Abstract
Insulin-specific CD4+ T cells are required for type 1 diabetes. How these cells are regulated and how tolerance breaks down are poorly understood because of a lack of reagents. Therefore, we used an enrichment method and tetramer reagents to track insulin-specific CD4+ T cells in diabetes-susceptible NOD and resistant B6 mice expressing I-Ag7. Insulin-specific cells were detected in both strains, but they only became activated, produced IFN-g, and infiltrated the pancreas in NOD mice. Unexpectedly, the majority of Ag-experienced cells in NOD mice displayed an anergic phenotype, but this population decreased with age as tolerance was lost. B6 mice expressing I-Ag7 were protected because insulin-specific cells did not become effector or anergic T cells but remained naive. These data suggest that NOD mice promote tolerance through anergy induction, but a small proportion of autoreactive T cells escape anergy to provoke type 1 diabetes.
Insulin is an immunodominant Ag during type 1 diabetes (T1D) (1-4). In NOD mice, >90% of insulin-specific CD4+ T cells in the pancreas are specific for the insulin B chain (InsB) peptide 9–23 (InsB9–23) (3), and these cells are required for T1D (5). In addition, tolerogenic immunization with InsB9–23 peptide delays or prevents T1D (6, 7). Despite the well-established role of insulin-specific CD4+ T cells during T1D, little is known about how this immune response develops because these cells have been difficult to track. There has not been an in-depth analysis of this critical CD4+ T cell population to understand how peripheral tolerance fails and T1D develops.
MHC class II tetramers are powerful reagents to track Agspecific CD4+ T cells. When coupled with magnetic enrichment, rare cells can be tracked with high precision (8, 9). However, a major challenge in generating MHC class II tetramers is determining the peptide-binding register. The relevant binding register for the InsB9–23 epitope is debated (10-13). However, there is evidence that the majority of InsB10–23-reactive CD4+ T cells recognize the 14–22 core segment ALYLVCGER (register 3) when mutated to optimize binding to I-Ag7 (11, 12). Therefore, we constructed a tetramer re-agent containing the modified register 3 epitope bound to I-Ag7 to define the dynamics of the insulin-specific CD4+ T cell response in diabetes-susceptible NOD mice, as well as resistant B6 mice expressing the I-Ag7 allele (B6.g7) (14). Our results led to the surprising conclusion that most InsB10–23r3: I-Ag7–specific T cells are anergic in NOD mice but are naive in B6.g7 mice.
Materials and Methods
Mice
NOD mice were purchased from Taconic. B6.g7 mice were generated by Zucchelli et al. (14). NOD.BDC2.5 mice were purchased from The Jackson Laboratory. NOD.BDC2.5 cells were isolated, as described (15), and 7500 naive T cells were transferred i.v. to 7–12-wk-old prediabetic NOD mice. Blood glucose ≥ 250 mg/dl indicated diabetes (LifeScan). All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
Insulin tetramer
The InsB10–23r3:I-Ag7 tetramer was constructed similarly as described (8). Briefly, I-Ag7 monomer containing the peptide HLVERLYLVCGEEG was produced and biotinylated in Drosophila S2 cells. Biotinylated monomer was purified on a monomeric avidin column (Thermo Scientific) and combined with streptavidin (SA)-PE and SA-allophycocyanin (Prozyme) to produce the tetramers. The National Institutes of Health tetramer core provided I-Ag7 henegg lysozyme (HEL)11–25 tetramer (AMKRHGLDNYRGYSL).
Flow cytometry
Single-cell suspensions were generated, as described (15). Tetramer-binding cells were enriched from the spleen and nondraining lymph nodes (nondLNs; periaortic, inguinal, brachial, cervical, axillary, and mesenteric lymph nodes) by incubation with 10 nM PE- or allophycocyanin-tetramer for 1 h at 25°C, followed by anti-PE and anti-allophycocyanin MicroBeads for 30 min at 4°C and prior to elution over magnetic columns (Miltenyi Biotec).
Samples were collected using a BD LSR II and Fortessa (BD Biosciences). Data were analyzed using FlowJo software (TreeStar). Cells were enumerated using AccuCheck Counting Beads (Life Technologies).
Cytokine stimulation and priming
Cytokines from insulin-specific CD4+ T cells were assessed in vitro in complete DMEM containing 100 ng/ml PMA, 1000 ng/ml ionomycin, and 10 mg/ml brefeldin A (Sigma) for 4 h (15). For BDC2.5 T cells, 500 μg acetylated p31 peptide (YVRPLWVRME) (Genemed Sythesis) was injected i.v. for 4 h. The modified InsB10–23 peptide (11) or HEL11–25 (Genemed Synthesis) was emulsified in CFA. Mice were immunized s.c. in the flank (100 μg).
Statistics
Unpaired two-tailed Student t tests were performed with a 95% confidence interval using GraphPad Prism 5 software.
Results and Discussion
Development of the InsB10–23r3:I-Ag7 tetramer reagent
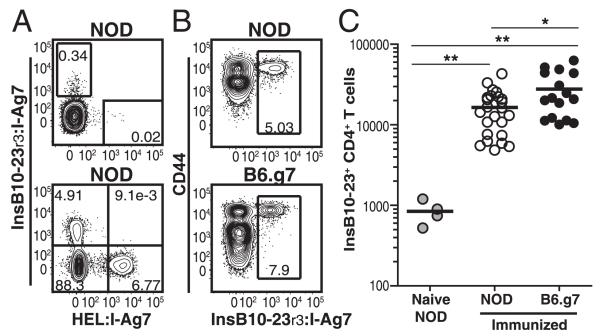
We produced an I-Ag7 tetramer containing a variant of InsB10–23 with substitutions (InsB10–23r3) to anchor the peptide in register 3, because previous work showed that this tetramer detects the majority of CD4+ T cells specific for the native peptide (11). Cells from NOD mice were enriched with InsB10–23r3:I-Ag7 or HEL11–25:I-Ag7 tetramers (control), as described (8). Small, but mutually exclusive, populations of tetramer-binding cells were detected among preimmune CD4+ T cells (Fig. 1A). As control, mice were primed with InsB10–23r3 and HEL11–25, and mutually exclusive tetramer populations expanded (Fig. 1A). Insulin-specific cells became activated, expressed CD44 (Fig. 1B, Supplemental Fig. 1C), and expanded (Fig. 1C).
FIGURE 1.
Generation of the InsB10–23r3:I-Ag7 tetramer reagent. (A) InsB10–23r3:I-Ag7 and HEL11–25:I-Ag7 tetramer staining from spleen and nondLNs of naive (top panel) or immunized (bottom panel) nondiabetic NOD mice. (B) CD44 and InsB10–23r3:I-Ag7 from NOD or B6.g7 mice 8 d after immunization with InsB10–23r3 or HEL11–25 in CFA. (C) Enumeration of InsB10–23r3-specific CD4+ T cells in SLOs from NOD (n = 24) or B6.g7 (n = 17) mice postimmunization with InsB10–23r3/CFA and control unimmunizedNOD mice (n = 4). Data in (B) and (C) are from five experiments. All flow cytometry was gated on singlet+, CD3+, B220−, CD11b−, CD11c−, and CD4+ cells. *p = 0.01–0.05, **p = 0.001–0.01.
A small degree of nonspecific binding of the InsB10–23r3:I-Ag7 tetramer was detected on CD8+ T cells (Supplemental Fig. 1A). To increase sensitivity, we stained cells from NOD mice simultaneously with InsB10–23r3:I-Ag7 /SA-PE and SA-allophycocyanin tetramers (Supplemental Fig. 1A). All double tetramer–binding cells expressed CD4 but not CD8. This approach was used for the remainder of the study to eliminate background.
Insulin-specific CD4+ T cells only infiltrate the NOD pancreas
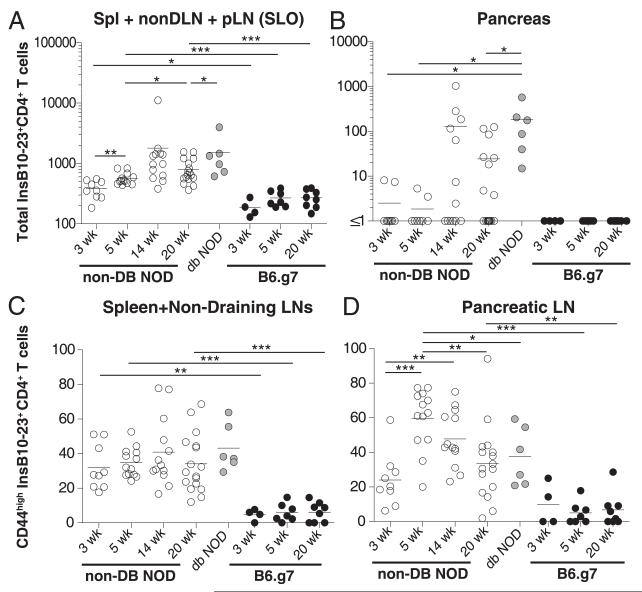
We next evaluated the dynamics of the InsB10–23r3:I-Ag7–specific CD4+ T cells in diabetes-susceptible NOD and resistant B6.g7 mice. Insulin-specific CD4+ T cells were detected in the pancreatic lymph nodes (pLNs), spleen, and non-dLNs of both strains, consistent with work examining BDC2.5 mimotope–specific CD4+ T cells (Fig. 2A, 2B, Supplemental Fig. 1B) (16). The number of insulin-specific CD4+ T cells increased in the secondary lymphoid organs (SLOs) of NOD mice with age, peaking at 14 wk (Fig. 2A, Supplemental Table IA). There were significantly more insulin-reactive cells in the SLOs of NOD mice at all ages examined compared with B6.g7 mice (Fig. 2A, Supplemental Table IA). In B6.g7 mice, there was a slight, but not significant, increase in cell number between weeks 3 and 5 of life (Fig. 2A). The number of insulin-specific CD4+ T cells did not change in B6.g7 mice between weeks 5 and 20 (Fig. 2A, Supplemental Table IA). Importantly, insulin-specific CD4+ T cells infiltrated the pancreas of NOD mice but not B6.g7 mice (Fig. 2B, Supplemental Fig. 1B). These data demonstrate that insulin-specific CD4+ T cells escape central deletion in both NOD and B6.g7 mice but only infiltrate the pancreas in NOD mice.
FIGURE 2.
Insulin-specific CD4+ T cells only become activated and infiltrate the pancreas of NOD mice. Enumeration of InsB10–23r3:I-Ag7 cells in the pLNs, spleen,and non-dLNs combined (SLOs) (A) or pancreas (B) from NOD and B6.g7 mice. Frequency of CD44high insulin-specific CD4+ T cells in the spleen and non-dLNs (C) and pLNs (D) from the NOD and B6.g7 mice shown in (A). Flow cytometry in (A) and (B) was gated on singlet+, CD3+B220−, CD11b−, CD11c−, CD4+, CD8a−, InsB10–23r3:I-Ag7 PE+, and allophycocyanin+; flow cytometry in (C) and (D) also included CD44. Nondiabetic NOD at 3 wk (n = 9), 5 wk (n = 13), 14 wk (n = 13), and 20 wk (n = 17). Diabetic NOD mice (n = 6). B6.g7 mice at 3 wk (n = 4), 5 wk (n = 7), and 20 wk (n = 8). Data are compiled from 15 experiments. *p = 0.01–0.05, **p = 0.001–0.01, ***p < 0.001.
We next examined the activation of insulin-specific CD4+ T cells by measuring CD44 expression. Work using BDC2.5 mice showed that activation can be impaired in diabetes-resistant hosts (17). We determined that there was a higher frequency (Fig. 2C, 2D) and number (Supplemental Table IB) of CD44high insulin-specific CD4+ T cells in NOD mice than in B6.g7 mice. The frequency of CD44high cells in the pLNs of NOD mice peaked between weeks 3 and 5, resulting in significantly more activated insulin-specific CD4+ T cells in NOD mice than in B6.g7 mice (Fig. 2D). These results suggest that InsB10–23r3:I-Ag7–specific T cells become activated in the pLNs of NOD mice but not B6.g7 mice.
Insulin-specific CD4+ T cells differentiate into IFN-γ–producing Th1 cells in NOD mice
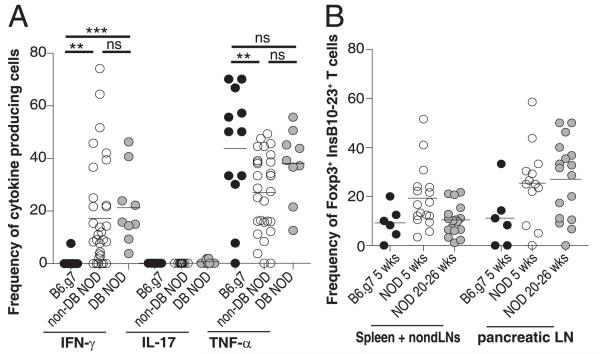
In NOD mice we observed a subpopulation of insulin-specific CD4+ T cells that became activated and infiltrated the pancreas. We speculated that these activated cells would produce IFN-γ, because this cytokine is involved in T1D pathogenesis (18, 19). Conversely, we predicted that IFN-γ would not be produced by insulin-specific CD4+ T cells in B6.g7 mice because these cells were a naive phenotype (Fig. 2C, 2D). We also examined TNF-α and IL-17A because of their predicted roles for T1D pathogenesis (20, 21). IFN-γ, TNF-α, and IL-17A were all detected in the polyclonal CD4+CD44high population in both NOD and B6.g7 mice (Supplemental Fig. 1F). Tetramer-binding cells in NOD mice produced IFN-γ and TNF-α but not IL-17A, whereas B6.g7 mice produced little to no IFN-γ or IL-17A (Fig. 3A). IFN-γ was enriched in CD44high cells in NOD mice (Supplemental Fig. 1D). TNF-α production by insulin-specific CD4+ T cells was detected in both strains, suggesting that the cells were viable (Fig. 3A) (22). These data support a model in which insulin-specific CD4+ T cells encounter Ag and differentiate into pathogenic Th1 cells in NOD mice, whereas failure to encounter autoantigen in B6.g7 mice leads to a lack of Th1 differentiation.
FIGURE 3.
Insulin-specific CD4+ T cells produce the pathogenic cytokine IFN-γ in NOD mice but not B6.g7 mice. (A) Frequency of insulin-specific CD4+ T cells from combined SLOs (Spl+nonDLN+pLN) producing IFN-γ, IL-17A, or TNF-α. Data are compiled from nine experiments with B6.g7 (n = 12), nondiabetic NOD (n = 28), and diabetic NOD (n = 9) mice. (B) Frequency of Foxp3+ insulin-specific CD4+ T cells in B6.g7 mice at 5 wk (n = 6), NOD mice at 5 wk (n = 17), and NOD mice at 20–26 wk (n = 17). Data are compiled from six experiments. Cells were gated on singlet+, B220−, CD11b−, CD11c−, CD4+, CD8a−, InsB10–23r3:I-Ag7-PE+ , and InsB10–23r3:I-Ag7-allophycocyanin+. **p = 0.001–0.01, ***p < 0.001. ns, Not significant.
Insulin-specific CD4+ T cells can differentiate into Foxp3+ regulatory T cells
Previous reports demonstrated that regulatory T cells (Tregs) influence the development of T1D (20). We examined the possibility that NOD mice had a decreased frequency of insulin-specific Tregs. However, Foxp3+ Tregs developed in both strains, and we did not measure a significant difference between NOD and B6.g7 mice (Fig. 3B). Therefore, we speculate that Ag-specific Tregs alone are not the main mechanism for diabetes resistance in the B6.g7 mouse. In fact, it appears that there are more Tregs in NOD mice compared with B6.g7 mice; however, this difference is not statistically significant.
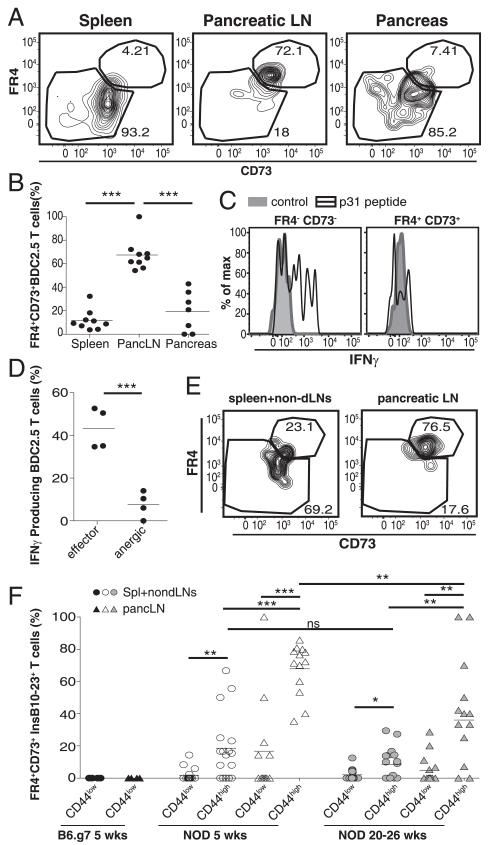
T cell anergy develops in the pLNs of NOD mice
It is thought that T1D develops as the result of the loss of peripheral tolerance. However, tolerance induction in the endogenous insulin-specific CD4+ T cell population has never been tested. We predicted that the majority of insulin-reactive T cells would escape tolerance and cause diabetes. However, we found that only a small subset of insulin-reactive T cells produced IFN-γ and infiltrated the pancreas (Figs. 2, 3). We speculated that Ag-experienced insulin-specific T cells might become anergized and be retained in the SLOs. To address this possibility, we took advantage of FR4 and CD73, which were recently identified as markers of T cell anergy on CD4+CD44highFoxp3− T cells (23). Recent experiments that we performed in healthy hosts also identified FR4+CD73+ anergic cells within polyclonal CD4+ T cells (L.A. Kalekar, K.E. Pauken, J.L. Linehan, B.T. Fife, M.K. Jenkins, and D.L. Mueller, unpublished observations). To test this in T1D, we used our recently characterized adoptive-transfer model to study islet-specific CD4+ T cells (15). In this model, a low number of naive T cells from the BDC2.5 mouse were transferred into prediabetic NOD mice to mimic the endogenous population (15). By 3 wk posttransfer, the BDC2.5 CD4+ T cells developed an anergic phenotype (Foxp3−FR41CD731) (Fig. 4A, 4B). Surprisingly, more BDC2.5 CD4+ T cells in the pLNs were anergic (Fig. 4A, 4B) than were effector phenotype cells (FR4−CD73−CD44highFoxp3−). In contrast, anergic phenotype cells were largely absent from the spleen and pancreas (Fig. 4A, 4B). To validate that coexpression of FR4 and CD73 correlated with functional anergy in diabetogenic T cells, we assessed IFN-γ in the pLNs. Following peptide challenge, IFN-γ was not produced by CD73+FR4+BDC2.5 T cells, suggesting that they were anergic (Fig. 4C, 4D).
FIGURE 4.
Anergy is induced in islet-reactive CD4+ T cells in NOD mice but not B6.g7 mice. (A) FR4 and CD73 expression on BDC2.5 T cells 17 d posttransfer. (B) Quantification of anergic BDC2.5 T cells in the spleen, pLNs, and pancreas 17–26 d posttransfer to nondiabetic NOD mice from three experiments. (C) IFN-γ production by effector (FR4−CD73−) and anergic (FR4+CD73+) phenotype BDC2.5 T cells in the pLNs at 56 d posttransfer. (D) Frequency of IFNg+ BDC2.5 T cells in the pLNs at 56 d posttransfer. Data in (C) and (D) are representative from three experiments (n = 3–4). (E) FR4 and CD73 on insulin-specific CD4+ T cells from a 5-wkold nondiabetic NOD mouse. (F) Frequency of anergic phenotype (Foxp3−FR4+CD73+) insulin-specific CD4+ T cells in the spleen+non-dLNs (circles) and the pLNs (triangles) from 5-wk-old B6.g7 mice (black, n = 6), 5-wk-old nondiabetic NOD mice (white, n = 17), and 20–26-wk-old nondiabetic NOD mice (gray, n = 17). Insulin-specific CD4+ T cells were further gated on CD44high versus CD44low cells. Data are compiled from six experiments. Cells are gated on singlet+, CD3+, B220−, CD11b−, CD11c−, CD4+, Foxp3− cells. Data in (A)–(D) also include Thy1.1+, CD44high cells, and data in (E) and (F) also include InsB10–23r3:I-Ag7 PE+ and allophycocyanin+ tetramers. *p = 0.01–0.05, **p = 0.001–0.01, ***p < 0.001. ns, Not significant.
We next examined endogenous insulin-specific CD4+ T cells in NOD and B6.g7 mice for the anergic phenotype. Similar to BDC2.5 cells, the majority of insulin-specific cells displayed the anergic phenotype within the pLNs of NOD mice, and these cells were largely absent from the spleen and non-dLNs (Fig. 4E, 4F). It was difficult to assess cytokine production from the insulin-specific population because of low cell numbers; however, anergic polyclonal CD4+ cells were functionally impaired compared with effector cells, similarly to BDC2.5 cells (Supplemental Fig. 1E). We speculate that the small population of effector cells in the pLNs have escaped tolerance and participate in T1D pathogenesis. The frequency of anergic phenotype insulin-specific T cells was significantly higher in the pLNs of young NOD mice (5 wk) compared with older NOD mice (20–26 wk), consistent with the idea that as mice age, more cells break tolerance. These data support a model in which anergy is induced in a large fraction of insulin-specific CD4+ T cells in the pLNs early in life. However, insulin-specific cells can be detected in the pancreas at this age (Fig. 2), demonstrating that tolerance is incomplete, and progression to T1D may require only a small fraction of self-reactive T cells escaping peripheral tolerance. Furthermore, these data suggest that anergy is not sustained as NOD mice age, which may contribute to enhanced infiltration of the pancreas, ultimately causing diabetes.
We next compared the frequency of anergic cells to ascertain whether anergic cells dominated the B6.g7 mice. B6.g7 mice did not have a significant population of anergic insulin-specific cells (Fig. 4F). These data suggest that B6.g7 mice are protected from T1D because insulin-specific cells do not become effector T cells and retain a naive phenotype rather than become anergic cells.
Although the mechanisms that limit autoimmunity in B6.g7 mice but fail in NOD mice remain to be fully elucidated, our data suggest that there is a substantial difference in the presentation of InsB10-23/MHC class II between these mouse strains. Our findings suggest that, in NOD mice, insulin-specific CD4+ T cells encountered Ag in the pLNs between weeks 3 and 5 of life, and a small subset differentiated into IFNg+ effector cells and infiltrated the pancreas, whereas another larger subset became anergic. Despite the induction of anergy in a large fraction of cells in the pLNs, insulin-specific CD4+ T cells in young NOD mice are capable of driving disease. Therefore, we speculate that only a small number of activated effector cells are responsible for T1D in NOD mice. We further speculate that B6.g7 mice are diabetes resistant because these cells fail to encounter autoantigen and remain naive.
In conclusion, we developed an MHC class II tetramer that allows tracking of InsB10–23r3-specific CD4+ T cells. Despite the presence of pathogenic CD4+ T cells in NOD mice, an anergic population emerged specifically in the pLNs. This population diminished over time and was absent from the pancreas. In B6.g7 mice, insulin-specific cells remained naive, suggesting an absence of Ag encounter. Studying how insulin-specific CD4+ T cells become activated in NOD mice, but not in B6.g7 mice, may provide new insight into how tolerance is breached during T1D. It would be interesting to determine whether other InsB9–23 registers have the same bias toward anergy induction or whether these cells are more pathogenic (12, 24). Understanding how peptide/MHC binding impacts the effector potential of diabetogenic CD4+ T cells may aid in our therapeutic efforts to restore tolerance in T1D patients.
Supplementary Material
Acknowledgments
We thank Dr. Vaiva Vezys and Jason Schenkel (Department of Microbiology, University of Minnesota) for critical review of the manuscript and James Heffernan (Department of Medicine, University of Minnesota) for animal care.
This work was supported by the Juvenile Diabetes Research Foundation (22011662 to B.T.F.), the American Diabetes Association (0709JF21 to B.T.F.), the National Institutes of Health (P01 AI35296 to B.T.F., D.L.M., and M.K.J.), and a Stanwood Johnston Fellowship (to K.E.P.)
Abbreviations used in this article
- B6.g7 mice
B6 mice expressing the I-Ag7 allele
- HEL
hen egg lysozyme
- InsB
insulin B chain
- non-dLN
nondraining lymph node
- pLN
pancreatic lymph node
- SA
streptavidin
- SLO
secondary lymphoid organ
- T1D
type 1 diabetes
- Treg
regulatory T cell
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati O, Raghu PK, Paquette TL. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983;222:1337–1339. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- 2.Wegmann DR, Norbury-Glaser M, Daniel D. Insulin-specific T cells are a predominant component of islet infiltrates in pre-diabetic NOD mice. Eur. J. Immunol. 1994;24:1853–1857. doi: 10.1002/eji.1830240820. [DOI] [PubMed] [Google Scholar]
- 3.Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur. J. Immunol. 1995;25:1056–1062. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Nakayama M, Eisenbarth GS. Insulin as an autoantigen in NOD/human diabetes. Curr. Opin. Immunol. 2008;20:111–118. doi: 10.1016/j.coi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, Yu L, Wegmann DR, Hutton JC, Elliott JF, Eisenbarth GS. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daniel C, Weigmann B, Bronson R, von Boehmer H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J. Exp. Med. 2011;208:1501–1510. doi: 10.1084/jem.20110574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Stadinski BD, Michels A, Kappler JW, Eisenbarth GS. Immunization with an insulin peptide-MHC complex to prevent type 1 diabetes of NOD mice. Diabetes Metab. Res. Rev. 2011;27:784–789. doi: 10.1002/dmrr.1252. [DOI] [PubMed] [Google Scholar]
- 8.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenkins MK, Moon JJ. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J. Immunol. 2012;188:4135–4140. doi: 10.4049/jimmunol.1102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levisetti MG, Suri A, Petzold SJ, Unanue ER. The insulin-specific T cells of nonobese diabetic mice recognize a weak MHC-binding segment in more than one form. J. Immunol. 2007;178:6051–6057. doi: 10.4049/jimmunol.178.10.6051. [DOI] [PubMed] [Google Scholar]
- 11.Stadinski BD, Zhang L, Crawford F, Marrack P, Eisenbarth GS, Kappler JW. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc. Natl. Acad. Sci. USA. 2010;107:10978–10983. doi: 10.1073/pnas.1006545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawford F, Stadinski B, Jin N, Michels A, Nakayama M, Pratt P, Marrack P, Eisenbarth G, Kappler JW. Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proc. Natl. Acad. Sci. USA. 2011;108:16729–16734. doi: 10.1073/pnas.1113954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohan JF, Petzold SJ, Unanue ER. Register shifting of an insulin peptide-MHC complex allows diabetogenic T cells to escape thymic deletion. J. Exp. Med. 2011;208:2375–2383. doi: 10.1084/jem.20111502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zucchelli S, Holler P, Yamagata T, Roy M, Benoist C, Mathis D. Defective central tolerance induction in NOD mice: genomics and genetics. Immunity. 2005;22:385–396. doi: 10.1016/j.immuni.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Pauken KE, Jenkins MK, Azuma M, Fife BT. PD-1, but not PDL1, expressed by islet-reactive CD4+ T cells suppresses infiltration of the pancreas during Type 1 Diabetes. Diabetes. 2013;62:2859–2869. doi: 10.2337/db12-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stratmann T, Martin-Orozco N, Mallet-Designe V, Poirot L, McGavern D, Losyev G, Dobbs CM, Oldstone MB, Yoshida K, Kikutani H, et al. Susceptible MHC alleles, not background genes, select an autoimmune T cell reactivity. J. Clin. Invest. 2003;112:902–914. doi: 10.1172/JCI18337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez X, Kreuwel HT, Redmond WL, Trenney R, Hunter K, Rosen H, Sarvetnick N, Wicker LS, Sherman LA. CD8+ T cell tolerance in nonobese diabetic mice is restored by insulin-dependent diabetes resistance alleles. J. Immunol. 2005;175:1677–1685. doi: 10.4049/jimmunol.175.3.1677. [DOI] [PubMed] [Google Scholar]
- 18.Hultgren B, Huang X, Dybdal N, Stewart TA. Genetic absence of gamma-interferon delays but does not prevent diabetes in NOD mice. Diabetes. 1996;45:812–817. doi: 10.2337/diab.45.6.812. [DOI] [PubMed] [Google Scholar]
- 19.Esensten JH, Lee MR, Glimcher LH, Bluestone JA. T-betdeficient NOD mice are protected from diabetes due to defects in both T cell and innate immune system function. J. Immunol. 2009;183:75–82. doi: 10.4049/jimmunol.0804154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu. Rev. Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 21.Joseph J, Bittner S, Kaiser FM, Wiendl H, Kissler S. IL-17 silencing does not protect nonobese diabetic mice from autoimmune diabetes. J. Immunol. 2012;188:216–221. doi: 10.4049/jimmunol.1101215. [DOI] [PubMed] [Google Scholar]
- 22.Priyadharshini B, Welsh RM, Greiner DL, Gerstein RM, Brehm MA. Maturation-dependent licensing of naive T cells for rapid TNF production. PLoS ONE. 2010;5:e15038. doi: 10.1371/journal.pone.0015038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez RJ, Zhang N, Thomas SR, Nandiwada SL, Jenkins MK, Binstadt BA, Mueller DL. Arthritogenic self-reactive CD4+ T cells acquire an FR4hiCD73hi anergic state in the presence of Foxp3+ regulatory T cells. J. Immunol. 2012;188:170–181. doi: 10.4049/jimmunol.1101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat. Immunol. 2010;11:350–354. doi: 10.1038/ni.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.