Abstract
Objective
To explore the extent and nature of cognitive deficits in patients with idiopathic intracranial hypertension (IIH) at the time of diagnosis and after 3 months of treatment.
Design
Prospective case–control study.
Setting
Neurological department, ophthalmological department and a tertiary headache referral clinic at a Danish university hospital.
Participants
31 patients with definite IIH referred from June 2011 to February 2013 and included within 1 week of diagnostic intracranial pressure (ICP) measurement. 29 patients completed re-examination at the 3-month follow-up. At the time of testing, none of the patients took medication potentially affecting cognitive function. Controls were 31 healthy age-matched and sex-matched volunteers from the local community.
Outcome measures
Executive function, working memory, visuospatial memory, processing speed, attention and reaction time assessed by a comprehensive neuropsychological test battery consisting of validated computerised (Cambridge neuropsychological test automated battery) and paper-and-pencil tests.
Results
Patients with IIH performed significantly worse than controls in four of six cognitive domains (p≤0.02). Deficits were most pronounced in reaction time (1.45 SD below controls 95% CI 2.10 to 0.85) and processing speed (1.48 SD below controls 95% CI 2.08 to 0.81). Despite marked improvement in ICP and headache, re-examination showed persistent cognitive dysfunction 3 months after diagnosis and start of treatment.
Conclusions
We demonstrate for the first time in a well-defined cohort of patients that IIH may be associated with cognitive dysfunction. This could explain the functional disability of patients with IIH. A focused multidisciplinary approach including neuropsychological rehabilitation, therefore, might be relevant in the treatment of patients with IIH.
Keywords: pseudotumor cerebri, cognition disorders, case-control studies
Strengths and limitations of this study.
The first study to assess a broad range of cognitive functions in more than 10 patients.
Prospective controlled design and a well-defined study population.
Controls were matched for age, sex and premorbid intelligence, and for comparisons of cognitive measures we adjusted for education and headache at time of testing.
The study was non-blinded and controls were not matched for body mass index.
Cognitive assessment by an automated computerised test battery reduced the influence of the non-blinded observer.
Introduction
Owing to a predilection for young individuals of working age, idiopathic intracranial hypertension (IIH) is a condition with substantial socioeconomic consequences. In the USA alone, the estimated annual costs exceed US$444 million (>US$17 000/patient).1 In addition to direct medical cost, the major expense is loss of wages caused by patients having to give up work or change profession due to IIH. Loss of income due to IIH is reported by 48% of patients,1 but the exact cause of this substantial disability is yet unknown.
Despite the obvious threat to visual function, compliance with long-term treatment is often poor. In our clinics, we experience a substantial lack of initiative and self-awareness in patients with IIH, which has raised the suspicion of prefrontal dysfunction. However, while numerous studies describe the visual and headache-related complications of IIH, very little is known about the cognitive implications of the disease. Except for a single memory test conducted in 85 patients,2 the cognitive function in IIH has only been tested in a few very small study populations.3–5 In all studies, apart from the case report by Kaplan et al,5 testing revealed significant cognitive deficits in patients with IIH. Especially within verbal tests and memory, deficits have been demonstrated.
The aim of this case–control study is to explore in detail the extent and nature of cognitive deficits in patients with IIH at the time of diagnosis and after 3 months of treatment.
Methods
Subjects
We recruited 31 consecutive patients with IIH referred to the Department of Neuro-Ophthalmology, the Department of Neurology or the Danish Headache Center, Glostrup hospital from June 2011 to February 2013. Sample size was determined by the number of cases referred in the inclusion period. Twenty-eight of the patients were newly diagnosed with IIH, three patients had a well-defined relapse of IIH after a minimum of 10 months (range 10–26 months) of medication-free remission (resolved headache and papilloedema). All patients had definite IIH according to the diagnostic criteria.6 7 We included only patients who could be tested within 7 days of confirmed diagnosis. Exclusion criteria were: other disorders or medication that could potentially affect cognition, decreased visual acuity or language skills (Danish) deemed insufficient for participation in the cognitive assessment.
Thirty-one healthy and headache-free (defined as less than 4 headache days/month) controls, matched for age and sex, were recruited by advertising at Glostrup hospital and on the website forsogspersonen.dk. Healthy controls were tested only once and did not have a lumbar puncture performed. Otherwise, the cognitive examination programme for patients and controls was identical.
Standard protocol approvals, registration and patient consents
All participants gave written, informed consent to participate in the study. The study was conducted in accordance with the Declaration of Helsinki and approved by the Regional Ethics Committee.
General examination
At the time of diagnosis, patients underwent a complete neurological examination including MRI/CT with venous sequences. All but one patient underwent a thorough standardised neuro-ophthalmological examination.8 One patient did not participate in the neuro-opthalmological evaluation in spite of numerous invitations. A general ophthalmological examination was, however, performed at the local referring ophthalmological department.
Treatment
After diagnostic lumbar puncture and after cognitive testing was completed, treatment with acetazolamide was initiated. From baseline to 3-month follow-up, doses were individually adjusted at doses of 750–2225 mg/day. Owing to intolerable side effects, acetazolamide was replaced by topiramate, 125 mg/day in one patient. Treatment with acetazolamide and topiramate was paused 3 and 7 days, respectively, before the 3-month follow-up examinations.
Infrequent (<14 days/month) use of simple analgesics (paracetamol and/or acetylsalicylic acid) was allowed. Treatment did not include use of opiate analgesics or tranquillisers.
Weight loss was strongly recommended and patients were offered dietician consultations.
Intracranial pressure
ICP was measured at baseline and at the 3-month follow-up. In one patient, ICP was measured by direct ICP monitoring. In the remaining patients (n=30), ICP was measured by standardised lumbar puncture manometry. Patients were placed in the lateral decubital position, had their legs straightened and were given a minimum of 10 min to relax before a stabilised pressure was recorded.
Cognitive testing
We assessed cognitive function by a neuropsychological test battery of validated computerised (Cambridge neuropsychological test automated battery (CANTAB))9 and paper-and-pencil tests.
Paper-and-pencil tests: (1) Rey-Osterrieth Complex Figure Test, testing visuospatial memory; (2) Trail making test A and B, primarily testing psychomotor speed; (3) Symbol digit modalities test, testing psychomotor speed and (4) Verbal fluency test, testing verbal semantic and phonological fluency. The letters ‘S’ and ‘A’ and the categories ‘animals’ and ‘items in a supermarket’ were used.
CANTAB computerised tests: (5) Motor screening test to familiarise participants with the touch screen; (6) Spatial span, assessing visuospatial working memory span; (7) Spatial working memory, testing the ability to retain and manipulate spatial information in working memory; (8) Stockings of Cambridge, assessing spatial planning ability; (9) Intra–extra dimensional set shift, testing cognitive flexibility, requiring the formation and shifting of attentional set; (10) Reaction time, assessing motor and reaction time latencies and (11) Rapid visual information processing, testing sustained attention with a working memory load.
The Danish Adult Reading Test (Danish version of the National Adult Reading Test) was applied as an estimate of premorbid intelligence.10
The test battery was administered in a fixed order by the same physician (HY), instructed and trained by experienced neuropsychologists (HF and BF). To ensure uniform test instructions, we used a written instruction manual during all sessions. Headache intensity at the time of testing was recorded by a 10-point visual analogue scale (VAS). Patients were retested at the 3-month follow-up.
Statistical analysis
Statistical analyses were conducted using SAS V.9.3. Significance levels were set at 0.05. Non-normal distributed data were logarithmically transformed to reduce skewness. Categorical data were investigated by χ2 test, Fisher’s exact test and McNemar test.
Test scores of patients and healthy controls were compared using a linear mixed model adjusting for education and headache at time of testing. Changes in patient test scores from baseline to follow-up were analysed in a linear mixed model for paired data adjusting for headache at time of testing. Test performance in patients with normalised ICP at follow-up and patients with continuous elevated ICP was compared in a mixed model using ICP ≤25 cm H2O and ICP >25 cm H2O as a binary categorical variable. In addition, the effect of ICP change (as a continuous variable) on difference in test performance from baseline to follow-up was analysed.
The effects of depression and chronic pain on cognitive performance within the patient group were explored in a model comparing participants with or without these traits, adjusting for education and headache at time of testing. The effect of body mass index (BMI) was explored in a similar model with BMI as a continuous variable.
To avoid effects of multiple comparisons in the analyses of cognitive function, the analyses were performed in mixed linear models including all 19 subtest scores into the same model.
For comparability of test scores and evaluation of effect sizes, test scores were standardised into z-scores. Z-scores were based on performance of the healthy controls, which by definition had a mean scale score of 0 and SD set to 1. All scales were computed so that a higher z-score indicated better performance.
We used standardised test scores to create composite domain scores, calculated by grouping selected tests, based on which cognitive domain they theoretically represented. Z-scores for cognitive domains were averaged and restandardised based on the composite domain average and SD of healthy controls.
Although they spoke Danish fluently, Trail Making Test scores and Verbal Fluency scores from non-native Danish speakers (n=2) were omitted from statistical analysis as these tests are potentially influenced by language fluency and familiarity with the Latin alphabet. In domain construction, the average of the remaining tests was used to determine the domain score.
Results
Demographics and clinical characteristics at baseline
Patients and healthy controls did not differ in demographics, household income, educational level or premorbid intelligence level (table 1). However, patients had significantly higher BMI and slightly less education counted in years than healthy controls.
Table 1.
Demographics and clinical characteristics for patients with IIH at baseline and at follow-up and healthy controls
| IIH baseline | IIH follow-up | Controls | Statistics |
||
|---|---|---|---|---|---|
| n=31 | n=29 | n=31 | p Value* | p Value† | |
| Demographics | |||||
| Age (SD), years | 31.0 (11.2) | 30.7 (11.2) | 0.91 | ||
| Gender, f/m | 31/0 | 31/0 | |||
| Danish adult reading test (SD), words | 22.9 (6.8) | 24.8 (5.3) | 0.15 | ||
| Education (SD), years | 11.2 (2.2) | 12.8 (2.1) | 0.001 | ||
| Educational level (n) | 0.38 | ||||
| Long cycle higher (≥5 years) | 0 | 3 | |||
| Medium cycle higher (3–5 years) | 4 | 7 | |||
| Short cycle higher (<3 years) | 4 | 4 | |||
| Vocational upper-secondary | 5 | 3 | |||
| Student | 10 | 10 | |||
| No education | 8 | 4 | |||
| Household income (n) | 0.81 | ||||
| High (>DKK 400 000/year) | 10 | 8 | |||
| Middle (DKK 200–400 000/year) | 12 | 12 | |||
| Low (<DKK 200 000/year) | 9 | 11 | |||
| Clinical characteristics | |||||
| BMI (SD), kg/m2 | 35.7 (6.2) | 34.0 (6) | 23.6 (4) | <0.001 | 0.009 |
| Headache at time of testing, n (%) | 22.0 (71) | 14.0 (48) | 0 | ||
| Mean headache intensity (SD), VAS | 2.64 (2.3) | 1.84 (2.4) | 0.01 | ||
| ICP ↔ cognitive testing‡ (SD), days | 3.0 (2.4) | 1.0 (1.6) | |||
| Mean ICP§ (SD), cm H2O | 41.0 (12.6) | 25.9 (5.5) | <0.001 | ||
| Memory difficulties¶, n (%) | 17.0 (55) | 18.0 (62) | 0.42 | ||
| Concentration difficulties¶, n (%) | 20.0 (65) | 15.0 (52) | 0.18 | ||
| Duration of IIH symptoms (SD), months | 4.3 (5.4) | ||||
χ2 Test was used for household income, Fisher's exact test for educational level and McNemar's test for paired categorical variables. Two-tailed t test was used for numerical variables. Significant p Values are printed in italic.
*p difference between patients at baseline and healthy controls.
†p difference between patients at baseline and follow-up.
‡Time span between ICP measurement and cognitive testing.
§ICP measured with direct ICP monitor (n=1) not included.
¶Subjective difficulties reported by the patients.
BMI, body mass index; DKK, Danish Krone; ICP, intracranial pressure; IIH, intracranial hypertension; VAS, visual analogue scale.
Headache at the time of testing was reported by the majority of patients, but by none of the controls (table 1). General headache disability in patients was heterogeneous. Eleven patients fulfilled the criteria of chronic headache (≥15 days/month for 3 months),7 4 patients had frequent headache (mean 4.5 days/month),7 13 patients only had headaches in the weeks up until diagnosis and 3 patients reported no headache at all. Healthy controls reported infrequent headaches with a mean frequency at 0.5 days/month.
Visual fields (Automated perimetry, Humphrey 30-2) were bilaterally normal in 14 patients and normal in at least one eye in another 9 patients. Seven patients had mild bilateral peripheral defects. One patient had bilateral concentric defects with remaining 15–20 central degrees of vision. In the cognitive tests, this patient performed equally well as the average patient. No photophobia or visual disturbances were reported during testing.
Depression (explicitly specified in the standardised interview) was reported by eight (26%) patients. Other comorbidities included tension-type headache (n=12), migraine (n=7), diabetes (n=2), hypertension (n=2), inflammatory bowel disease (n=2), mild personality disorder (n=1), asthma (n=1), fibromyalgia (n=1), small pineal gland cyst (n=1; asymptomatic, discovered on routine MRI at the time of IIH diagnosis), sequela after monocular central serous chorioretinopathy (n=1), intermittent claudication (n=1) and lumbar disc herniation (n=1).
Twenty-three patients were on either short-term (n=19) or long-term sick leave (n=4), 5 were unemployed and 3 had retired from work for reasons other than IIH.
Cognitive function in patients at baseline
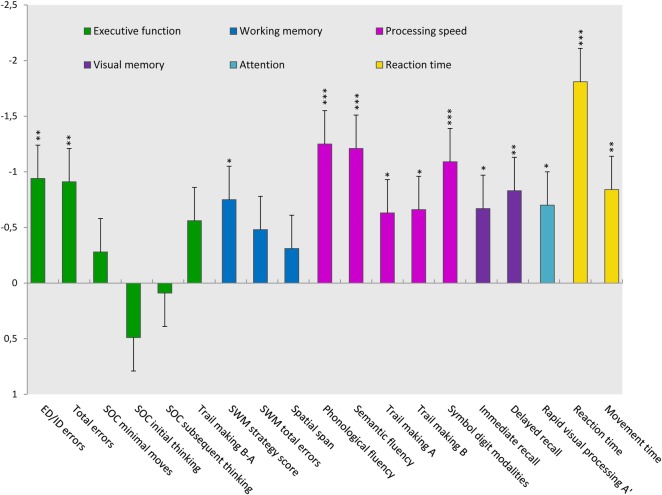
Patients with IIH performed significantly worse than controls in 4 of 6 cognitive domains and in 13 of 19 subtests (table 2). The most pronounced deficits were found in the domains of processing speed and reaction time (figure 1). Even though deficits in executive functions only reached trend levels of significance, patients scored significantly worse in the subtest measuring cognitive flexibility (ID/ED errors). Likewise, patients performed significantly worse in the subtest measuring spatial working memory strategy, although no overall deficits in working memory were found.
Table 2.
Cognitive test scores and composite domain scores at baseline compared to healthy controls
| Test variables | Raw scores |
Z-scores and statistics |
|||
|---|---|---|---|---|---|
| IIH baseline | Healthy controls | ||||
| n=31 | n=31 | Z | 95% CI | p Value | |
| Executive function | −0.61 | −1.25 to 0.02 | 0.059 | ||
| Intra–extra dimensional set shift | |||||
| ID/ED errorslog | 8.1 (0–32) | 4.0 (0–25) | −0.94 | −1.54 to −0.35 | 0.002 |
| Total errors adjustedlog | 20.9 (7–177) | 12.2 (7–55) | −0.91 | −1.50 to −0.32 | 0.003 |
| Stockings of Cambridge | |||||
| Solved in minimum moves | 9.61 (2) | 10.19 (1.7) | −0.28 | −0.87 to 0.31 | 0.31 |
| Initial thinking timelog, s | 6.5 (2–18.3) | 8.2 (3.1–40.7) | 0.49 | −0.11 to 1.08 | 0.11 |
| Subsequent thinking timelog, s | 0.013 (0–3.7) | 0.011 (0–3) | 0.09 | −0.51 to 0.68 | 0.77 |
| Trail Making Test* | |||||
| Trail Making B-Alog, s | 39.2 (14.7–101.1) | 30.6 (16.3–98.4) | −0.56 | −1.1 to 0.09 | 0.07 |
| Working memory | −0.56 | −1.19 to 0.08 | 0.08 | ||
| Spatial working memory | |||||
| Strategy scorelog | 29.9 (20–42) | 24.8 (19–40) | −0.75 | −1.35 to −0.16 | 0.01 |
| Total errorslog | 10.2 (0–79) | 4.7 (0–70) | −0.48 | −1.07 to 0.12 | 0.11 |
| Spatial span | |||||
| Span length | 6.4 (1.3) | 7.0 (1.4) | −0.31 | −0.9 to 0.28 | 0.31 |
| Processing speed | −1.45 | −2.08 to −0.81 | <0.0001 | ||
| Verbal Fluency* | |||||
| Letters | 19.4 (7) | 30.3 (8.3) | −1.25 | −1.84 to −0.65 | <0.0001 |
| Categories | 39.8 (9.9) | 55.5 (12.3) | −1.21 | −1.81 to −0.61 | <0.0001 |
| Trail Making Test* | |||||
| Trail Making Alog, s | 31.5 (18–68.1) | 25.2 (12.8–51.4) | −0.63 | −1.22 to −0.02 | 0.04 |
| Trail Making Blog, s | 73.5 (40.9–169.2) | 52.2 (31.2–131.1) | −0.66 | −1.26 to −0.07 | 0.02 |
| Symbol digit modalities | |||||
| Correct symbols | 47.8 (10.2) | 58.7 (9) | −1.09 | −1.68 to −0.49 | 0.0003 |
| Visuospatial memory | −0.74 | −1.32 to −0.05 | 0.02 | ||
| Rey-Osterrieth figure | |||||
| Immediate recall, score | 24.5 (5.4) | 28.0 (4.3) | −0.67 | −1.26 to −0.08 | 0.03 |
| Delayed recall, score | 23.8 (5) | 28.0 (4.4) | −0.83 | −1.42 to −0.24 | 0.006 |
| Attention | |||||
| Rapid visual processing | |||||
| A’ sensitivity to target | 0.9 (0.1) | 0.93 (0.1) | −0.7 | −1.3 to −0.11 | 0.01 |
| Reaction time | −1.48 | −2.1 to −0.85 | <0.0001 | ||
| Reactionlog, ms | 428.7 (264.9–988.6) | 330.0 (247.6–464.1) | −1.81 | −2.4 to −1.22 | <0.0001 |
| Movement, ms | 417.8 (86.3) | 338.3 (80.1) | −0.84 | −1.43 to −0.25 | 0.006 |
Normally distributed raw score variables are shown as mean (SD). Logarithmically transformed variableslog are shown as arithmetic mean (range). Z-scores and test statistics are given in estimates from a linear mixed model adjusting for education and headache at the time of testing and multiple testing. Significant p Values are printed in italic.
*n=29, as Trail Making Test scores and Verbal Fluency scores from non-native Danish speakers (n=2) were omitted from analysis.
IIH, intracranial hypertension.
Figure 1.
Cognitive function in patients with intracranial hypertension (IIH) is shown in SDs from healthy controls (z-score). Error bars represent SEM. Colours indicate which domain the tests represent. *p<0.05, **p<0.005, ***p<0.0005.
Subanalyses within the patient group showed no significant difference between patients with or without depression (mean overall test difference 0.05 SD 95% CI −0.42 to 0.53, p=0.83) or between patients with or without chronic headache (mean overall test difference 0.34 SD 95% CI −0.18 to 0.83, p=0.19). Performance in cognitive tests within the patient group was not related to BMI (ranging from 24.2 to 48.8 kg/m2) (difference per kg/m2: 0.05 SD 95% CI −0.02 to 0.04, p=0.60). Differences for each of the 19 individual subtest variables are specified in online supplementary table S4.
Clinical characteristics at follow-up
In spite of several invitations to attend a follow-up examination, two patients dropped out from baseline to follow-up. Clinical characteristics and baseline test scores in these two patients did not differ from the rest of the patient group.
Twenty-nine patients were re-examined at the 3-month follow-up. One patient refused to have lumbar puncture performed at follow-up. A normalised ICP was found in 14 of the remaining 28 patients. Less than half of the patients had a headache during cognitive retesting (table 1). Visual fields were either stable or had improved from baseline.
Fourteen of 31 patients had resumed work/school, 11 patients were now on long-term sick leave, 1 patient had reduced and altered the work schedule due to IIH and 2 patients were unemployed.
Cognitive function at follow-up
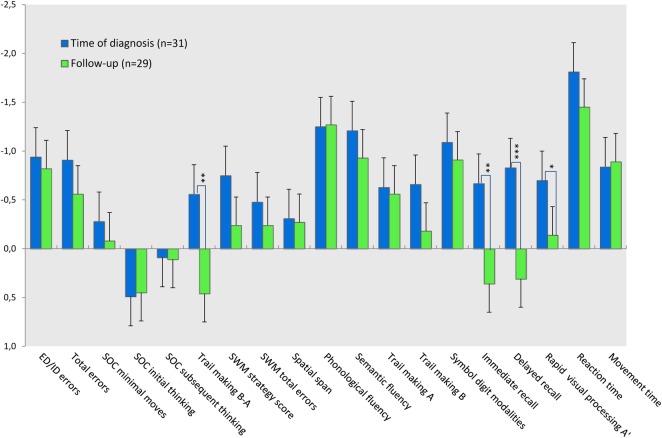
After 3 months of treatment, statistically significant improvement was detected in two domains (table 3). Attention scores (RVP A’) had practically normalised while performance in visuospatial memory tests improved to a level above performance in healthy controls.
Table 3.
Cognitive test scores and composite domain scores at follow-up compared to baseline
| Test variables | Raw scores |
Z-scores and statistics |
|||
|---|---|---|---|---|---|
| IIH baseline | IIH follow-up | ||||
| n=31 | n=29 | Z* | 95% CI | p Value | |
| Executive function | −0.18 | −0.77 to 0.42 | 0.16 | ||
| Intra–extra dimensional set shift | |||||
| ID/ED Errorslog | 8.1 (0–32) | 5.8 (1–32) | −0.82 | −1.40 to −0.25 | 0.77 |
| Total errors adjustedlog | 20.9 (7–177) | 14.4 (7–68) | −0.56 | −1.14 to 0.01 | 0.26 |
| Stockings of Cambridge | |||||
| Solved in minimum moves | 9.61 (2) | 19.9 (2) | −0.08 | −0.66 to 0.49 | 0.55 |
| Initial thinking timelog, s | 6.5 (2–18.3) | 6.7 (2.5–18.4) | 0.45 | −0.14 to 1.02 | 0.98 |
| Subsequent thinking timelog, s | 0.013 (0–3.7) | 0.013 (0–3.7) | 0.11 | −0.47 to 0.68 | 0.85 |
| Trail Making Test† | |||||
| Trail Making B-Alog, s | 39.2 (14.7–101.1) | 33.1 (1.3–79.5) | 0.46 | −0.12 to 1.05 | 0.002 |
| Working memory | −0.33 | −0.84 to 0.18 | 0.44 | ||
| Spatial working memory | |||||
| Strategy scorelog | 29.9 (20–42) | 27.9 (19–42) | −0.24 | −0.81 to 0.34 | 0.1 |
| Total errorslog | 10.2 (0–79) | 10.1 (0–61) | −0.24 | −0.81 to 0.34 | 0.5 |
| Spatial span | |||||
| Span length | 6.4 (1.3) | 6.4 (1.3) | −0.27 | −0.85 to 0.31 | 0.96 |
| Processing speed | −1.23 | −1.83 to −0.64 | 0.49 | ||
| Verbal Fluency† | |||||
| Letters | 19.4 (7.0) | 18.6 (6.6) | −1.27 | −1.86 to −0.69 | 0.88 |
| Categories | 39.8 (9.9) | 42.5 (10.8) | −0.93 | −1.51 to −0.34 | 0.41 |
| Trail Making Test† | |||||
| Trail Making Alog, s | 31.5 (18.0–68.1) | 32.9 (9.8) | −0.56 | −1.15 to 0.02 | 0.95 |
| Trail Making Blog, s | 73.5 (40.9–169.2) | 66.1 (38.7–125.4) | −0.18 | −0.79 to 0.4 | 0.16 |
| Symbol digit modalities | |||||
| Correct symbols | 47.8 (10.2) | 49.1 (12.3) | −0.91 | −1.49 to −0.33 | 0.5 |
| Visuospatial memory | 0.39 | −0.17 to 1.02 | 0.0005 | ||
| Rey-Osterrieth figure | |||||
| Immediate recall, score | 24.5 (5.4) | 28.9 (4.1) | 0.36 | −0.22 to 0.93 | 0.002 |
| Delayed recall, score | 23.8 (5) | 28.8 (3.8) | 0.31 | −0.26 to 0.89 | 0.0002 |
| Attention | |||||
| Rapid visual processing | |||||
| A’ sensitivity to target | 0.9 (0.1) | 0.92 (0.04) | −0.14 | −0.71 to 0.43 | 0.03 |
| Reaction time | −1.31 | −1.90 to −0.71 | 0.9 | ||
| Reactionlog, ms | 428.7 (264.9–988.6) | 387.4 (393–710.1) | −1.45 | −2.02 to −0.88 | 0.68 |
| Movement, ms | 417.8 (86.3) | 412.3 (72.1) | −0.89 | −1.46 to −0.31 | 0.32 |
Normally distributed raw score variables are shown as mean (SD). Logarithmically transformed variableslog are shown as arithmetic mean (range). Z-scores and test statistics are given in estimates from a linear mixed model adjusting for education and headache at the time of testing and multiple testing. Significant p values are printed in italic.
*Z Patients at follow-up compared to healthy controls.
†n=29, as Trail Making Test scores and Verbal Fluency scores from non-native Danish speakers (n=2) were omitted from analysis.
IIH, intracranial hypertension.
No overall change was detected in the domains of executive function, working memory, processing speed and reaction time (figure 2). Patients in whom ICP had normalised (<25 cm H2O) did not perform better than patients in whom elevated ICP persisted (ICP>25 cm H2O), and performance was not significantly associated with intensity or presence/absence of headache during the test. No correlation was found between change in cognitive performance and difference in ICP from baseline.
Figure 2.
Test performance in patients with intracranial hypertension (IIH) is shown in SDs from healthy controls (z-score). Error bars represent SEM. *p<0.05, **p<0.005, ***p<0.001.
Discussion
This study is the first to comprehensively explore the cognitive functions in a cohort of more than 10 patients with IIH. We examined 31 patients and found deficits in four of six cognitive domains, suggesting that IIH is associated with a global cognitive dysfunction.
Cognitive function in IIH has only been reported in three studies2–4 in addition to a single case report.5 One study2 examined 85 patients but applied only a single memory test and the methodology was not described in details. The remaining studies performed more extensive cognitive testing, but in contrast to our study were uncontrolled and included only respectively 1, 5 and 10 patients3–5 Prior studies were, in addition, based on patients with a wide range of disease duration (6–98 months) and only one study3 reported ICP at the time of testing. Our study is the first to assess the cognitive function in a well-defined group of patients with newly diagnosed disease (n=29) or relapse (n=2).
While the case study of Kaplan et al5 found no convincing cognitive deficits, Arseni et al2 and Kharkar et al4 reported substantial deficits in memory. We found deficits in visuospatial memory and in spatial working memory strategy, but detected no overall difference in working memory. Verbal memory (measured by Wecheler Memory Scale) was by far the most affected parameter in the study of Kharkar et al and similarly was reported to be moderate to severe in 90% of the patients studied by Arseni et al. Although we did not test verbal memory, we found significant deficits in other verbal functions (verbal fluency). This is in line with the study of Sorensen et al3 reporting verbal deficits in all their five patients. Deficits in phonological fluency, which were substantial in our patients, have been shown to relate to frontal lobe damage, reflecting an additional executive component.11
The most severe deficits in our study were found in the domains of reaction time and processing speed, which is consistent with the study of Sorensen et al.3 In addition, we found significant impairment in cognitive flexibility. Cognitive flexibility is fundamental to effective decision-making and the ability to learn and adapt to environmental changes, but has never been tested previously in patients with IIH.
Although overall working memory was not affected in our study, patients did score significantly worse in the working memory strategy. This may reflect an executive component consistent with other executive deficits detected in our patients.
The deficits we detected in the domains of reaction time, processing speed, visuospatial memory and attention were equivalent to those found in patients with first episode schizophrenia.12 In addition, deficits in cognitive flexibility were similar to those (measured by Wisconsin card sort, a task conceptionally akin to the intra–extra dimensional set shift test) found in a meta-analysis of patients with schizophrenia in general.13 Verbal fluency in our patients was affected to the same extent as reported for patients with schizophrenia13 as well as patients with congenital hydrocephalus.11 Furthermore, deficits in verbal phonological fluency and processing speed (measured by symbol digit modalities test) were in the range found in patients with multiple sclerosis.14–16
Despite marked improvement in ICP and headache, we found no convincing signs of overall cognitive improvement at the 3-month follow-up as the improvement seen in the visuospatial tests could be explained by the test–retest effect (familiarisation with the Rey-Osterrieth complex figure).
Sorensen et al3 reported that although signs of cognitive dysfunction were only minor, four of their five patients were unable to manage work and/or everyday activities. In our study, 12 of the 31 patients were either on long-term sick leave or had a reduced and altered work schedule due to IIH at follow-up 3 months after diagnosis. Short follow-up and coexistent headache symptoms limit the interpretation of the socioeconomic impact of cognitive dysfunction demonstrated in our study. However, in other well-recognised diseases such as schizophrenia, a robust relationship between global and specific cognitive deficits and functional outcome has been demonstrated consistently.17 18
The cause of cognitive impairment in IIH remains speculative. Theories could involve dysfunction of grey and/or white matter substance due to mechanical compression as proposed in normal pressure hydrocephalus,11 dysfunction related to axonal flow as in optic nerve swelling and dysfunction19 or release of cytotoxic substances as is seen in other conditions with cognitive decline.20 Until now, there is no plausible evidence for brain damage in IIH,21 and as brain volume seems to be normal in IIH,22 we would expect any structural change that could explain the cognitive deficits found in this study to be subtle.
The strengths of the study are the prospective and controlled design, the broad range of cognitive tests, a relatively large study population, and the use of a culturally blind and computerised test battery that by automatic test conduction and score recording reduced the influence of the non-blinded observer. In addition, the study population was well defined with cognitive testing performed in close relation to IIH diagnosis and ICP measurement. As patients were enrolled consecutively from neurological and ophthalmological departments, our study population reflects representative patients with IIH and not a selected group of cognitively symptomatic patients.
We recognise limitations to our study. First, the design was the non-blinded design and we did not perform a retest of healthy controls. Second, the follow-up period was relatively short and may very well explain why we, unlike others,3 failed to demonstrate improvement in cognitive function. Most importantly, although we adjusted for many of the most important confounders, our controls were not matched for BMI, headache or history of depression. The effect of headache on cognitive function has been debated,23–25 but a recent comprehensive review concluded that there is no evidence of cognitive dysfunction in patients with migraine in general.26 On the other hand, there seems to be evidence that chronic pain is associated with mild cognitive impairment in selected domains.27 28 However, it is unclear if the cognitive impairment is attributed to the pain itself or more likely mediated by coexistent depression.29 Headache was chronic in 10 (32%) of our patients and depression was reported by 8 (26%) patients. Neither depression nor chronic pain was associated with poorer cognitive performance when compared within the patient group. BMI in our patients ranged from normal to morbidly obese (24.2–48.8 kg/m2). Patients with higher BMI did not perform worse than the less obese. Although it thus seems less likely that chronic pain, depression or obesity accounts for our findings of impaired cognition, subanalyses were limited by the small sample and statistical uncertainty. We acknowledge that to account for the influence of these potential confounders we ideally should have included an additional control group of obese patients with frequent headache. However, the wide range of factors potentially affecting performance in cognitive tests, and the great variation within the patient group, makes an ideal match very difficult to achieve. For future studies, a feasible approach to this challenge could be to recruit participants with suspected IIH, but in whom the diagnosis is declined after appropriate investigations.
In conclusion, this study strongly suggests that IIH is associated with cognitive deficits. The results, in addition, indicate that the cognitive deficits are long-lasting, not paralleling ICP and headache reduction, and are not sufficiently treated by diuretics and weight loss. Contrary to our hypothesis, executive and memory functions were only moderately affected. Nevertheless, we found substantial deficits in processing speed and reaction time which could explain some of the difficulties that patients encounter in work and daily activities. A focused multidisciplinary approach including neuropsychological rehabilitation, therefore, might be relevant in the treatment of patients with IIH.
Supplementary Material
Acknowledgments
The authors thank Winnie G Nielsen, Lene Elkjær and especially Hanne Andresen for their tireless effort and technical assistance during data collection (intracranial pressure measurements). They also thank neuro-ophthalmologists Marianne Wegener and Steffen Hamann for thorough neuro-ophthalmological examination and evaluation supporting the diagnosis of intracranial hypertension in our patients.
Footnotes
Contributors: HMY made a substantive intellectual contribution to the design of the study, acquisition, analysis and interpretation of the data, and the drafting and revision of the manuscript. BF and HBF made a substantive intellectual contribution to the design of the study, the interpretation of the data and the revision of the manuscript. . RHJ made a substantive intellectual contribution to the conceptualisation and design of the study, the interpretation of the data and the revision of the manuscript.
Funding: This work was supported by Region Hovedstadens Forskningsfond and Fonden til Lægevidenskabens Fremme, grant number 12–375.
Competing interests: HMY has received honoraria for consultant work from Neurocore and a travel grant from Berlin-Chemi Menarini. RHJ has received honoraria for lectures and patient leaflets from MSD, Berlin-Chemie Menarini, ATI and Pfizer and serves on medical advisory boards for LindeGas, ATI and Neurocore.
Patient consent: Obtained.
Ethics approval: The Regional Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Friesner D, Rosenman R, Lobb BM, et al. Idiopathic intracranial hypertension in the USA: the role of obesity in establishing prevalence and healthcare costs. Obes Rev 2011;12:e372–80 [DOI] [PubMed] [Google Scholar]
- 2.Arseni C, Simoca I, Jipescu I, et al. Pseudotumor cerebri: risk factors, clinical course, prognostic criteria. Rom J Neurol Psychiatry 1992;30:115–32 [PubMed] [Google Scholar]
- 3.Sorensen PS, Thomsen AM, Gjerris F. Persistent disturbances of cognitive functions in patients with pseudotumor cerebri. Acta Neurol Scand 1986;73:264–8 [DOI] [PubMed] [Google Scholar]
- 4.Kharkar S, Hernandez R, Batra S, et al. Cognitive impairment in patients with pseudotumor cerebri syndrome. Behav Neurol 2011;24:143–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan CP, Miner ME, McGregor JM. Pseudotumour cerebri: risk for cognitive impairment? Brain Inj 1997;11:293–303 [DOI] [PubMed] [Google Scholar]
- 6.Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology 2002;59:1492–5 [DOI] [PubMed] [Google Scholar]
- 7.Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 2013;33:629–808 [DOI] [PubMed] [Google Scholar]
- 8.Yri HM, Wegener M, Sander B, et al. Idiopathic intracranial hypertension is not benign: a long-term outcome study. J Neurol 2012;259:886–94 [DOI] [PubMed] [Google Scholar]
- 9.Levaux MN, Potvin S, Sepehry AA, et al. Computerized assessment of cognition in schizophrenia: promises and pitfalls of CANTAB. Eur Psychiatry 2007;22:104–15 [DOI] [PubMed] [Google Scholar]
- 10.O'Carroll R. The assessment of premorbid ability: A critical review. Neurocase 1995;1:83–9. [Google Scholar]
- 11.Iddon JL, Pickard JD, Cross JJ, et al. Specific patterns of cognitive impairment in patients with idiopathic normal pressure hydrocephalus and Alzheimer's disease: a pilot study. J Neurol Neurosurg Psychiatry 1999;67:723–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen R, Fagerlund B, Rasmussen H, et al. Cognitive effects of six months of treatment with quetiapine in antipsychotic-naive first-episode schizophrenia. Psychiatry Res 2011;187:49–54 [DOI] [PubMed] [Google Scholar]
- 13.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 1998;12:426–45 [DOI] [PubMed] [Google Scholar]
- 14.Foong J, Rozewicz L, Quaghebeur G, et al. Executive function in multiple sclerosis. The role of frontal lobe pathology. Brain 1997;120(Pt 1):15–26 [DOI] [PubMed] [Google Scholar]
- 15.Ruet A, Deloire MS, Charre-Morin J, et al. A new computerised cognitive test for the detection of information processing speed impairment in multiple sclerosis. Mult Scler 2013;19:1665–72 [DOI] [PubMed] [Google Scholar]
- 16.Lapshin H, Lanctot KL, O'Connor P, et al. Assessing the validity of a computer-generated cognitive screening instrument for patients with multiple sclerosis. Mult Scler 2013;19:1905–12 [DOI] [PubMed] [Google Scholar]
- 17.Green MF, Kern RS, Braff DL, et al. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull 2000;26:119–36 [DOI] [PubMed] [Google Scholar]
- 18.Jaeger J, Tatsuoka C, Berns S, et al. Associating functional recovery with neurocognitive profiles identified using partially ordered classification models. Schizophr Res 2006;85:40–8 [DOI] [PubMed] [Google Scholar]
- 19.Tso MO, Hayreh SS. Optic disc edema in raised intracranial pressure. III. A pathologic study of experimental papilledema. Arch Ophthalmol 1977;95:1448–57 [DOI] [PubMed] [Google Scholar]
- 20.Beeri MS, Moshier E, Schmeidler J, et al. Serum concentration of an inflammatory glycotoxin, methylglyoxal, is associated with increased cognitive decline in elderly individuals. Mech Ageing Dev 2011;132:583–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wall M, Dollar JD, Sadun AA, et al. Idiopathic intracranial hypertension. Lack of histologic evidence for cerebral edema. Arch Neurol 1995;52:141–5 [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann J, Huppertz HJ, Schmidt C, et al. Morphometric and volumetric MRI changes in idiopathic intracranial hypertension. Cephalalgia 2013;33:1075–84 [DOI] [PubMed] [Google Scholar]
- 23.Mulder EJ, Linssen WH, Passchier J, et al. Interictal and postictal cognitive changes in migraine. Cephalalgia 1999;19:557–65 [DOI] [PubMed] [Google Scholar]
- 24.Schmitz N, Arkink EB, Mulder M, et al. Frontal lobe structure and executive function in migraine patients. Neurosci Lett 2008;440:92–6 [DOI] [PubMed] [Google Scholar]
- 25.Le PF, Zappala G, Giuffrida S, et al. Memory disturbances in migraine with and without aura: a strategy problem? Cephalalgia 2000;20:475–8 [DOI] [PubMed] [Google Scholar]
- 26.Rist PM, Kurth T. Migraine and cognitive decline: a topical review. Headache 2013;53:589–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Block C, Cianfrini L. Neuropsychological and neuroanatomical sequelae of chronic non-malignant pain and opioid analgesia. NeuroRehabilitation 2013;33:343–66 [DOI] [PubMed] [Google Scholar]
- 28.Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol 2011;93:385–404 [DOI] [PubMed] [Google Scholar]
- 29.Brown SC, Glass JM, Park DC. The relationship of pain and depression to cognitive function in rheumatoid arthritis patients. Pain 2002;96:279–84 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.