Abstract
Transcranial two-photon microscopy allows long-term imaging of neurons, glia, and vasculature in the intact cortex of living animals. So far, this technique has been primarily used to acquire images in anesthetized animals. Here, we describe a detailed protocol for high-resolution two-photon imaging of neuronal structures in the cortex of awake head-restrained mice. Surgery is done within 1 h in anesthetized mice. After animals recover from anesthesia, two-photon imaging can be performed multiple times over minutes to days, allowing longitudinal studies of synaptic plasticity and pathology without the complication induced by anesthesia reagents.
Keywords: Two photon laser scanning microscopy, In vivo imaging, Dendritic spine, Dendritic filopodia, Synaptic plasticity
1 Introduction
Transcranial two-photon laser scanning microscopy (TPLSM) is a minimally invasive technique for imaging brain structures at high optical resolution over intervals ranging from seconds to years [1–20]. By creating a thinned-skull cranial window with skull thickness ~20 μm [2, 20], it is possible to image fluorescently labeled synaptic structures within the cortex located as deep as 300–400 μm from the pial surface [1, 2, 6, 10]. This approach has significantly contributed to our understanding of synapse development and maintenance in the mouse cortex under normal and pathological conditions [2–4, 6, 7, 10, 11, 17–21]. To date, transcranial two-photon imaging studies have been performed predominantly with mice under general anesthesia, which is critical for acquiring high resolution images with minimum motion artifacts. While anesthesia is important for reducing motion artifacts during imaging acquisition, it alters the normal patterns of brain activity and has transient effects on dendritic spines and filopodia in the developing mouse cortex [22]. Here, we describe a method for transcranial two-photon imaging of synaptic structures in awake head-restrained mice. This method can be used to study the dynamics of individual dendritic protrusions and axonal varicosities repeatedly over intervals of minutes to days in the cortex of awake mice. It provides an important tool to investigate synaptic plasticity and pathology in health and disease.
2 Materials
2.1 Experimental Animals
Transgenic mice expressing fluorescent proteins in cortical neurons (e.g., thy1-YFP line) [23, 24] at 3–4 weeks of age.
2.2 Surgical Reagents
Ketamine–Xylazine mix (KX): 20 mg/ml Ketamine (Fort Dodge, Iowa, USA) and 3 mg/ml Xylazine (Shenandoah, Iowa, USA) in saline. Store at room temperature.
Sterile lubricant eye ointment (DEL Pharmaceuticals).
Double edge shaving blades (CAMB Machine Knives International LLC, Cat. No. CMK169S).
Sterile alcohol prep pad (Fisherbrand).
High speed micro drill (Fine Science Tools).
Micro drill steel burrs (Fine Science Tools).
Microsurgical blades (Surgistar, #6900).
Two steel bars: 30 mm long and 1.6 mm in diameter.
Cyanoacrylate glue (Loctite 495).
Dental acrylate resin (Motloid): Mix right before use.
Artificial cerebrospinal fluid (ACSF): 119 mM NaCl, 26.2 mM NaHCO3, 2.5 mM KCl, 1 mM NaH2PO4, 1.3 mM MgCl2, 10 mM glucose; Gas with 5% CO2/95% O2 for 10–15 min, then add 2.5 mM CaCl2. Filter-sterilized with a 0.22 μm filter apparatus and stored at 4°C.
Silicone low viscosity kit (World Precision Instruments): Mix right before use.
2.3 Imaging Equipment
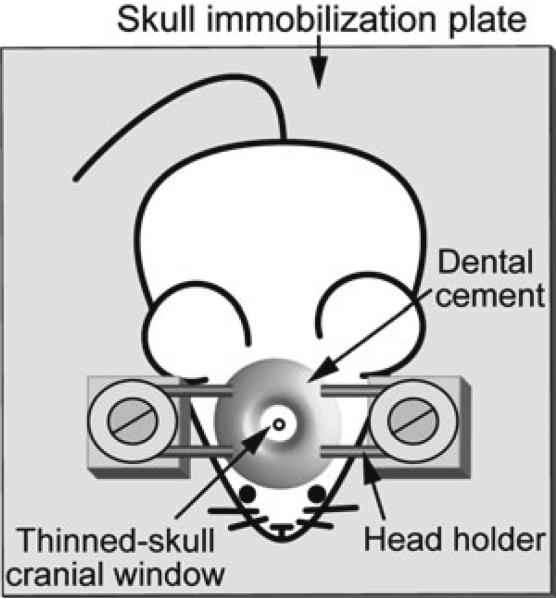
Head immobilization plate (Fig. 1; see also ref. 20): Glue two 18 × 18 × 18 mm steel blocks to a 14 × 10 × 0.1 cm steel plate. The blocks are placed about 2 cm from one of the short sides of the plate and 2 cm from each other. A hole with internal thread is drilled on each block to accommodate the ¼ in. screw. Each screw has a washer.
Dissecting stereomicroscope with attached CCD camera.
TPLSM microscope with water-immersion objective: We have used either a Bio-Rad 2001 multi-photon microscope or a custom-built multi-photon microscope equipped with a mode-locked laser system. For both systems, the laser system (Tsunami and Millennia Xs, Spectra Physics, Mountain View, California, USA) is tunable from 690 to 1,000 nm wavelength with 80 MHz pulse repeat and <100 fs pulse width. The laser-scanning system is coupled to an upright fluorescence microscope. For the custom-built unit, an Olympus laser scanning system is used and detectors (photomultiplier tubes) are placed close to the objective to facilitate the signal detection. The microscope is equipped with 10× air and 60× Water-immersion objectives.
Fig. 1.
Schematic diagram of a head restrained, awake animal preparation. A skull holder includes two parallel steel bars firmly attached to the skull with both cyanoacrylate glue and dental cement. The brain region of interest is exposed in the center and a circular area of skull (typically ~0.5–1 mm in diameter) is thinned to approximately 20 μm. During imaging, the skull holder is tightened to the steel blocks of the skull immobilization plate to reduce motion artifacts
3 Methods
All animal experiments related to surgery and imaging should comply with relevant institutional and national animal care guidelines.
3.1 Attaching the Head Holder
Anesthetize mice by intraperitoneal injection (5–6 μl/g body weight) of KX. Wait for 5–10 min until a surgical level of anesthesia has been reached (see Note 1) and place the mouse on a cotton pad. Lubricate both eyes with a drop of eye ointment (see Note 2). A heating pad may be inserted under the cotton pad in order to maintain a body temperature of ~37°C.
Thoroughly shave the hair over most of the scalp with a double edged razor blade. Remove the residual hair and clean the scalp with a sterile alcohol prep pad.
Perform a midline scalp incision which extends approximately from the neck region (between the ears) to the frontal portion of the head (between the eyes).
Carefully disrupt the fascia located between the scalp and the underlying muscle and skull with a pair of spring scissors, taking care not to sever blood vessels.
Remove connective tissues on the surface of the skull gently with the high-speed micro-drill. Avoid contacting the midline, bregma, and lambda sutures, as well as the temporal and occipital muscles.
Localize the brain area to be imaged based on stereotactic coordinates and mark the area with a pencil.
Position two steel bars, approximately 1 cm apart, centered over the pencil mark. Make sure that the bars are oriented tangentially to the marked region of the skull (Fig. 1) (see Note 3).
Apply a thin layer of cyanoacrylate glue to the skull surface and the surface of the two steel bars contacting the skull (see Note 4).
Before the layer of cyanoacrylate glue dries completely, quickly apply a thick layer of freshly mixed dental cement (see Note 5). Avoid applying dental cement directly over the marked skull region (see Note 6).
3.2 Creating the Thinned-Skull Cranial Window
Wait ~15 min until the dental cement layer has solidified and the head holder is well bonded to the skull. Attach the head holder to the skull immobilization plate by gently inserting both ends of the steel bars between the steel blocks and washers of the skull immobilization plate. Tighten both screws to completely immobilize the head (Fig. 1).
Remove any remaining cyanoacrylate glue covering the marked skull region using a high-speed micro-drill.
Immerse the exposed area of the skull with a drop of ACSF. Use a high-speed micro-drill to thin a circular area of skull (typically ~0.5–1 mm in diameter; Fig. 1) over the marked region under a dissecting microscope (see Note 7). Perform drilling intermittently during the thinning procedure to avoid friction-induced overheating. ACSF helps soften the bone and absorbs heat. Replace the ACSF periodically and wash away the bone debris.
Remove the external layer of compact bone and most of the spongy bone with the drill. Some bleeding from the blood vessels running through the spongy bone may occur during the thinning process. This bleeding will usually stop spontaneously within a few minutes.
After removing the majority of the spongy bone, remaining concentric cavities within the bone can usually be seen under the dissecting microscope, indicating that drilling is approaching the internal compact bone layer. At this stage, skull thickness should still be more than 50 μm. Continue skull-thinning with a microsurgical blade to obtain a very thin (~20 μm) and smooth preparation (~200 μm in diameter) (see Note 8). During the thinning process, repeatedly examine the preparation with a conventional fluorescence microscope until the dendrites and spines in the area of interest can be clearly visualized.
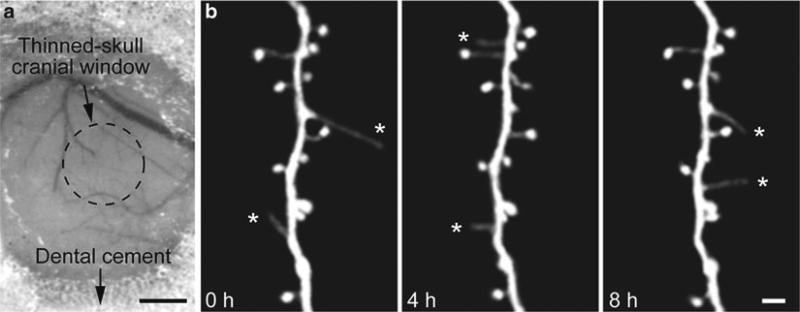
After finishing the skull thinning, take a high quality picture of the brain vasculature with a CCD camera attached to a stereo dissecting microscope (Fig. 2a). This picture is used to label the imaged area in the coming experiment.
Apply a layer of silicone gel on top of the skull to protect the thinned region and release the mouse to the home cage for recovery (see Note 9).
Fig. 2.
Repetitive transcranial TPLSM imaging of fine neuronal structures from an awake head-restrained animal. (a) A CCD camera view of a thinned-skull cranial window in a head-restrained, awake animal preparation. The cortical vasculature can be clearly seen through the thinned skull and used as a landmark to relocate the imaged region at subsequent time points. (b) In vivo time-lapse imaging of the same dendritic segment over 4 and 8 h in the primary somatosensory cortex of an awake animal at 1 month of age (adapted from ref. 22). A majority of dendritic spines remained stable over 8 h whereas dendritic filopodia (asterisks) underwent rapid turnover. Two-dimensional projections of three-dimensional image stacks containing dendritic segments of interest were displayed. Scale bar: 500 μm (a), 2 μm (b)
3.3 Habituation
After the mice wake up from the surgery, habituate the animals to the imaging apparatus before the experiments start.
Attach the mouse's head holder to the skull immobilization plate, and then secure the apparatus to the TPLSM microscope stage. Body restraint may be used depending on the age and weight of the animal (see Note 10) [25].
Keep the awake head-restrained mice in the dark for ~10 min and then release the animal to its home cage.
Repeat steps 1 and 2 for each habituation session. Up to three spaced habitation sessions are done before the experiment.
3.4 TPLSM Imaging
Attach the head holder of the animals to the skull immobilization plate, and then carefully peel off the silicone gel covering the skull. Clean the thinned skull region with ACSF and secure the skull immobilization plate to the TPLSM microscope stage.
Select a properly thinned area for imaging under a fluorescence microscope and mark the selected area on the CCD brain vasculature map (see Subheading 3.2, step 6) by observing the pattern of blood vessels adjacent to it (Fig. 2).
Tune the TPLSM to the appropriate wavelength (e.g., 920 nm for YFP). When possible, use high numerical aperture water-immersion objectives (e.g., 60×, 1.1 N.A.) to acquire images.
Obtain a low-magnification stack of fluorescently labeled neuronal processes (e.g., 60× objective; 200 μm × 200 μm; 512 × 512 pixel; 2 μm step size), which serves as a finer map for accurate relocation of the same region at later time points in conjunction with the CCD brain vasculature map (Fig. 2a) (see Notes 11 and 12).
Without changing the position of the stage, take high-magnification images (e.g., 66.7 μm × 66.7 μm; 512 × 512 pixel; 0.75 μm step size: Fig. 2b) from the same area. The stack is typically taken within ~100 μm below the pial surface for spine imaging (see Notes 11 and 12).
After imaging, apply the silicone gel on top of the exposed skull, and release the mouse to its home cage until the next imaging session (see Note 9).
3.5 Re-imaging
Awake animal imaging is suited for multi-session imaging without the interference of anesthesia. Depending on the design of the experiment, reimaging can be obtained minutes, hours to days after the first view (see Note 13).
Carefully remove the silicone gel covering the skull and find the thinned region based on the brain vasculature map, and check the image quality with the TPLSM microscope. Skull re-thinning may be needed if the reimaging is done 3 days after the previous imaging.
Find the previously imaged region under the fluorescence microscope. Align the region according to the low magnification map under TPLSM, and then zoom in to higher magnification to further align the area. After the region is precisely aligned with the first view, take images with TPLSM.
Acknowledgments
This work was supported by grants from AFAR and the Alzheimer's Association (NIRG-11-205362) to G.Y.; NIH (R01 NS047325) and the Alzheimer's Association (IIRG) to W.B.G.
Footnotes
Continuously monitor the depth of anesthesia by testing the animal's reflexes during the surgery by pinching the animal's foot with a blunt pair of forceps and checking for the absence of the eye blinking reflex. Inject more KX when necessary.
Dehydration of eye tissue can cause permanent damage to the eyes.
1–1/4 in. wire brads can be used as steel bars to create a head holder for young mice (less than 15 g). The objective may not be able to focus into the brain during the imaging if the two bars are placed too close to each other.
Skull immobilization is critical for imaging awake, head-restrained animals. An air duster may be used to make sure the skull is completely dry before applying the glue. The skull holder may detach from the head during the imaging if it is not well bonded to the skull.
Use dental cement to create a well surrounding the marked skull region. This helps hold the ACSF in place during imaging when using a water immersion lens.
If the dental cement covers the marked brain region by accident, scrape it off immediately with a microsurgery blade before the cement solidifies.
Minimize the area of the skull to be thinned for imaging as thinning a large area will increase the chance of causing damage to the underlying cortex.
Hold the microsurgical blade at ~45° angle during thinning and take great care not to push the skull downwards against the brain surface or to break through the bone, as minor brain trauma or bleeding may potentially cause inflammation and disruption of neuronal structures. The thickness of the skull can be directly measured by imaging the skull auto-fluorescence with the TPLSM. Periodic measurement of the skull thickness during thinning may help the novice user in preventing skull over-thinning.
Prolonged exposure of the thinned skull region to air without any protection (ACSF or silicone gel) may cause damage to the brain tissue beneath.
Stable images of dendritic spines can be obtained from 3-week-old and younger mice without body restraint. For mice heavier than 12 g, a half-cut plastic cylinder (~3 cm in diameter) may be placed on the back of the mouse to reduce the body movement during imaging.
Mouse ACSF should be used at all times during imaging for objective immersion. If there is a sudden deterioration of imaging quality, check that the lens remains fully immersed in ACSF.
We typically use laser intensities in the range of 10–30 mW (measured at the sample) to minimize phototoxicity.
Silicone gel may need to be replaced if the interval between two adjacent imaging sessions is more than 2 days apart.
References
- 1.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 2.Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- 3.Majewska AK, Newton JR, Sur M. Remodeling of synaptic structure in sensory cortical areas in vivo. J Neurosci. 2006;26:3021–3029. doi: 10.1523/JNEUROSCI.4454-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishiyama H, Fukaya M, Watanabe M, et al. Axonal motility and its modulation by activity are branch-type specific in the intact adult cerebellum. Neuron. 2007;56:472–487. doi: 10.1016/j.neuron.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stosiek C, Garaschuk O, Holthoff K, et al. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci USA. 2003;100:7319–7324. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai J, Grutzendler J, Duff K, et al. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci. 2004;7:1181–1183. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- 7.Xu HT, Pan F, Yang G, et al. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat Neurosci. 2007;10:549–551. doi: 10.1038/nn1883. [DOI] [PubMed] [Google Scholar]
- 8.Yoder EJ, Kleinfeld D. Cortical imaging through the intact mouse skull using two-photon excitation laser scanning microscopy. Microsc Res Tech. 2002;56:304–305. doi: 10.1002/jemt.10002. [DOI] [PubMed] [Google Scholar]
- 9.Zhang ZG, Zhang L, Ding G, et al. A model of mini-embolic stroke offers measurements of the neurovascular unit response in the living mouse. Stroke. 2005;36:2701–2704. doi: 10.1161/01.STR.0000190007.18897.e3. [DOI] [PubMed] [Google Scholar]
- 10.Zuo Y, Lin A, Chang P, et al. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Zuo Y, Yang G, Kwon E, et al. Long-term sensory deprivation prevents dendritic spine loss in primary somatosensory cortex. Nature. 2005;436:261–265. doi: 10.1038/nature03715. [DOI] [PubMed] [Google Scholar]
- 12.Kim JV, Kang SS, Dustin ML, et al. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009;457:191–195. doi: 10.1038/nature07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wake H, Moorhouse AJ, Jinno S, et al. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haynes SE, Hollopeter G, Yang G, et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 15.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fl uorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 16.Theer P, Hasan MT, Denk W. Two-photon imaging to a depth of 1000 microm in living brains by use of a Ti:Al2O3 regenerative amplifier. Opt Lett. 2003;28:1022–1024. doi: 10.1364/ol.28.001022. [DOI] [PubMed] [Google Scholar]
- 17.Wu SH, Arevalo JC, Sarti F, et al. Ankyrin Repeat-rich Membrane Spanning/Kidins220 protein regulates dendritic branching and spine stability in vivo. Dev Neurobiol. 2009;69:547–557. doi: 10.1002/dneu.20723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan F, Aldridge GM, Greenough WT, et al. Dendritic spine instability and insensitivity to modulation by sensory experience in a mouse model of fragile X syndrome. Proc Natl Acad Sci USA. 2010;107:17768–17773. doi: 10.1073/pnas.1012496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang G, Pan F, Parkhurst CN, et al. Thinned-skull cranial window technique for long-term imaging of the cortex in live mice. Nat Protoc. 2010;5:201–208. doi: 10.1038/nprot.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S, Murphy TH. Imaging the impact of cortical microcirculation on synaptic structure and sensory-evoked hemodynamic responses in vivo. PLoS Biol. 2007;5:e119. doi: 10.1371/journal.pbio.0050119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang G, Chang PC, Bekker A, et al. Transient effects of anesthetics on dendritic spines and filopodia in the living mouse cortex. Anesthesiology. 2011;115:718–726. doi: 10.1097/ALN.0b013e318229a660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng G, Mellor RH, Bernstein M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 24.Jung S, Aliberti J, Graemmel P, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fl uorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferris CF, Stolberg T. Imaging the immediate non-genomic effects of stress hormone on brain activity. Psychoneuroendocrinology. 2010;35:5–14. doi: 10.1016/j.psyneuen.2009.09.003. [DOI] [PubMed] [Google Scholar]