Abstract
Acute kidney injury (AKI) is a disease with mitochondrial dysfunction and a newly established risk factor for the development of chronic kidney disease (CKD) and fibrosis. We examined mitochondrial homeostasis in the folic acid (FA)-induced AKI model that develops early fibrosis over a rapid time course. Mice given a single dose of FA had elevated serum creatinine (3-fold) and urine glucose (2.2-fold) 1 and 2 d after injection that resolved by 4 d. In contrast, peroxisome proliferator gamma coactivator 1α (PGC-1α) and mitochondrial transcription factor A (TFAM), critical transcriptional regulators of mitochondrial biogenesis (MB), were down-regulated ~80% 1 d after FA injection and remained depressed through 14 d. Multiple electron transport chain and ATP synthesis genes were also down-regulated from 1–14 d after FA, including NADH dehydrogenase (ubiquinone) 1 beta subcomplex 8 (NDUFβ8), ATP synthase subunit β (ATPS-β), and cytochrome C oxidase subunit I (COXI). Mitochondrial DNA copy number was reduced ~50% from 2–14 d after FA injection. Protein levels of early fibrosis markers α-smooth muscle actin and transforming growth factor β1 were elevated at 6 and 14 d after FA. Picro-sirius red staining and collagen 1A2 (COL1A2) IHC revealed staining for mature collagen deposition at 14 d. We propose that mitochondrial dysfunction induced by AKI is a persistent cellular injury that promotes progression to fibrosis and CKD, and that this model can be used to test mitochondrial therapeutics that limit progression to fibrosis and CKD.
Keywords: Acute kidney injury, mitochondrial biogenesis, fibrosis, folic acid
1. Introduction
Acute kidney injury (AKI) is defined as a rapid and reversible decline in renal function. AKI affects 40–60% of intensive-care unit patients and incidence rates remain unchanged (Bellomo et al., 2012; Thadhani et al., 1996). Drugs, toxicants, ischemia/reperfusion (I/R), and sepsis are common causes of AKI and lead to reduced glomerular filtration and tubular necrosis. One mechanism of drug and toxicant-induced renal injury is crystal formation in the lumen, as observed with uric acid, acyclovir, and calcium oxalate, the molecule responsible for renal toxicity of ethylene glycol (Thadhani et al., 1996). When given intraperitoneally at high doses (e.g. 250 mg/kg), folic acid (FA) causes AKI in rodents (Brade et al., 1970; Long et al., 2008). The mechanism of FA nephropathy may be due to the formation of luminal crystals at these doses (Schmidt et al., 1973); however, FA also has direct toxicity on the tubular epithelium at high doses (Fink et al., 1987). The Reference Daily Intake for adult humans provided by the Food and Drug Administration is 400 μg. While renal toxicity of FA has not been reported in humans, FA-induced nephrotoxicity has been used as a model of AKI.
CKD is defined as a consistent reduction in the glomerular filtration rate and typically progresses to end stage renal disease (ESRD) (Levey and Coresh, 2012). CKD affects over 750,000 people in the US and the incidence is rising due to increasing rates of diabetes and hypertension (Levey and Coresh, 2012). Tubulointerstitial fibrosis (TIF) is the best predictive marker of progression to ESRD (Fine and Norman, 2008) and development of TIF is observed following I/R- and toxicant-induced AKI (Kim et al., 2009; Yang et al., 2010). Fibrotic lesions damage the peritubular vasculature (Choi et al., 2000; Yuan et al., 2003), and lead to a state of chronic hypoxia in the nephron, promoting sustained oxidative stress (Kim et al., 2009; Seok et al., 2008).
Recent advances in nephrology research demonstrate a causal role for AKI in CKD development. For example, AKI is a major risk factor for CKD, increasing the risk of progression to CKD as much as 28-fold (Lo et al., 2009). In addition, the severity of injury in the acute phase of AKI is a highly effective indicator of progression to CKD (Chawla et al., 2011). In mice, CKD and fibrosis have been shown to follow AKI caused by FA, I/R, and aristolichic acid (Leelahavanichkul et al., 2010; Yang et al., 2010). CKD progression in the models is dependent upon the extent of AKI (Yang et al., 2010) and preventable with therapeutic intervention during the acute phase (Kapitsinou et al., 2012). These studies form the basis of our analysis of mitochondrial dysfunction in the AKI to CKD continuum.
Mitochondrial dysfunction is a recognized pathogenic element of AKI and cause of tubular cell dysfunction and death. Increased reactive oxygen species (ROS) and reactive nitrogen species (RNS) production, decreased ATP production, and cytochrome c release are frequently associated with epithelial cell injury in AKI (Plotnikov et al., 2007; Szeto et al., 2011; Zager et al., 2004). Our group recently reported persistent disruption of mitochondrial homeostasis and suppression of mitochondrial biogenesis (MB) following I/R- and glycerol-induced AKI (Funk and Schnellmann, 2011). In both models, renal mitochondrial proteins cytochrome c oxidase subunit I (COXI), ATP synthase subunit β (ATPS-β) and NADH dehydrogenase (ubiquinone) 1 beta subcomplex 8 (NDUFβ8) were depleted, indicative of mitochondrial damage and suppressed MB (Funk and Schnellmann, 2011). Over-expression of PGC-1α, the master regulator or MB, in renal proximal tubule cells restored mitochondrial and cellular functions after oxidant exposure, demonstrating the importance of MB in recovery from cellular injury (Rasbach and Schnellmann, 2007). While the mechanisms of maladaptive repair of the tubular epithelium after AKI are still unclear, it can lead to TIF through paracrine activation of resident fibroblasts and epithelial-mesenchymal transition (EMT) of renal epithelial cells (Iwano et al., 2002; Lan et al., 2012). Interestingly, mitochondrial-derived ROS can induce EMT in renal tubular cells in vitro, and restoration of functional mitochondria and antioxidant mechanisms by induction of PGC-1α attenuates this transition (Hallman et al., 2008; Yuan et al., 2012). However, little is known about the role of mitochondrial function in renal fibrosis. Here we report that persistent mitochondrial dysfunction is linked to early renal fibrosis in a model of FA-induced AKI.
2. Methods
2.1. Animal Model
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina and all efforts were made to minimize animal suffering. Male CD-1 mice (8–10 weeks of age, Harlan Laboratories) were injected with a single intraperitoneal dose of 250 mg/kg FA (Sigma) dissolved in 300 mM NaHCO3 as previously described (Wen et al., 2012). At 0, 1, 2, 4, 6, 10, and 14 d after treatment, serum and urine samples were collected. Urine was collected overnight from mice housed in metabolic cages (18 h collection period). Serum and urine creatinine, blood urea nitrogen (BUN), and urine glucose levels were measured using Quantichrom Assay Kits (BioAssay Sytems). Urine glucose was normalized to urine creatinine. Urine osmolality was measured using the 5004 Micro-Osmette (Precision Systems). Mice were euthanized 0, 1, 2, 6, and 14 d after treatment by CO2 asphyxiation. These time points were chosen to capture the acute, recovery and chronic phases of FA-induced AKI. Kidneys were removed and preserved either by snap freezing or formalin fixation and paraffin embedding.
2.2. Histological Analysis
Kidneys were sectioned and stained with Periodic Acid Schiff (PAS) for the evaluation of histology. Fibrillar collagen content was evaluated using picro-sirius red staining. Briefly, slides were deparaffinized and rehydrated, stained with Weigert’s Iron hematoxylin for 10 min, and washed with water for 10 min. Slides were then stained with picro-sirius red (0.1% direct red 80 CI#35780 in saturated aqueous picric acid) for 1 h, washed twice in 1% glacial acetic acid, dehydrated, cleared with xylene, mounted with Permount and examined under plane polarized light. Immunohistochemistry was performed as previously described (Korrapati et al., 2012) using the collagen 1A2 (COL1A2) antibody (Santa Cruz).
2.3. mRNA Analysis
Total RNA was isolated from renal cortex with Trizol (Invitrogen) and reverse transcription was performed using the iScript Advanced cDNA Synthesis Kit (Bio-Rad) with 2 μg RNA. qPCR was performed using SsoAdvanced SYBR Green Supermix (Bio-Rad). mRNA expression of all genes was calculated using the 2-ΔΔCT method normalized to β-actin. Primer sequences for PGC-1α, TFAM, NDUFβ8, COX1, and ATPS-β were described previously (Funk and Schnellmann, 2011). Additional primer sequences were as follows: COL1A2: sense 5′-TGTTGGCCCATCTGGTAAAGA-3′, antisense 5′-CAGGGAATCCGATGTTGCC-3′; β-actin: sense 5′-GGGATGTTTGCTCCAACCAA-3′, antisense 5′-GCGCTTTTGACTCAAGGATTTAA-3′.
2.4. Immunoblot Analysis
Protein lysates were isolated from renal cortex using RIPA buffer containing mammalian protease inhibitor cocktail, 1 mM sodium orthovanadate, and 10 mM sodium fluoride (Sigma). Proteins were separated on a 4–15% SDS-PAGE gel and transferred to a nitrocellulose membrane before blocking in 2.5% BSA. Membranes were incubated with primary antibody at 4°C overnight. Primary antibodies used were α-smooth muscle actin (α-SMA) (1:1000, Sigma), transforming growth factor β1 (TGF-β1) (1:1000, Abcam), neutrophil gelatinase-associated lipocalin 2 (NGAL) (1:1000, Abcam) and β-actin (1:500, Santa Cruz). Membranes were then incubated with an appropriate horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h. Bound antibody was imaged following chemiluminescent visualization using a GE ImageQuant LAS4000 standalone imaging system. Densitometry of Western blots was performed using NIH Image J software.
2.5. Mitochondrial DNA Content
qPCR was used to determine relative quantities of mitochondrial DNA content in renal cortex as previously described (Funk et al., 2010). Briefly, the DNeasy Blood and Tissue kit (QIAGEN, Valencia, CA) was used for genomic DNA extraction and 5 ng of total DNA was used for qPCR. Mitochondrially encoded NADH dehydrogenase 1 (ND1) was used to measure mitochondrial copy number and was normalized to nuclear β-actin. Primer sequences were as follows: ND1: sense 5′-TAGAACGCAAAATCTTAGGG-3′, antisense 5′-TGCTAGTGTGAGTGATAGGG-3′; β-actin: sense 5′-GGGATGTTTGCTCCAACCAA-3′, antisense 5′-GCGCTTTTGACTCAAGGATTTAA-3′.
2.6. Statistical Analysis
Data are presented as means ± SEM and were subjected to one-way analysis of variance (ANOVA). Multiple means were compared using Student-Newman-Keuls test with p<0.05 considered to be a statistically significant difference between means.
3. Results and Discussion
3.1. FA Induces AKI with Rapid Functional Recovery
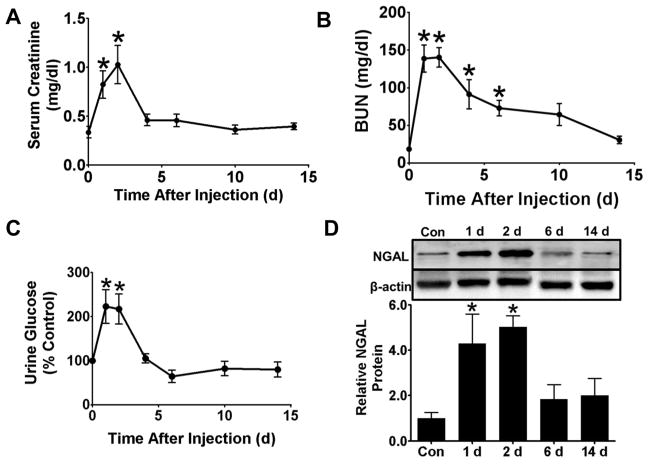
FA decreased glomerular function as serum creatinine levels increased ~3-fold versus controls 1–2 d after FA injection and returned to control levels at 4 d (Fig 1A). BUN concentrations increased ~8-fold versus controls 1–2 d after FA injection, and did not return to control levels until 10 d (Fig 1B). Over 90% of mice developed AKI, defined as a doubling of serum creatinine or BUN levels at 2 d. As an indicator of tubular function we measured urinary glucose concentrations, which increased 2-fold over controls 1–2 d after FA injection (Fig 1C). Urinary glucose concentrations returned to control levels at 4 d. Renal NGAL protein levels also increased ~4-fold 1–2 d after FA injection and returned to control levels at 6 d (Fig 1D).
Figure 1.
Folic acid (FA) causes AKI. Two days after a 250 mg/kg intraperitoneal dose of FA, serum creatinine (A), BUN (B), and urine glucose (C) levels were maximally elevated. Both serum creatinine and urine glucose concentrations returned to control levels at 4 d. BUN concentrations returned to control levels at 10 d. Protein levels of renal NGAL increased at 1 and 2 d but returned to control levels at 6 and 14 d (D). Data are represented as mean ± SEM. *, p < 0.05 vs control. N = 8–19 (A and B), 7–21 (C), 4–7 (D).
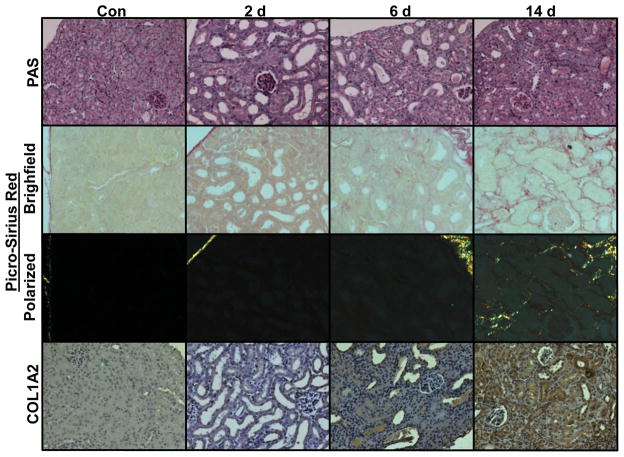
Renal histopathology was examined using PAS staining at 2, 6, and 14 d. At 2 d the renal cortex displayed tubular dilatation, brush border loss, cast formation, and accumulation of debris in the lumen. At 6 d there was a partial restoration of normal renal architecture characterized by repopulation of the tubular epithelial cells, but brush border restoration remained incomplete at 14 d (Fig 2).
Figure 2.
Histopathology of progressive FA nephropathy. Kidneys display massive tubular dilatation and brush border loss at 2 d via PAS staining (top row) that partially recovered at 6 and 14 d. Picro-sirius red staining showed deposition of fibrillar collagen at 14 d using brightfield (second row) and polarized (third row) light microscopy. Immunohistochemical detection of COL1A2 (bottom row) showed strong staining only at 14 d after FA injection. Representative images shown, N = 3–8.
In summary, peak renal dysfunction occurred 2 d after FA administration, as indicated by maximal levels of serum creatinine, BUN, urine glucose, and the injury marker NGAL. All markers returned to control levels by 4 d except BUN, which gradually decreased over time and returned to control levels at 10 d. In contrast to the recovery of glomerular and tubular function, persistent disruption of normal tubular morphology was observed in PAS-stained kidneys through 14 d, revealing incomplete recovery.
3.2 FA-Induced AKI Suppresses Mitochondrial Homeostasis and Biogenesis Rapidly and Persistently
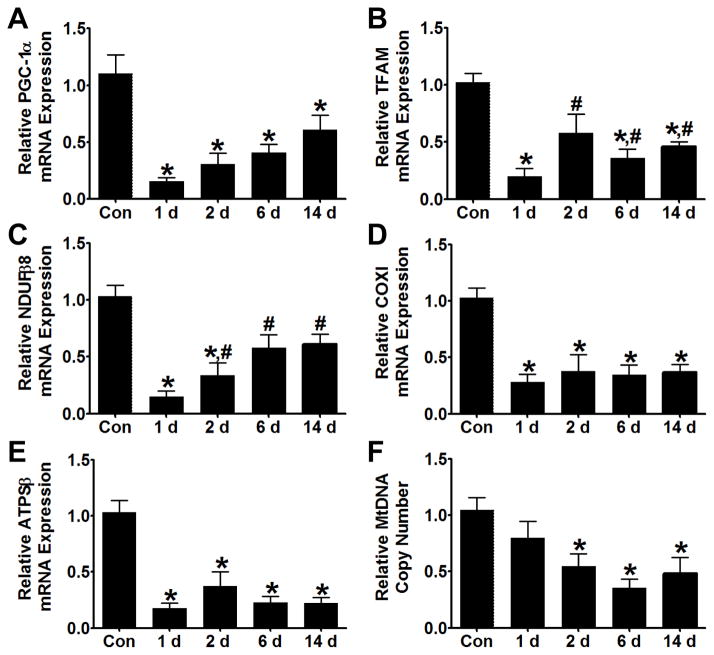
To determine if FA nephrotoxicity disrupts mitochondrial homeostasis and biogenesis, transcript levels of mitochondrial markers of homeostasis and biogenesis were measured in renal cortex over time. mRNA levels of the transcription factor coactivator PGC-1α and mitochondrial transcription factor A (TFAM) decreased by ~80% 1 d after FA treatment (Fig. 3A, B). PGC-1α levels remained reduced throughout the 14 d experiment while TFAM levels partially recovered to ~60% of controls at 2 d and remained depressed at 6 and 14 d. One day after FA injection, transcript levels of NDUFβ8, ATPS-β, and COXI decreased 85%, 83%, and 72%, respectively (Fig, 3C-E). NDUFβ8 levels partially recovered at 6 d but remained depressed at 14 d. COXI and ATPS-β transcript levels remained depressed throughout the 14 d of the experiment. Finally, we measured mitochondrial DNA copy number as an indicator of mitochondrial content. Mitochondrial DNA copy number was reduced ~50% 2 d after FA injection and remained depressed through 14 d (Fig 3F). To control for possible variation in expression of the mitochondrial markers over the course of the study, a separate experiment was performed using time-matched controls. Over 14 d mRNA expression did not change in control mice, while mitochondrial markers were suppressed in FA-treated mice (Fig S1).
Figure 3.
Mitochondrial biogenesis is persistently down-regulated during FA nephropathy. mRNA levels of PGC-1α (A) and TFAM (B) were reduced 1 d after FA injection. PGC-1α remained depressed while TFAM mRNA partially recovered at 2 d and did not increase further through 14 d. Expression of mitochondrial homeostasis markers NDUFβ8 (C), COX1 (D), and ATPSβ (E) was also strongly suppressed at 1 d, and only NDUFβ8 reached a partial recovery at 6 and 14 d. Mitochondrial copy number was reduced by ~50% 2 d after FA injection and remained persistently depressed at 6 and 14 d (F). Data are represented as mean ± SEM. *, p < 0.05 vs control, # p < 0.05 vs 1 d. N = 4–8.
In summary, numerous markers of mitochondrial homeostasis were strongly suppressed at 1 d and remained suppressed through 14 d. It should be noted that TFAM and NDUFβ8 partially recovered to 50% and 60% of control, respectively. Importantly, mitochondrial DNA copy number, an indirect marker of mitochondrial content was also decreased from 2–14 d. These data reveal that the disruption of mitochondrial homeostasis and content begins with the onset of AKI and persists, consistent with the disruption of tubular morphology. The persistent loss of mitochondrial homeostasis in this model is similar to that reported in AKI caused by I/R and myoglobulinuria (Funk and Schnellmann, 2011). Furthermore, it is interesting that the early recovery of glomerular and tubular function is not dependent on the up-regulation of these genes.
3.3. FA-Induced AKI and Mitochondrial Dysfunction is Followed by Fibrosis
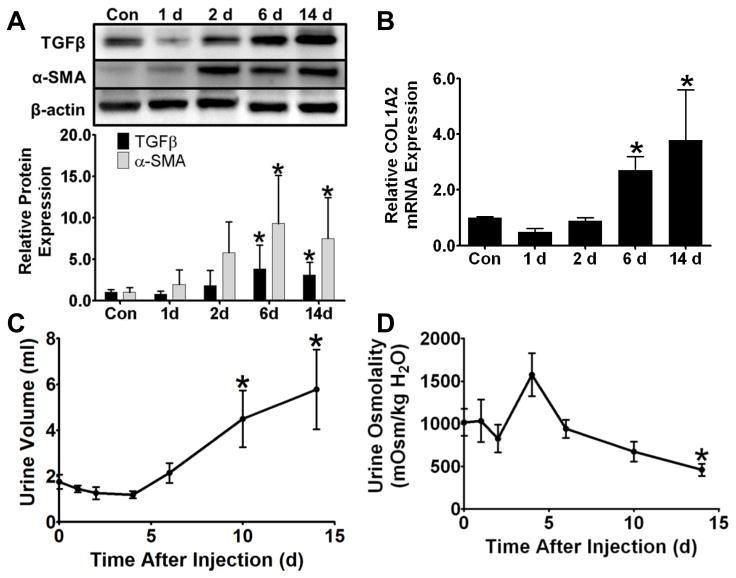
Protein levels of early fibrosis markers TGF-β1 and α-SMA increased at 6 and 14 d after FA injection with a maximum induction of ~4- and ~9-fold, respectively (Fig 4A). Transcript levels of COL1A2 (Fig 4B), a primary transcriptional target of TGF-β1 and pathological molecule in fibrosis, increased at 6 and 14 d, in concert with the increases in TGF-β1. COL1A2 mRNA also increased versus 14 d controls in the separate time-matched study (Fig S1).
Figure 4.
Early fibrosis and CKD in FA nephropathy. TGF-β1 and α-SMA protein remained at control levels through 2 d but increased at 6 and 14 d (A). COL1A2 mRNA was significantly increased in renal cortex 6 and 14 d after FA treatment (B). Urine volume increased 10 and 14 d after FA injection (C), while urine osmolality decreased at 14 d (D). Data are represented as mean ± SEM. *, p < 0.05 vs control. N = 5–8 (A), 4–10 (B), 8–20 (C), 6–7 (D).
We measured urine output and osmolality as indicators of CKD. Urine output was similar to controls from 1–6 d after FA injection but increased at 10 and 14 d. (Fig 4C). Urine osmolality decreased 14 d after FA injection (Fig 4D).
Kidney sections were stained with picro-sirius red to examine the development of TIF. When examined using bright-field microscopy, the red staining is indicative of general ECM deposition (Fig 2). We observed very low levels of red staining in control kidneys and in kidneys 2 and 6 d after FA injection. However, at 14 d there was a marked increase in red staining in the tubulointerstitium. We also examined the picro-sirius red staining under polarized light, which allows specific visualization of fibrillar collagen (Junqueira et al., 1979). There was no detectable kidney collagen staining in controls or at 2–6 d following FA treatment, but there was an intense tubulointerstitial staining pattern at 14 d (Fig 2). We used immunohistochemistry to examine the levels of COL1A2. Low levels of staining were observed through 6 d after FA injection, but at 14 d there was a strong increase in the COL1A2 positive staining in the renal cortex (Fig 2).
In conclusion, while glomerular and tubular function recovered, early fibrosis occurred at 6 d and continued through 14 d following FA treatment. Molecular markers of fibrosis including TGF-β1 and α-SMA increased 6 and 14 d after FA, and picro-sirius red staining and IHC for COL1A2 showed increases in staining 14 d after FA injection. The changes in these markers are consistent with the persistent disruption of tubular morphology. Additionally, increases in urine volume were observed at 6 and 14 d, and urine osmolality decreased at 14 d. Decreased ability to concentrate the urine is seen in CKD patients (Schrier, 2010). CKD has been characterized in the FA model, with decreased glomerular function and microalbuminuria at 2 and 12 weeks (Doi et al., 2008; Leelahavanichkul et al., 2010). We did not detect increases in urinary albumin or total protein in FA-treated mice at 14 d (Fig S2). This finding could be due to the early stage of fibrosis at 14 d in this study. In summary, the FA model under these conditions represents early AKI with recovery of multiple functions within 4 d that is followed by early fibrosis and CKD.
The above studies provide the first evidence of a link between suppression of the MB program and the AKI to CKD continuum. However, while the sustained suppression of MB is correlated with development of early fibrosis and CKD, it is not clear if early fibrosis and CKD are dependent on MB suppression. Hickey et al., reported increased PGC-1α protein in a unilateral ureteral obstruction (UUO) model of renal fibrosis (2011). However, the authors did not measure any PGC-1α targets or functional mitochondrial parameters in vivo. In skeletal muscle, it has been shown that tissue-specific over-expression of PGC-1α slows the age-dependent development of fibrosis (Wenz et al., 2009). In addition, the severe cardiomyopathy induced by anthracycline, which includes fibrosis as a hallmark, is associated with decreased cardiac MB and increased oxidative stress (Suliman et al., 2007). These studies support our findings that the suppression of MB by AKI is involved in the development of renal fibrosis.
The AKI-CKD continuum is recently established and many questions remain regarding clinical progression and pathophysiological mechanisms. A single episode of AKI is known to increase the risk of CKD (Ishani et al., 2009; Wald et al., 2009), and the severity of the acute injury is predictive of progression to CKD (Chawla et al., 2011). TIF, a pathological hallmark of CKD, is an effective predictor of declining renal function (Farris et al., 2011), and work in animal models has addressed mechanisms of fibrogenesis after AKI. Inhibition of prolyl-4-hydroxylase domain (PHD)-containing dioxygenases, which promote degradation of hypoxia inducible factors (HIF) 1 and 2, reduces I/R-induced AKI and subsequent fibrogenesis (Kapitsinou et al., 2012). The effect was only observed when PHD inhibitors were administered before I/R (Kapitsinou et al., 2012) and suggests that the well-known activity of HIF in regulating cellular metabolism could be a critical factor in the early response to AKI and the maladaptive repair and fibrogenesis that develop afterwards. In addition, repeated, selective injury of renal epithelial cells was sufficient to cause fibrosis using a genetically engineered model expressing the diphtheria toxin (DT) receptor in the renal epithelium (Grgic et al., 2012). This suggests a key role for epithelia in renal fibrogenesis. Finally, hyper-methylation of the promoter for RASAL1, an inhibitor of the RAS oncoprotein, was also found to promote renal fibrosis after FA-induced AKI and was attenuated by 5-azacytidine or DNA methytransferase 1 haploinsufficiency (Bechtel et al., 2010). We did not examine promoter methylation in this study, but these findings raise the possibility that epigenetic mechanisms could play a role in silencing expression of MB pathway genes and progression to fibrosis.
Supplementary Material
Supplementary Figure 1. Time-matched control study. mRNA levels of mitochondrial genes PGC-1α, ATPSβ, and COXI were equivalent in control mice at day 0 and day 14, and decreased in FA mice at day 14. The fibrosis marker COL1A2 increased 14 d after FA (B). Data are represented as mean ± SEM. *, p < 0.05 vs 0 d and 14 d Con. N = 5–6.
Supplementary Figure 2. Measurement of Urinary Albumin and Protein in FA Nephropathy. There was no statistical change in the albumin:creatinine ratio (A) or protein:creatinine ratio (B) in the urine of mice 14 d after FA injection. Data are represented as mean ± SEM. N = 4 (A) and 8 (B).
Highlights.
Folic acid causes robust acute kidney injury with functional recovery in six days.
Renal mitochondrial biogenesis is suppressed from 1 – 14 d after injury.
Renal fibrosis develops two weeks after folic acid treatment.
We report the first connection between AKI, mitochondrial biogenesis, and fibrosis.
Acknowledgments
This study was supported by the National Institutes of Health [R01 GM084147 to R.G.S., T32 HL007260 and F32DK098053 to L.J.S., and F30DK096964 to R.M.W.],, and by the Biomedical Laboratory Research and Development Program of the Department of Veterans Affairs [BX000851]. This publication was supported, in part, by the SC COBRE in Oxidants, Redox Balance and Stress Signaling [P20 GM103542], and the South Carolina Clinical and Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina CTSA, and funded by the National Institutes of Health [UL1 RR029882].
Footnotes
5. Conflict of Interest Statemnt: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
L. Jay Stallons, Email: stallons@musc.edu.
Ryan M. Whitaker, Email: whitakr@musc.edu.
References
- Bechtel W, McGoohan S, Zeisberg EM, Muller GA, Kalbacher H, Salant DJ, Muller CA, Kalluri R, Zeisberg M. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med. 2010;16:544–550. doi: 10.1038/nm.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- Brade W, Herken H, Merker HJ. Regeneration of renal tubular cells after lesion by temporary ischaemia, folic acid, and 2,4,5-triamino 6-styrylpyrimidine. Naunyn-Schmiedebergs Archiv fur Pharmakologie. 1970;266:95–100. doi: 10.1007/BF00997785. [DOI] [PubMed] [Google Scholar]
- Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79:1361–1369. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Chakraborty S, Nguyen V, Nguyen C, Kim BK, Shim SI, Suki WN, Truong LD. Peritubular capillary loss is associated with chronic tubulointerstitial injury in human kidney: altered expression of vascular endothelial growth factor. Hum Pathol. 2000;31:1491–1497. doi: 10.1053/hupa.2000.20373. [DOI] [PubMed] [Google Scholar]
- Doi K, Leelahavanichkul A, Hu X, Sidransky KL, Zhou H, Qin Y, Eisner C, Schnermann J, Yuen PS, Star RA. Pre-existing renal disease promotes sepsis-induced acute kidney injury and worsens outcome. Kidney Int. 2008;74:1017–1025. doi: 10.1038/ki.2008.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris AB, Adams CD, Brousaides N, Della Pelle PA, Collins AB, Moradi E, Smith RN, Grimm PC, Colvin RB. Morphometric and visual evaluation of fibrosis in renal biopsies. J Am Soc Nephrol. 2011;22:176–186. doi: 10.1681/ASN.2009091005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine LG, Norman JT. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 2008;74:867–872. doi: 10.1038/ki.2008.350. [DOI] [PubMed] [Google Scholar]
- Fink M, Henry M, Tange JD. Experimental folic acid nephropathy. Pathology. 1987;19:143–149. doi: 10.3109/00313028709077125. [DOI] [PubMed] [Google Scholar]
- Funk JA, Odejinmi S, Schnellmann RG. SRT1720 induces mitochondrial biogenesis and rescues mitochondrial function after oxidant injury in renal proximal tubule cells. J Pharmacol Exp Ther. 2010;333:593–601. doi: 10.1124/jpet.109.161992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk JA, Schnellmann RG. Persistent Disruption of Mitochondrial Homeostasis after Acute Kidney Injury. Am J Physiol Renal Physiol. 2011;302:F853–F864. doi: 10.1152/ajprenal.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, Humphreys BD, Bonventre JV. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallman MA, Zhuang S, Schnellmann RG. Regulation of dedifferentiation and redifferentiation in renal proximal tubular cells by the epidermal growth factor receptor. J Pharmacol Exp Ther. 2008;325:520–528. doi: 10.1124/jpet.107.134031. [DOI] [PubMed] [Google Scholar]
- Hickey FB, Corcoran JB, Docherty NG, Griffin B, Bhreathnach U, Furlong F, Martin F, Godson C, Murphy M. IHG-1 promotes mitochondrial biogenesis by stabilizing PGC-1alpha. J Am Soc Nephrol. 2011;22:1475–1485. doi: 10.1681/ASN.2010111154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Kapitsinou PP, Jaffe J, Michael M, Swan CE, Duffy KJ, Erickson-Miller CL, Haase VH. Preischemic targeting of HIF prolyl hydroxylation inhibits fibrosis associated with acute kidney injury. Am J Physiol Renal Physiol. 2012;302:F1172–1179. doi: 10.1152/ajprenal.00667.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Seok YM, Jung KJ, Park KM. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol. 2009;297:F461–470. doi: 10.1152/ajprenal.90735.2008. [DOI] [PubMed] [Google Scholar]
- Korrapati MC, Shaner BE, Schnellmann RG. Recovery From Glycerol-Induced Acute Kidney Injury Is Accelerated By Suramin. J Pharmacol Exp Ther. 2012;341:126–136. doi: 10.1124/jpet.111.190249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R, Geng H, Polichnowski AJ, Singha PK, Saikumar P, McEwen DG, Griffin KA, Koesters R, Weinberg JM, Bidani AK, Kriz W, Venkatachalam MA. PTEN loss defines a TGF-beta-induced tubule phenotype of failed differentiation and JNK signaling during renal fibrosis. Am J Physiol Renal Physiol. 2012;302:F1210–1223. doi: 10.1152/ajprenal.00660.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leelahavanichkul A, Yan Q, Hu X, Eisner C, Huang Y, Chen R, Mizel D, Zhou H, Wright EC, Kopp JB, Schnermann J, Yuen PS, Star RA. Angiotensin II overcomes strain-dependent resistance of rapid CKD progression in a new remnant kidney mouse model. Kidney Int. 2010;78:1136–1153. doi: 10.1038/ki.2010.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Hsu CY. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76:893–899. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long DA, Price KL, Ioffe E, Gannon CM, Gnudi L, White KE, Yancopoulos GD, Rudge JS, Woolf AS. Angiopoietin-1 therapy enhances fibrosis and inflammation following folic acid-induced acute renal injury. Kidney Int. 2008;74:300–309. doi: 10.1038/ki.2008.179. [DOI] [PubMed] [Google Scholar]
- Plotnikov EY, Kazachenko AV, Vyssokikh MY, Vasileva AK, Tcvirkun DV, Isaev NK, Kirpatovsky VI, Zorov DB. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int. 2007;72:1493–1502. doi: 10.1038/sj.ki.5002568. [DOI] [PubMed] [Google Scholar]
- Rasbach KA, Schnellmann RG. PGC-1alpha over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun. 2007;355:734–739. doi: 10.1016/j.bbrc.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Schmidt U, Torhorst J, Huguenin M, Dubach UC. Acute renal failure after folate: NaK ATPase in isolated rat renal tubule. Ultramicrochemical and clinical studies. European journal of clinical investigation. 1973;3:169–178. doi: 10.1111/j.1365-2362.1973.tb00346.x. [DOI] [PubMed] [Google Scholar]
- Schrier RW, editor. Renal and Electrolyte Disorders. Lippincott Williams & Wilkins; Philadelphia, PA: 2010. [Google Scholar]
- Seok YM, Kim J, Park MJ, Boo YC, Park YK, Park KM. Wen-pi-tang-Hab-Wu-ling-san attenuates kidney fibrosis induced by ischemia/reperfusion in mice. Phytother Res. 2008;22:1057–1063. doi: 10.1002/ptr.2440. [DOI] [PubMed] [Google Scholar]
- Suliman HB, Carraway MS, Ali AS, Reynolds CM, Welty-Wolf KE, Piantadosi CA. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest. 2007;117:3730–3741. doi: 10.1172/JCI32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto HH, Liu S, Soong Y, Wu D, Darrah SF, Cheng FY, Zhao Z, Ganger M, Tow CY, Seshan SV. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 2011;22:1041–1052. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA-J Am Med Assoc. 2009;302:1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- Wen X, Peng Z, Li Y, Wang H, Bishop JV, Chedwick LR, Singbartl K, Kellum JA. One dose of cyclosporine A is protective at initiation of folic acid-induced acute kidney injury in mice. Nephrol Dial Transplant. 2012;27:3100–3109. doi: 10.1093/ndt/gfr766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A. 2009;106:20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–543. doi: 10.1038/nm.2144. 531p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan HT, Li XZ, Pitera JE, Long DA, Woolf AS. Peritubular capillary loss after mouse acute nephrotoxicity correlates with down-regulation of vascular endothelial growth factor-A and hypoxia-inducible factor-1 alpha. Am J Pathol. 2003;163:2289–2301. doi: 10.1016/s0002-9440(10)63586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Chen Y, Zhang P, Huang S, Zhu C, Ding G, Liu B, Yang T, Zhang A. Mitochondrial dysfunction accounts for aldosterone-induced epithelial-to-mesenchymal transition of renal proximal tubular epithelial cells. Free Radic Biol Med. 2012;53:30–43. doi: 10.1016/j.freeradbiomed.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Zager RA, Johnson AC, Hanson SY. Proximal tubular cytochrome c efflux: determinant, and potential marker, of mitochondrial injury. Kidney Int. 2004;65:2123–2134. doi: 10.1111/j.1523-1755.2004.00638.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Time-matched control study. mRNA levels of mitochondrial genes PGC-1α, ATPSβ, and COXI were equivalent in control mice at day 0 and day 14, and decreased in FA mice at day 14. The fibrosis marker COL1A2 increased 14 d after FA (B). Data are represented as mean ± SEM. *, p < 0.05 vs 0 d and 14 d Con. N = 5–6.
Supplementary Figure 2. Measurement of Urinary Albumin and Protein in FA Nephropathy. There was no statistical change in the albumin:creatinine ratio (A) or protein:creatinine ratio (B) in the urine of mice 14 d after FA injection. Data are represented as mean ± SEM. N = 4 (A) and 8 (B).