Abstract
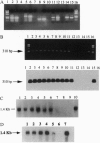
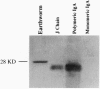
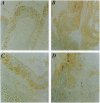
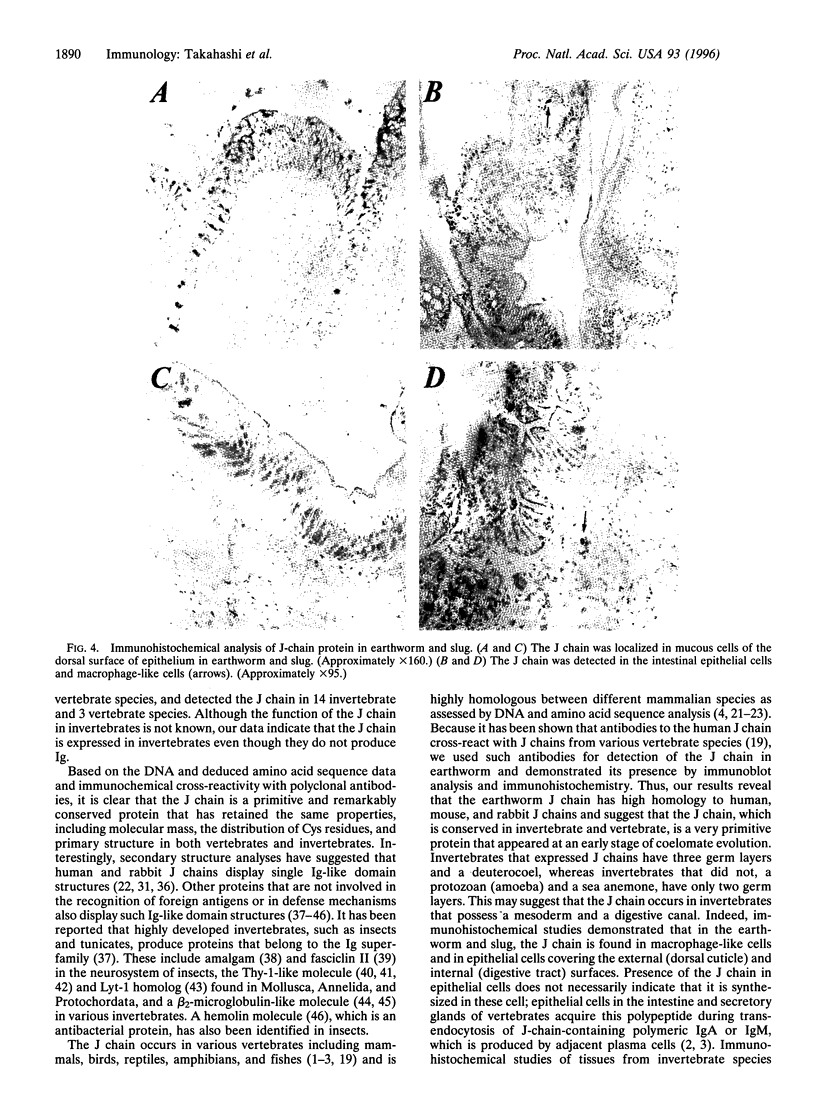
Joining (J) chain is a component of polymeric, but not monomeric, immunoglobulin (Ig) molecules and may play a role in their polymerization and transport across epithelial cells. To date, study of the J chain has been confined to vertebrates that produce Ig and in which the J chain displays a considerable degree of structural homology. The role of the J chain in Ig polymerization has been questioned and, since the J chain can be expressed in lymphoid cells that do not produce Ig, it is possible that the J chain may have other functions. To explore this possibility, we have surveyed J-chain gene, mRNA, and protein expression by using reverse transcriptase-coupled PCR, Northern blot analysis, and immunoblot analysis in invertebrate species that do not produce Ig. We report that the J-chain gene is expressed in invertebrates (Mollusca, Annelida, Arthropoda, Echinodermata, and Holothuroidea), as well as in representative vertebrates (Mammalia, Teleostei, Amphibia). Furthermore, J-chain cDNA from the earthworm has a high degree of homology (68-76%) to human, mouse, and bovine J chains. Immunohistochemical studies reveal that the J chain is localized in the mucous cells of body surfaces, intestinal epithelial cells, and macrophage-like cells of the earthworm and slug. This study suggests that the J chain is a primitive polypeptide that arose before the evolution of Ig molecules and remains highly conserved in extent invertebrates and vertebrates.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandtzaeg P. Presence of J chain in human immunocytes containing various immunoglobulin classes. Nature. 1974 Nov 29;252(5482):418–420. doi: 10.1038/252418a0. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Role of J chain and secretory component in receptor-mediated glandular and hepatic transport of immunoglobulins in man. Scand J Immunol. 1985 Aug;22(2):111–146. doi: 10.1111/j.1365-3083.1985.tb01866.x. [DOI] [PubMed] [Google Scholar]
- Carayannopoulos L., Max E. E., Capra J. D. Recombinant human IgA expressed in insect cells. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8348–8352. doi: 10.1073/pnas.91.18.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A., Neuberger M. S. Polymeric immunoglobulin M is secreted by transfectants of non-lymphoid cells in the absence of immunoglobulin J chain. EMBO J. 1987 Sep;6(9):2753–2758. doi: 10.1002/j.1460-2075.1987.tb02569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cooper E. L., Rinkevich B., Uhlenbruck G., Valembois P. Invertebrate immunity: another viewpoint. Scand J Immunol. 1992 Mar;35(3):247–266. doi: 10.1111/j.1365-3083.1992.tb02857.x. [DOI] [PubMed] [Google Scholar]
- Davis A. C., Roux K. H., Pursey J., Shulman M. J. Intermolecular disulfide bonding in IgM: effects of replacing cysteine residues in the mu heavy chain. EMBO J. 1989 Sep;8(9):2519–2526. doi: 10.1002/j.1460-2075.1989.tb08389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskeland T., Brandtzaeg P. Does J chain mediate the combination of 19S IgM and dimeric IgA with the secretory component rather than being necessary for their polymerization? Immunochemistry. 1974 Mar;11(3):161–163. doi: 10.1016/0019-2791(74)90214-6. [DOI] [PubMed] [Google Scholar]
- Furuta E., Yamaguchi K., Aikawa M., Shimozawa A. Phagocytosis by hemolymph cells of the land slug, Incilaria fruhstorferi Collinge (Gastropoda: Pulmonata). Anat Anz. 1987;163(2):89–99. [PubMed] [Google Scholar]
- Hajdu I., Moldoveanu Z., Cooper M. D., Mestecky J. Ultrastructural studies of human lymphoid cells. mu and J chain expression as a function of B cell differentiation. J Exp Med. 1983 Dec 1;158(6):1993–2006. doi: 10.1084/jem.158.6.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes G. J., Frutiger S., Paquet N., Jaton J. C. The amino acid sequence of rabbit J chain in secretory immunoglobulin A. Biochem J. 1990 Nov 1;271(3):641–647. doi: 10.1042/bj2710641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji H., Parkhouse R. M. Intracellular J chain in mouse plasmacytomas secreting IgA, IgM and IgG. Nature. 1974 May 3;249(452):45–47. doi: 10.1038/249045a0. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Vaerman J. P., Bazin H., LeBacq-Verheyden A. M., Heremans J. F. Identification of J-chain in polymeric immunoglobulins from a variety of species by cross-reaction with rabbit antisera to human J-chain. J Immunol. 1973 Nov;111(5):1590–1594. [PubMed] [Google Scholar]
- Koshland M. E. The coming of age of the immunoglobulin J chain. Annu Rev Immunol. 1985;3:425–453. doi: 10.1146/annurev.iy.03.040185.002233. [DOI] [PubMed] [Google Scholar]
- Kubagawa H., Burrows P. D., Grossi C. E., Mestecky J., Cooper M. D. Precursor B cells transformed by Epstein-Barr virus undergo sterile plasma-cell differentiation: J-chain expression without immunoglobulin. Proc Natl Acad Sci U S A. 1988 Feb;85(3):875–879. doi: 10.1073/pnas.85.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutteh W. H., Moldoveanu Z., Prince S. J., Kulhavy R., Alonso F., Mestecky J. Biosynthesis of J-chain in human lymphoid cells producing immunoglobulins of various isotypes. Mol Immunol. 1983 Sep;20(9):967–976. doi: 10.1016/0161-5890(83)90037-8. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Ma J. K., Hiatt A., Hein M., Vine N. D., Wang F., Stabila P., van Dolleweerd C., Mostov K., Lehner T. Generation and assembly of secretory antibodies in plants. Science. 1995 May 5;268(5211):716–719. doi: 10.1126/science.7732380. [DOI] [PubMed] [Google Scholar]
- Mansour M. H., DeLange R., Cooper E. L. Isolation, purification, and amino acid composition of the tunicate hemocyte Thy-1 homolog. J Biol Chem. 1985 Mar 10;260(5):2681–2686. [PubMed] [Google Scholar]
- Mather E. L., Alt F. W., Bothwell A. L., Baltimore D., Koshland M. E. Expression of J chain RNA in cell lines representing different stages of B lymphocyte differentiation. Cell. 1981 Feb;23(2):369–378. doi: 10.1016/0092-8674(81)90132-x. [DOI] [PubMed] [Google Scholar]
- Matsuuchi L., Cann G. M., Koshland M. E. Immunoglobulin J chain gene from the mouse. Proc Natl Acad Sci U S A. 1986 Jan;83(2):456–460. doi: 10.1073/pnas.83.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Korsmeyer S. J. Human J chain gene. Structure and expression in B lymphoid cells. J Exp Med. 1985 Apr 1;161(4):832–849. doi: 10.1084/jem.161.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Fu S. M., Kunkel H. G. J chain biosynthesis in pre-B cells and other possible precursor B cells. J Exp Med. 1981 Jul 1;154(1):138–145. doi: 10.1084/jem.154.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Mestecky J., Preud'homme J. L., Crago S. S., Mihaesco E., Prchal J. T., Okos A. J. Presence of J chain in human lymphoid cells. Clin Exp Immunol. 1980 Feb;39(2):371–385. [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., Winchester R. J., Hoffman T., Kunkel H. G. Parallel synthesis of immunoglobulins and J chain in pokeweed mitogen-stimulated normal cells and in lymphoblastoid cell lines. J Exp Med. 1977 Mar 1;145(3):760–765. doi: 10.1084/jem.145.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikoryak C. A., Margolies M. N., Steiner L. A. J chain in Rana catesbeiana high molecular weight Ig. J Immunol. 1988 Jun 15;140(12):4279–4285. [PubMed] [Google Scholar]
- Negm H. I., Mansour M. H., Cooper E. L. Serological characterization and partial purification of an Lyt-1 homolog in tunicate hemocytes. Biol Cell. 1991;72(3):249–257. doi: 10.1111/j.1768-322x.1991.tb03021.x. [DOI] [PubMed] [Google Scholar]
- Randall T. D., Brewer J. W., Corley R. B. Direct evidence that J chain regulates the polymeric structure of IgM in antibody-secreting B cells. J Biol Chem. 1992 Sep 5;267(25):18002–18007. [PubMed] [Google Scholar]
- Raschke W. C., Mather E. L., Koshland M. E. Assembly and secretion of pentameric IgM in a fusion between a nonsecreting B cell lymphoma and an IgG-secreting plasmacytoma. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3469–3473. doi: 10.1073/pnas.76.7.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roch P., Cooper E. L., Eskinazi D. P. Serological evidences for a membrane structure related to human beta 2-microglobulin expressed by certain earthworm leukocytes. Eur J Immunol. 1983 Dec;13(12):1037–1042. doi: 10.1002/eji.1830131216. [DOI] [PubMed] [Google Scholar]
- Saiuchi M., Nunoura N., Kim J. Preparation of a monoclonal antibody and expression of its antigen associated with myogenic differentiation on spontaneous and artificial myotubes derived from avian myoblasts. Cell Struct Funct. 1993 Oct;18(5):285–296. doi: 10.1247/csf.18.285. [DOI] [PubMed] [Google Scholar]
- Seeger M. A., Haffley L., Kaufman T. C. Characterization of amalgam: a member of the immunoglobulin superfamily from Drosophila. Cell. 1988 Nov 18;55(4):589–600. doi: 10.1016/0092-8674(88)90217-6. [DOI] [PubMed] [Google Scholar]
- Shalev A., Greenberg A. H., Lögdberg L., Björck L. beta 2-Microglobulin-like molecules in low vertebrates and invertebrates. J Immunol. 1981 Sep;127(3):1186–1191. [PubMed] [Google Scholar]
- Sun S. C., Lindström I., Boman H. G., Faye I., Schmidt O. Hemolin: an insect-immune protein belonging to the immunoglobulin superfamily. Science. 1990 Dec 21;250(4988):1729–1732. doi: 10.1126/science.2270488. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C. E., 3rd, Koshland M. E. Molecular size and shape of the J chain from polymeric immunoglobulins. Biochemistry. 1973 Aug 14;12(17):3218–3224. doi: 10.1021/bi00741a012. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Tse A. G., Gagnon J. Squid glycoproteins with structural similarities to Thy-1 and Ly-6 antigens. Immunogenetics. 1988;27(4):265–272. doi: 10.1007/BF00376121. [DOI] [PubMed] [Google Scholar]
- Zikan J., Novotny J., Trapane T. L., Koshland M. E., Urry D. W., Bennett J. C., Mestecky J. Secondary structure of the immunoglobulin J chain. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5905–5909. doi: 10.1073/pnas.82.17.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]