway overrides the effect of Hh inhibition and restores proliferative signals.
(A-C) Immunohistochemical analyses of proliferating cells using anti-BrdU (green) sera in control (A), cyclopamine (B) and cyclopamine+BIO (C) treated tissues. (D) Quantitative analysis of the BrdU labeled cells from control, cyclopamine and cyclopamine+BIO treated tissues (n=4; **, p<0.005). (E) Cell cycle kinetics of cells treated with DMSO (white bars), cyclopamine (black bars) and cyclopamine+BIO (grey bars). Note the relative increase in S- and G2/M- phase cells upon activating Wnt signaling in the background of Hh inhibition. Data points represent average cell populations from all experiments (n=3; *, p<0.01; **, p<0.005). Error bars indicate s.e.m.
Hh signaling modulates β-catenin dependent TCF4 activity
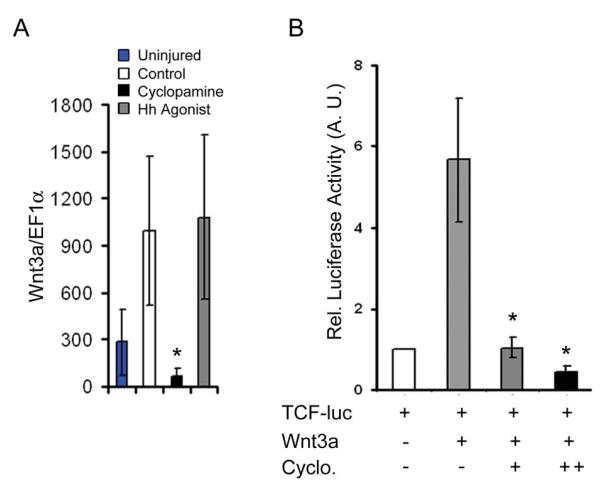
Additional experiments were performed to gain insight into the interaction of these two signaling pathways during limb regeneration. Quantitative RT-PCR analysis indicated that injury results in increased expression of Wnt3a transcript in the regenerating tissue. Furthermore, activating Hh signals increases Wnt3a expression, while Hh inhibition results in reduced expression relative to uninjured tissue (Fig. 9A) indicating the possibility of Hh dependent transcription of secreted signals. In addition, luciferase assays utilizing a TCF4-reporter construct, demonstrate that activation of Wnt3a ligands mediated TCF4 activity was suppressed to basal levels upon inhibition of Hh signaling, suggesting that Hh pathway modulates TCF4 activity. Furthermore, luciferase activity was inversely proportional to the increasing concentration of cyclopamine (Fig. 9B). Collectively, our results indicated that Hh signaling is required for the activation of Wnt signaling during regeneration.
Fig. 9. Hh signaling modulates Wnt-mediated transcriptional activity.
(A) qRT-PCR analysis of Wnt3a transcript expression from the regenerating limb tissue at 21d. Expression of Wnt3a mRNA is significantly reduced upon Hh inhibition relative to the controls (n=3; *, p<0.01). (B) Transcriptional assay using TCF-reporter construct in myoblast cells in the presence of Wnt3a alone or in combination with increasing concentrations of cyclopamine. Wnt3a mediated activation of reporter activity is significantly reduced upon inhibition of Hh signaling in a dose dependent manner (n=3; *, p < 0.01). Error bars indicate s.e.m.
Discussion
Amphibians have a remarkable capacity to regenerate an amputated limb with complete restoration of the cellular architecture and patterning of the injured limb (Brockes and Kumar, 2002; Gardiner et al., 2002; Singh et al., 2010). In this regenerative step, the dedifferentiated progenitors and resident stem cells interact and contribute to the repair and regeneration of the transected limb, however, the signaling pathways that govern this process and cell populations are incompletely defined. In this study, we examined the role of the hedgehog (Hh) and Wnt signaling pathways and made three fundamental discoveries which enhance our understanding of limb regeneration. These discoveries emphasized that Hh is dispensable for dedifferentiation of mature lineages but regulates the proliferation and migration of progenitors during limb regeneration. In addition, our results further define a hierarchical cascade between Hh and Wnt signaling during the regenerative program. Collectively, these results support the notion that signaling pathways function to coordinately regulate embryogenesis and regeneration of the limb.
Hh signaling has been shown to play important developmental roles during limb development (Chiang et al., 2001; McGrew and Pourquie, 1998; Zeller et al., 2004, 2009). Previous studies have demonstrated a Shh gradient in the developing posterior limb bud margin (Harfe et al., 2004; Tickle, 2006). This Shh gradient is part of the Shhgremlin-Fgf feedback loop that regulates proliferation of mesenchymal cells in the developing limb (Lewandoski et al., 2000; Panman et al., 2006; Verheyden and Sun, 2008; Zuniga et al., 1999). Another member of hedgehog signaling, Ihh, has been shown to be involved in bone formation and chrondrocyte proliferation in a FGF-BMP4 dependent pathway (Minina et al., 2002). In addition to the involvement of Ihh in bone and skeletal development, recent studies have indicated its role in muscle formation (Bren-Mattison et al., 2011). Studies have demonstrated that Hh regulates the expression of the myogenic regulatory factors, myf5 and Myod, in proliferating myoblasts during embryogenesis (Bren-Mattison and Olwin, 2002; Koleva et al., 2005). These roles for Hh are further evident as pharmacological perturbation of the Hh signaling pathway using cyclopamine resulted in limb malformation (Scherz et al., 2007; Stopper and Wagner, 2007). In addition, genetic mouse studies have revealed Hand2 and Hoxd dependent Shh expression in the developing limb as Hand2 or Hoxd knockout mouse models had decreased Shh activity and perturbed limb development (te Welscher et al., 2002). The developmental impact of the Shh pathway in the limb bud was evident as Shh null embryos had abnormal limb development with an absence of limb muscles (Chiang et al., 2001). In the present study, we identified dual roles for Hh signaling and defined the molecular mechanisms that were regulated by Hh morphogens during limb regeneration.
Our first discovery determined that Hh signal is essential for limb regeneration and cellular proliferation. We observed that Ihh and Shh morphogens were expressed early in the regenerates, which supported the notion that Hh signal governs the initial stages of regeneration. We demonstrated that the pharmacological inhibition of the Hh signaling results in stump formation and an absence of regeneration. Our studies further suggest several possible mechanisms for Hh and limb regeneration. For example, the presence of a thickened layer between the blastema and the epidermis following Hh inhibition may serve as a barrier for signaling cues (such as Igf2b, Fgf8, Wnt ligands) and/or this thickened membrane may limit cellular migration (Campbell and Crews, 2008; Chablais and Jazwinska, 2010). Furthermore, reduction in blastemal cellular pool in the cyclopamine treated regenerates further indicates the importance of the cross-talk between AEC and the regenerating blastema. Based on these data, we hypothesized that Hh signaling supports the intrinsic interaction between the blastema and wound epidermis to facilitate the regenerative program.
Studies from mammalian model systems reveal the importance of Shh morphogens in specifying the digit patterning (Tickle, 2006; Zeller, 2004). Here, we have established the biphasic functional role of Hh in regulating anterior-posterior (AP) patterning and the cellular expansion process in regenerative growth. Temporal inhibition of Hh signaling indicates that Hh morphogens are required early and transiently (0-14d) for patterning; thereafter it is mainly needed for cellular proliferation. Therefore, our study supports a dual functional role for Hh which appears to be temporally uncoupled. In addition to the role for Hh signaling during limb patterning, this signaling pathway plays a critical role in the survival and cellular proliferation of limb bud mesenchyme (Duprez et al., 1998; te Welscher et al., 2002; Zuniga et al., 1999). This is further supported by the finding of retarded limb growth following cyclopamine treatment. Our data suggest that Hh morphogens may function as a direct mitogen for the proliferation of the blastemal cells as Hh inhibition resulted in increased p21 expression and cell cycle arrest. Hh signaling may have an indirect role and function upstream of factors such as Wnt3a ligands that may in turn regulate blastemal cell proliferation, or alternatively it may be required to promote differentiation. Since we could not detect any differences in the dedifferentiation process in the Hh inhibited regenerates, we speculate that unidentified signals are required in combination with these factors to regulate this process. Future studies will be needed to further dissect and define the Hh mediated mechanisms that govern limb regeneration.
Our second discovery established a role for Hh as a regulator of Pax7 expressing myogenic progenitors. Following Hh inhibition, we observed a significant decrease in the number of Pax7+ myogenic progenitors at 7d after limb amputation. Our studies support a role for Hh in cellular migration and proliferation of the myogenic progenitors during limb repair/regeneration. The impact of Hh inhibition on Pax7 expressing myogenic progenitors may further explain the perturbed limb regeneration following cyclopamine treatment. In the present study, it is unclear whether impaired regeneration in the cyclopamine treated animals is due to reduced proliferation of Pax7+ cells or due to a block in differentiation (or a combination of reduced proliferation and impaired differentiation). Our study supports the hypothesis that Pax7 expression marks myogenic progenitors which contributes to muscle regeneration in a similar fashion to mammalian muscle regeneration (Charge and Rudnicki, 2004; Morrison et al., 2006). Moreover, it is likely that both activated myogenic progenitors (i.e. satellite cells) and dedifferentiated muscle fibers contribute to the formation of the blastema during limb regeneration. Collectively, these observations favor the hypothesis that Hh signaling pathways have common and overlapping roles that govern muscle development and limb regeneration.
In the present study, we examined the coordinated regulation of limb regeneration by the convergence of both the Hh and Wnt pathways. Our third discovery defined a hierarchical Hh-Wnt pathway interaction during limb regeneration. Accumulating evidence supports a role for the Wnt signaling pathway and tissue regeneration (Yokoyama et al., 2007, 2011). For example, the Wnt signaling pathway has been shown to be important in lens, blood and muscle regeneration. Our studies demonstrate that the Hh signaling functions upstream of the Wnt/β-catenin canonical pathway. This study provides a mechanism through which Hh and Wnt activities are integrated to control proliferative signals and progression through cell-cycle during regeneration. A number of interaction/s between these two pathways is possible and likely during limb regeneration. For example, Hh signaling may regulate TCF transcriptional activity by modulating Wnt3a expression; alternatively, it may serve as upstream regulator of TCF4 activity. Future studies will be needed once species specific antisera are developed to examine these regulatory nodes and to define interactions with additional signaling pathways to modulate limb regeneration.
In summary, our studies defined the importance of signaling pathways during limb regeneration and deciphered a hierarchical Hh-Wnt cascade that modulates limb regeneration. These studies further emphasize the importance of using animal models that have a remarkable capacity for limb patterning and regeneration following amputation.
Supplementary Material
Fig. S1. Activation of Hh signaling results in enhanced regeneration. (A) qRT-PCR analysis of Ptch1 transcript expression in the regenerating limb tissue at 21d. Treatment with cyclopamine results in significantly reduced Ptch1 expression (n=3; **, p<0.005). (B G) Whole mount and skeletal analysis of regenerating limbs in control (B-D) and Hh agonist treated (E-G) animals at different time periods. Arrowheads indicate the point of amputation. (H-K, I’-K’) Schematic representation and immunohistochemical staining of Shh (red) in uninjured (I, I’), control (J, J’) regenerating limb at 7d. Nuclei are stained with DAPI (blue). Panel I’-K’ shows Shh immunostaining at higher magnification. Expression of Shh is confined to the posterior region of the limb. Scale bars: 2 mm (B-G), 200 μm (IK; I’-K’).
Fig. S2. Hh signaling is required early during limb regeneration. (A-F) Gross morphology and skeletal analysis of the regenerating limbs of the control (A-C) and cyclopamine treated (D-F) animals between 0-21 days [Cyclo (0-21d)]. (G) Quantification of the regenerating limb from control (white bar), cyclopamine (0-21d) (black bar) treated regenerates at specified time periods. Data points represent average length from multiple experiments (n=12 animals; **, p<0.005). Error bars indicate s.e.m. Scale bars: 2 mm.
Fig. S3. Hh is not required for the dedifferentiation process. (A-D) TEM images of the tissues from control (A, C) and cyclopamine (B, D) treated injured limbs. Injury results in disorganized sacromeres and loss of Z-bands along with large intracellular spaces. (E, H) H & E staining of the regenerating tissue from control (E) and cyclopamine (H) treated animals show fragmented myofibers. (F, I) MHC staining (green) of the regenerating tissue from control (F) and cyclopamine (I) treated animals. Fragmented myofibers stained positive for MHC (white arrows) can be seen in the regenerating area. (G, J) TEM images of the tissue from control (G) and cyclopamine (J) treated animals. Cell membrane bound cellular components were seen in both control and cyclopamine treated tissues. Scale bars: 1 μm (A, B); 2 μm (D, G); 5 μm (C, J); 200 μm (E, F, H, I).
Fig. S4. Hh signaling alters myogenic proliferation. (A-D) Immunohistochemical analysis of the regenerating tissues using anti-BrdU sera (red) display reduced proliferation upon Hh inhibition (C, D). (E) CyclinD1-reporter (CCND1-luc) transcriptional assay using C2C12 myoblast cells untreated and treated with cyclopamine. Inhibition of Hh signaling resulted in marked reduction in the luciferase activity. Error bars indicate s.e.m. (F-K) shows the immunostaining of MHC (green) and BrdU (red) in the regenerating tissue. Note the absence of MHC stained cells together with BrdU, indicating lack of proliferation in mature myofibers. Scale bar: 50 μm.
Fig. S5. Wnt signaling is essential for limb regeneration. (A-F) Whole mount and skeletal analysis of the regenerating limbs at 7d and 60d from BIO (Wnt activator) (A-C), and Wnt inhibitor (D-F) treated animals. Arrowheads indicate the point of amputation. (G L) Histological analysis of the regenerating limb from control, BIO and cyclopamine+BIO treated animals at lower magnification (G, I, K) and at higher magnification (H, J, L) showing absence of thickened basement membrane between wound epithelium and underlying mesenschyme. Scale bars: 2 mm (A-F), 200 μm (G, L).
Table S1: PCR Primers used for Quantitative RT-PCR analysis
Shh signaling is essential for limb regeneration.
Reduced Shh signaling does not affect the dedifferentiation process.
Shh is required for AP patterning at early stages and for cellular expansion at later stages of regeneration.
Wnt signaling functions downstream of Shh signaling during regeneration.
Acknowledgements
We would like to acknowledge Fang Zhou (Characterization Facility, University of Minnesota) for conducting transmission electron microscopic studies. We acknowledge the support of Jennifer L. Fricton (HistoCore, Lillehei Heart Institute, University of Minnesota) and Kathy M. Bowlin for their assistance with the immunohistochemical analyses. Funding support was obtained from the National Institutes of Health (AR055906 and AR047850).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akimenko MA, Mari-Beffa M, Becerra J, Geraudie J. Old questions, new tools, and some answers to the mystery of fin regeneration. Dev. Dyn. 2003;226:190–201. doi: 10.1002/dvdy.10248. [DOI] [PubMed] [Google Scholar]
- Alvarez-Medina R, Le Dreau G, Ros M, Martí E. Hedgehog activation is required upstream of Wnt signaling to control neural progenitor proliferation. Development. 2009;136:3301–3309. doi: 10.1242/dev.041772. [DOI] [PubMed] [Google Scholar]
- Bren-Mattison Y, Olwin BB. Sonic hedgehog inhibits the terminal differentiation of limb myoblasts committed to the slow muscle lineage. Dev. Biol. 2002;242:130–148. doi: 10.1006/dbio.2001.0528. [DOI] [PubMed] [Google Scholar]
- Bren-Mattison Y, Hausburg M, Olwin BB. Growth of limb muscle is dependent on skeletal-derived Indian hedgehog. Dev. Biol. 2011;356:486–495. doi: 10.1016/j.ydbio.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat. Rev. Mol. Cell Biol. 2002;3:566–574. doi: 10.1038/nrm881. [DOI] [PubMed] [Google Scholar]
- Campbell LJ, Crews CM. Wound epidermis formation and function in urodele amphibian limb regeneration. Cell Mol. Life. Sci. 2008;65:73–79. doi: 10.1007/s00018-007-7433-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimir CM, Gates PB, Patient RK, Brockes JP. Evidence for dedifferentiation and metaplasia in amphibian limb regeneration from inheritance of DNA methylation. Development. 1988;104:657–668. doi: 10.1242/dev.104.4.657. [DOI] [PubMed] [Google Scholar]
- Caubit X, Nicolas S, Le Parco Y. Possible roles for Wnt genes in growth and axial patterning during regeneration of the tail in urodele amphibians. Dev. Dyn. 1997;210:1–10. doi: 10.1002/(SICI)1097-0177(199709)210:1<1::AID-AJA1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Chablais F, Jazwinska A. IGF signaling between blastema and wound epidermis is required for fin regeneration. Development. 2010;137:871–879. doi: 10.1242/dev.043885. [DOI] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, Beachy PA, Fallon JF. Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev. Biol. 2001;236:421–435. doi: 10.1006/dbio.2001.0346. [DOI] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Duprez D, Fournier-Thibault C, Le Douarin N. Sonic Hedgehog induces proliferation of committed skeletal muscle cells in the chick limb. Development. 1998;125:495–505. doi: 10.1242/dev.125.3.495. [DOI] [PubMed] [Google Scholar]
- Echeverri K, Clarke JD, Tanaka EM. In vivo imaging indicates muscle fiber dedifferentiation is a major contributor to the regenerating tail blastema. Dev. Biol. 2001;236:151–64. doi: 10.1006/dbio.2001.0312. [DOI] [PubMed] [Google Scholar]
- Endo T, Yokoyama H, Tamura K, Ide H. Shh expression in developing and regenerating limb buds of Xenopus laevis. Dev. Dyn. 1997;209:227–232. doi: 10.1002/(SICI)1097-0177(199706)209:2<227::AID-AJA8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Gardiner DM, Endo T, Bryant SV. The molecular basis of amphibian limb regeneration: integrating the old with the new. Semin. Cell Dev. Biol. 2002;13:345–352. doi: 10.1016/s1084952102000903. [DOI] [PubMed] [Google Scholar]
- Globus M, Vethamany-Globus S, Lee YC. Effect of apical epidermal cap on mitotic cycle and cartilage differentiation in regeneration blastemata in the newt, Notophthalmus viridescens. Dev. Biol. 1980;75:358–372. doi: 10.1016/0012-1606(80)90169-4. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the hedgehog/patched signaling pathway from flies to mice: induction of a mouse patched gene by Hedgehog. Genes Dev. 1996;10:301–312. doi: 10.1101/gad.10.3.301. [DOI] [PubMed] [Google Scholar]
- Hanken J, Wassersug R. The visible skeleton. Funct. Photog. 1981;16:22–26. [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Imokawa Y, Yoshizato K. Expression of Sonic hedgehog gene in regenerating newt limb blastemas recapitulates that in developing limb buds. Proc. Natl. Acad. Sci. 1997;94:9159–9164. doi: 10.1073/pnas.94.17.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovine MK. Conserved mechanisms regulate outgrowth in zebrafish fins. Nat. Chem. Biol. 2007;3:613–618. doi: 10.1038/nchembio.2007.36. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Rodriguez Esteban C, Raya M, Kawakami H, Marti M, Dubova I, Izpisua Belmonte JC. Wnt/beta-catenin signaling regulates vertebrate limb regeneration. Genes Dev. 2006;20:3232–3237. doi: 10.1101/gad.1475106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf F, Hammond C, Chekuru A, Kurth T, Hans S, Weber CW, Mahatma G, Fisher S, Brand M, Schulte-Merker S, Weidinger G. Bone regenerates via dedifferentiation of osteoblasts in the zebrafish fin. Dev. Cell. 2011;20:713–724. doi: 10.1016/j.devcel.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Koleva M, Kappler R, Vogler M, Herwig A, Fulda S, Hahn H. Pleiotropic effects of sonic hedgehog on muscle satellite cells. Cell Mol. Life. Sci. 2005;62:1863–1870. doi: 10.1007/s00018-005-5072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragl M, Knapp D, Nacu E, Khattak S, Maden M, Epperlein HH, Tanaka EM. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460:60–65. doi: 10.1038/nature08152. [DOI] [PubMed] [Google Scholar]
- Lentz TL. Cytological studies of muscle dedifferentiation and differentiation during limb regeneration of the newt Triturus. Am. J. Anat. 1969;124:447–479. doi: 10.1002/aja.1001240404. [DOI] [PubMed] [Google Scholar]
- Lewandoski M, Sun X, Martin GR. Fgf8 signalling from the AER is essential for normal limb development. Nature Genet. 2000;26:460–463. doi: 10.1038/82609. [DOI] [PubMed] [Google Scholar]
- Lin G, Slack JM. Requirement for Wnt and FGF signaling in Xenopus tadpole tail regeneration. Dev Biol. 2008;316:323–335. doi: 10.1016/j.ydbio.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Lo DC, Allen F, Brockes JP. Reversal of muscle differentiation during urodele limb regeneration. Proc. Natl. Acad. Sci. 1993;90:7230–7234. doi: 10.1073/pnas.90.15.7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann CJ, Odelberg SJ, Keating MT. Mammalian myotube dedifferentiation induced by newt regeneration extract. Proc. Natl. Acad. Sci. 2001;98:13699–13704. doi: 10.1073/pnas.221297398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A. Interaction of FGF, Ihh/Pthlh, and BMP Signaling Integrates Chondrocyte Proliferation and Hypertrophic Differentiation. Dev. Cell. 2002;3:1–20. doi: 10.1016/s1534-5807(02)00261-7. [DOI] [PubMed] [Google Scholar]
- Morrison JI, Loof S, He P, Simon A. Salamander limb regeneration involves the activation of a multipotent skeletal muscle satellite cell population. J. Cell Biol. 2006;172:433–440. doi: 10.1083/jcb.200509011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panman L, Galli A, Lagarde N, Michos O, Soete G, Zuniga A, Zeller R. Differential regulation of gene expression in the digit forming area of the mouse limb bud by SHH and gremlin 1/FGF-mediated epithelial-mesenchymal signalling. Development. 2006;133:3419–3428. doi: 10.1242/dev.02529. [DOI] [PubMed] [Google Scholar]
- Poss KD, Shen J, Keating MT. Induction of lef1 during zebrafish fin regeneration. Dev. Dyn. 2000;219:282–286. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1045>3.3.co;2-3. [DOI] [PubMed] [Google Scholar]
- Poss KD, Keating MT, Nechiporuk A. Tales of regeneration in zebrafish. Dev. Dyn. 2003;226:202–210. doi: 10.1002/dvdy.10220. [DOI] [PubMed] [Google Scholar]
- Schnapp E, Kragl M, Rubin L, Tanaka EM. Hedgehog signaling controls dorsoventral patterning, blastema cell proliferation and cartilage induction during axolotl tail regeneration. Development. 2005;132:3243–3253. doi: 10.1242/dev.01906. [DOI] [PubMed] [Google Scholar]
- Scherz PJ, McGlinn E, Nissim S, Tabin CJ. Extended exposure to Sonic hedgehog is required for patterning the posterior digits of the vertebrate limb. Dev. Biol. 2007;308:343–354. doi: 10.1016/j.ydbio.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BN, Koyano-Nakagawa N, Garry JP, Weaver CV. Heart of newt: a recipe for regeneration. J. Cardiovasc. Transl. Res. 2010;3:397–409. doi: 10.1007/s12265-010-9191-9. [DOI] [PubMed] [Google Scholar]
- Slack JM. Amphibian muscle regeneration—dedifferentiation or satellite cells? Trends Cell Biol. 2006;16:273–275. doi: 10.1016/j.tcb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Stoick-Cooper CL, Weidinger G, Riehle KJ, Hubbert C, Major MB, Fausto N, Moon RT. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- Stopper GF, Wagner GP. Inhibition of Sonic hedgehog signaling leads to posterior digit loss in Ambystoma mexicanum: parallels to natural digit reduction in urodeles. Dev. Dyn. 2007;236:321–331. doi: 10.1002/dvdy.21025. [DOI] [PubMed] [Google Scholar]
- Tanaka EM. Cell differentiation and cell fate during urodele tail and limb regeneration. Curr. Opin. Genet. Dev. 2003;13:497–501. doi: 10.1016/j.gde.2003.08.003. [DOI] [PubMed] [Google Scholar]
- te Welscher P, Zuniga A, Kuijper S, Drenth T, Goedemans HJ, Meijlink F, Zeller R. Progression of vertebrate limb development through SHH-mediated counteraction of GLI3. Science. 2002;298:827–830. doi: 10.1126/science.1075620. [DOI] [PubMed] [Google Scholar]
- ten Berge D, Brugmann SA, Helms JA, Nusse R. Wnt and FGF signals interact to coordinate growth with cell fate specification during limb development. Development. 2008;135:3247–3257. doi: 10.1242/dev.023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickle C. Making digit patterns in the vertebrate limb. Nat. Rev. Mol. Cell Biol. 2006;7:45–53. doi: 10.1038/nrm1830. [DOI] [PubMed] [Google Scholar]
- Tsonis PA, Vergara MN, Spence JR, Madhavan M, Kramer EL, Call MK, Santiago WG, Vallance JE, Robbins DJ, Del Rio-Tsonis K. A novel role of the hedgehog pathway in lens regeneration. Dev Biol. 2004;267:450–461. doi: 10.1016/j.ydbio.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Verheyden JM, Sun X. An Fgf/Gremlin inhibitory feedback loop triggers termination of limb bud outgrowth. Nature. 2008;454:638–641. doi: 10.1038/nature07085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace H. Vertebrate Limb Regeneration. Wiley and Sons; New York: 1981. [Google Scholar]
- Whitehead GG, Makino S, Lien CL, Keating MT. fgf20 is essential for initiating zebrafish fin regeneration. Science. 2005;310:1957–1960. doi: 10.1126/science.1117637. [DOI] [PubMed] [Google Scholar]
- Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yokoyama H, Ogino H, Stoick-Cooper CL, Grainger RM, Moon RT. Wnt/beta-catenin signaling has an essential role in the initiation of limb regeneration. Dev Biol. 2007;306:170–178. doi: 10.1016/j.ydbio.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama H, Maruoka T, Ochi H, Aruga A, Ohgo S, Ogino H, Tamura K. Different Requirement for Wnt/β-Catenin Signaling in Limb Regeneration of Larval and Adult Xenopus. PLoS One. 2011;6:e21721. doi: 10.1371/journal.pone.0021721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller R. It takes time to make a pinky: unexpected insights into how SHH patterns vertebrate digits. Sci. STKE. 2004;259:pe53. doi: 10.1126/stke.2592004pe53. [DOI] [PubMed] [Google Scholar]
- Zeller R, Lopez-Rios J, Zuniga A. Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat. Rev. Genet. 2009;10:845–858. doi: 10.1038/nrg2681. [DOI] [PubMed] [Google Scholar]
- Zuniga A, Haramis AP, McMahon AP, Zeller R. Signal relay by BMP antagonism controls the SHH/FGF4 feedback loop in vertebrate limb buds. Nature. 1999;401:598–602. doi: 10.1038/44157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Activation of Hh signaling results in enhanced regeneration. (A) qRT-PCR analysis of Ptch1 transcript expression in the regenerating limb tissue at 21d. Treatment with cyclopamine results in significantly reduced Ptch1 expression (n=3; **, p<0.005). (B G) Whole mount and skeletal analysis of regenerating limbs in control (B-D) and Hh agonist treated (E-G) animals at different time periods. Arrowheads indicate the point of amputation. (H-K, I’-K’) Schematic representation and immunohistochemical staining of Shh (red) in uninjured (I, I’), control (J, J’) regenerating limb at 7d. Nuclei are stained with DAPI (blue). Panel I’-K’ shows Shh immunostaining at higher magnification. Expression of Shh is confined to the posterior region of the limb. Scale bars: 2 mm (B-G), 200 μm (IK; I’-K’).
Fig. S2. Hh signaling is required early during limb regeneration. (A-F) Gross morphology and skeletal analysis of the regenerating limbs of the control (A-C) and cyclopamine treated (D-F) animals between 0-21 days [Cyclo (0-21d)]. (G) Quantification of the regenerating limb from control (white bar), cyclopamine (0-21d) (black bar) treated regenerates at specified time periods. Data points represent average length from multiple experiments (n=12 animals; **, p<0.005). Error bars indicate s.e.m. Scale bars: 2 mm.
Fig. S3. Hh is not required for the dedifferentiation process. (A-D) TEM images of the tissues from control (A, C) and cyclopamine (B, D) treated injured limbs. Injury results in disorganized sacromeres and loss of Z-bands along with large intracellular spaces. (E, H) H & E staining of the regenerating tissue from control (E) and cyclopamine (H) treated animals show fragmented myofibers. (F, I) MHC staining (green) of the regenerating tissue from control (F) and cyclopamine (I) treated animals. Fragmented myofibers stained positive for MHC (white arrows) can be seen in the regenerating area. (G, J) TEM images of the tissue from control (G) and cyclopamine (J) treated animals. Cell membrane bound cellular components were seen in both control and cyclopamine treated tissues. Scale bars: 1 μm (A, B); 2 μm (D, G); 5 μm (C, J); 200 μm (E, F, H, I).
Fig. S4. Hh signaling alters myogenic proliferation. (A-D) Immunohistochemical analysis of the regenerating tissues using anti-BrdU sera (red) display reduced proliferation upon Hh inhibition (C, D). (E) CyclinD1-reporter (CCND1-luc) transcriptional assay using C2C12 myoblast cells untreated and treated with cyclopamine. Inhibition of Hh signaling resulted in marked reduction in the luciferase activity. Error bars indicate s.e.m. (F-K) shows the immunostaining of MHC (green) and BrdU (red) in the regenerating tissue. Note the absence of MHC stained cells together with BrdU, indicating lack of proliferation in mature myofibers. Scale bar: 50 μm.
Fig. S5. Wnt signaling is essential for limb regeneration. (A-F) Whole mount and skeletal analysis of the regenerating limbs at 7d and 60d from BIO (Wnt activator) (A-C), and Wnt inhibitor (D-F) treated animals. Arrowheads indicate the point of amputation. (G L) Histological analysis of the regenerating limb from control, BIO and cyclopamine+BIO treated animals at lower magnification (G, I, K) and at higher magnification (H, J, L) showing absence of thickened basement membrane between wound epithelium and underlying mesenschyme. Scale bars: 2 mm (A-F), 200 μm (G, L).
Table S1: PCR Primers used for Quantitative RT-PCR analysis