SUMMARY
Reactivation of a silent transcriptional program is a critical step in successful axon regeneration following injury. Yet how such program is unlocked after injury remains largely unexplored. We found that axon injury in peripheral sensory neurons elicits a back-propagating calcium wave that invades the soma and causes nuclear export of HDAC5 in a PKCµ–dependent manner. Injury-induced HDAC5 nuclear export enhances histone acetylation to activate a pro-regenerative gene expression program. HDAC5 nuclear export is required for axon regeneration, since expression of a nuclear-trapped HDAC5 mutant prevents axon regeneration, whereas enhancing HDAC5 nuclear export promotes axon regeneration in vitro and in vivo. Components of this HDAC5 pathway failed to be activated in a model of central nervous system injury. These studies reveal a signaling mechanism from the axon injury site to the soma that controls neuronal growth competence and suggest a role for HDAC5 as a transcriptional switch controlling axon regeneration.
INTRODUCTION
Injured peripheral neurons successfully activate intrinsic signaling pathways to enable axon regeneration (Liu et al., 2011). Within hours of a peripheral nerve injury, damaged axon tips are transformed into new growth-cone-like structures, and the expression of regeneration-associated genes in the cell body enhances axon regenerative capacity. In contrast, neurons within the central nervous system (CNS) typically fail at these tasks, leading to permanent neurological impairments. Defining how these intrinsic regenerative pathways are initiated may thus suggest novel therapeutic approaches to improve neuronal recovery following axon injury.
Activation of a genetic regeneration program is an important determinant of successful axon regeneration (Smith and Skene, 1997; Tedeschi, 2011). During development, multiple transcriptional pathways regulate the genes that control axons’ intrinsic growth capacity. Once axons have reached their targets, however, these transcriptional pathways are turned off and the growth program is shut down. Peripheral neurons are able to successfully reactivate this growth program by expressing regeneration-associated genes that allow for robust axonal re-growth (Smith and Skene, 1997; Tedeschi, 2011), whereas CNS neurons are typically unable to do so. Activation of such pro-regenerative program is illustrated by the conditioning injury paradigm, in which a sensory neuron exposed to a prior peripheral lesion exhibits a dramatic improvement in axon regeneration compared to that of a naive neuron (McQuarrie and Grafstein, 1973; Richardson and Issa, 1984; Smith and Skene, 1997).
Activation of pro-regenerative pathways requires injury signals to be sent back to the cell body to be converted to a transcriptional response (Abe and Cavalli, 2008; Rishal and Fainzilber, 2009). Three types of retrograde injury signals have been proposed to inform the cell body about a distantly located axon injury and to trigger the activation of a regenerative program (Ambron and Walters, 1996). First, positive injury signals comprise microtubule-dependent retrograde transport of protein complexes from the injured axon back to the cell body (Abe and Cavalli, 2008; Rishal and Fainzilber, 2009). Blocking the retrograde transport of such injury signals inhibits the activation of certain transcription factors and prevents efficient regeneration (Shin et al., 2012) (Perry et al., 2012; Ben-Yaakov et al., 2012). Second, the reduction in target-derived trophic factors is another mechanism for signaling axon injury to the cell body (Shadiack et al., 2001). Third, axon injury triggers a rapid depolarization and intracellular calcium influx that propagates along the axon in Aplysia, C. elegans and mammalian neurons (Ghosh-Roy et al., 2010) (Mandolesi et al., 2004) (Ziv and Spira, 1993).
Although many studies have identified injury signals and transcriptional signaling pathways activated by nerve injury, the epigenetic mechanisms that control the switch from silent to growth-competent state following injury remain largely unexplored. Here we reveal that axon injury elicits a back-propagating calcium wave invading the soma and causing nuclear export of HDAC5 in a PKCµ–dependent manner, leading to enhanced histone acetylation. Importantly, injury-triggered PKCµ activation and increased histone acetylation fail to occur in retinal ganglion cells (RGCs), a model of CNS neurons. Promoting HDAC5 nuclear export mimics the conditioning injury paradigm and accelerates axon regeneration, whereas expression of an HDAC5 mutant that is retained in the nucleus prevents axon regeneration. Our results suggest that injury-induced HDAC5 nuclear export underlies an epigenetic switch controlling regenerative competence in adult sensory neurons.
RESULTS
Axon injury causes elevation of calcium levels and PKC activation in DRG cell bodies
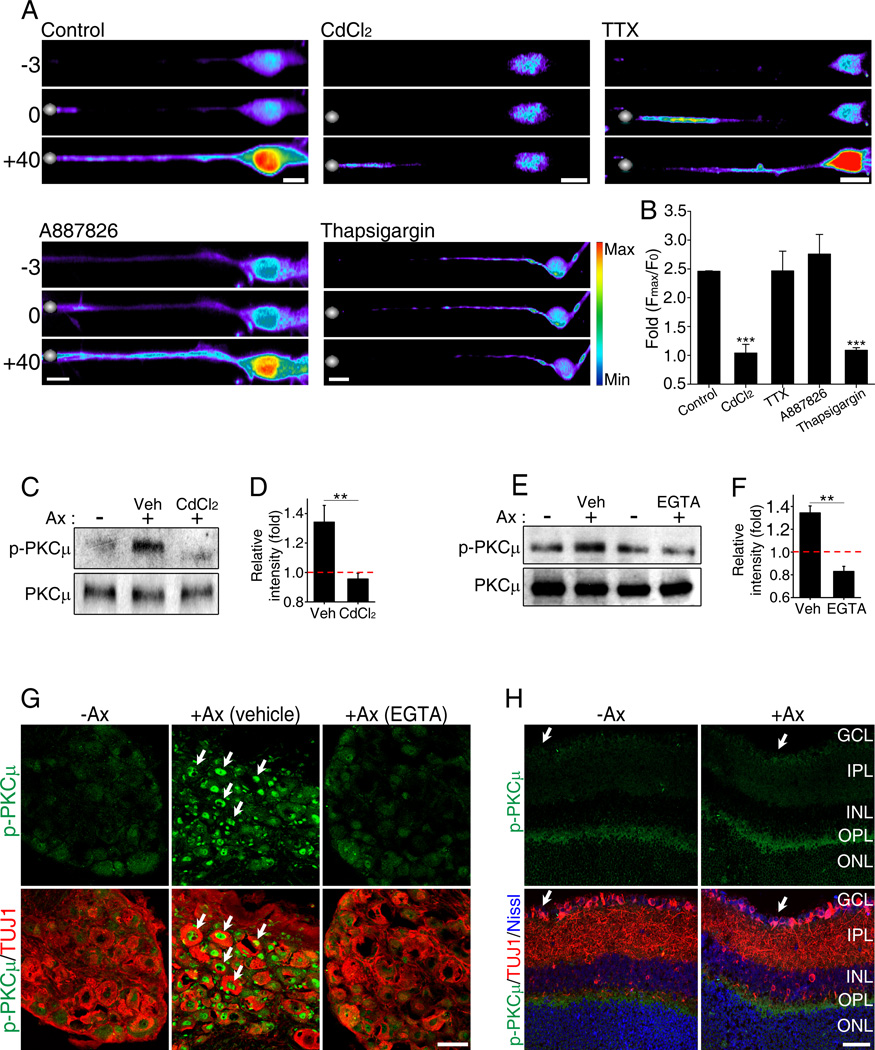
Calcium signals have been implicated in axon regeneration in many systems (Ambron and Walters, 1996; Ghosh-Roy et al., 2010; Kamber et al., 2009) and we have shown that axotomy of DRG neurons causes elevation of the intracellular calcium levels at the site of injury and in the cell soma (Cho and Cavalli, 2012). We hypothesized that a back-propagating calcium wave may serve as a rapid signaling mechanism informing the cell body about distant axon injury. We first examined the mechanisms regulating axotomy-induced calcium back-propagation in DRG neurons. We found that inhibition of VGCCs with CdCl2 completely blocked the calcium wave propagation from the axotomy site back to the cell body (Figures 1A and 1B; Movies S1 and S2). In contrast, blocking VGSCs with TTX had little effect on calcium wave back-propagation and calcium levels in cell body compared to control (Figure1A and 1B; Movies S3). Because DRG neurons contain TTX-resistant sodium channels, we used A887826 (Zhang et al., 2010) to block Nav1.8, one of the most prominent TTX-resistant sodium channels present in the majority of DRG neurons (Shields et al., 2012). A887826, similar to TTX, did not significantly block calcium back propagation and accumulation in the soma (Figure1A and 1B, Movie S4). Next we tested the role of calcium release from internal stores using thapsigargin to deplete internal calcium stores. Thapsigargin completely blocked the calcium back propagation wave and accumulation in the cell soma following axon injury (Figure1A and 1B; Movies S5). Together these data suggest that calcium release from internal stores together with VGCCs mediate injury-induced calcium back propagation along DRG axons. We noted that the intracellular calcium wave propagated at a rate of ~10 µm/sec, which is comparable to what has been observed previously in Aplysia neurons (Ziv and Spira, 1993) and in C. elegans (Ghosh-Roy et al., 2010).
Figure 1. Axotomy induces a back propagating calcium wave causing PKCµ activation in the soma.
(A) Calcium influx induced by laser axotomy was monitored in cultured embryonic DRG neurons using Fluo-4AM. Silver dots: laser axotomy site, time in seconds. Scale, 10µm. (B) Maximum fold changes (Fmax/F0) in DRG cell bodies (n=8, 10, 8, 6 and 8 respectively; ***p<0.001). (C) DRG spot cultures collected in the absence of axotomy (−) or 1 hour after axotomy (+) in the presence vehicle (Veh) or of 1mM CdCl2 were analyzed by western blot. (D) Quantification of (C) (n=5; **p<0.01). (E) Sciatic nerve was axotomized (+) with locally applied vehicle (Veh) or 10mM EGTA (100µl), DRG collected 2 hours later and analyzed by western blot. (F) Quantification of (E) (n=4; **p<0.01). (G) Sciatic nerve were axotomized (+Ax) with locally applied vehicle or 10mM EGTA. Sections of DRGs collected 24 hours later were stained with the indicated antibodies. Scale, 50µm. (H) Sections of retina that received or not a prior (24 hours) optic nerve axotomy were stained with indicated antibodies. Scale, 50µm. GCL, ganglion cells layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer. Data are represented as mean±SEM.
Calcium transients induced by axon injury in C. elegans have been associated with cAMP and DLK signaling (Ghosh-Roy et al., 2010). However, elevation of cAMP only partly recapitulates a peripheral nerve injury response in rats (Blesch et al., 2012), suggesting that other signaling pathways functioning downstream of calcium influx activate a regenerative response. We have shown that PKCµ (also known as PKD) is activated in axons via injury-induced calcium influx (Cho and Cavalli, 2012). We thus tested whether PKCµ is also activated in DRG cell bodies following nerve injury in a calcium-dependent manner. Spot-cultured DRG neurons were axotomized and analyzed 1 hour post axotomy for the levels of phosphorylated PKCµ (p-PKCµ) (Figures 1C and 1D). Axotomy-induced PKCµ phosphorylation was reduced by pre-incubation of DRG neurons with CdCl2 to prevent calcium back-propagation (Figures 1C and 1D). To further test whether activation of PKCµ in the cell body results from the actual back propagation of a calcium wave or by motor-dependent retrograde transport of injury signals, we performed a sciatic nerve injury in the presence or absence of locally applied calcium chelator EGTA and analyzed the levels of p-PKCµ in DRGs 2 hours after injury. The presence of EGTA at the site of nerve injury strongly reduced phosphorylation of PKCµ in DRG cell bodies (Figures 1E and 1F). Given that the site of injury in the sciatic nerve is located ~ 2 cm away from the DRG cell body, it would take a retrograde motor moving at the average in vivo speed of 1.5 µm/sec (Bilsland et al., 2010) at least ~ 4 hours to cover the distance between injury site and DRG cell body. This data suggest that a calcium wave initiated at the site of injury activates PKCµ in DRG cell body.
Activation of PKCµ also correlates with its translocation from the cytoplasm to the nucleus (Wang, 2006). Consistently, we observed that nerve injury induced re-localization of p-PKCµ to the nucleus of DRG neurons, which was blocked when EGTA was applied locally at the site of nerve injury (Figure 1G). In contrast, phosphorylation of PKCµ was not observed in retinal ganglion cells (RGC), a model of CNS neurons, following optic nerve injury (Figure 1H). These observations indicate that axon injury in DRG neurons causes the back-propagation of a calcium wave that invades the soma and leads to PKCµ activation, whereas optic nerve injury fails to elicit PKCµ activation in RGC neurons.
Axon injury stimulates HDAC5 nuclear export in a calcium- and PKC-dependent manner
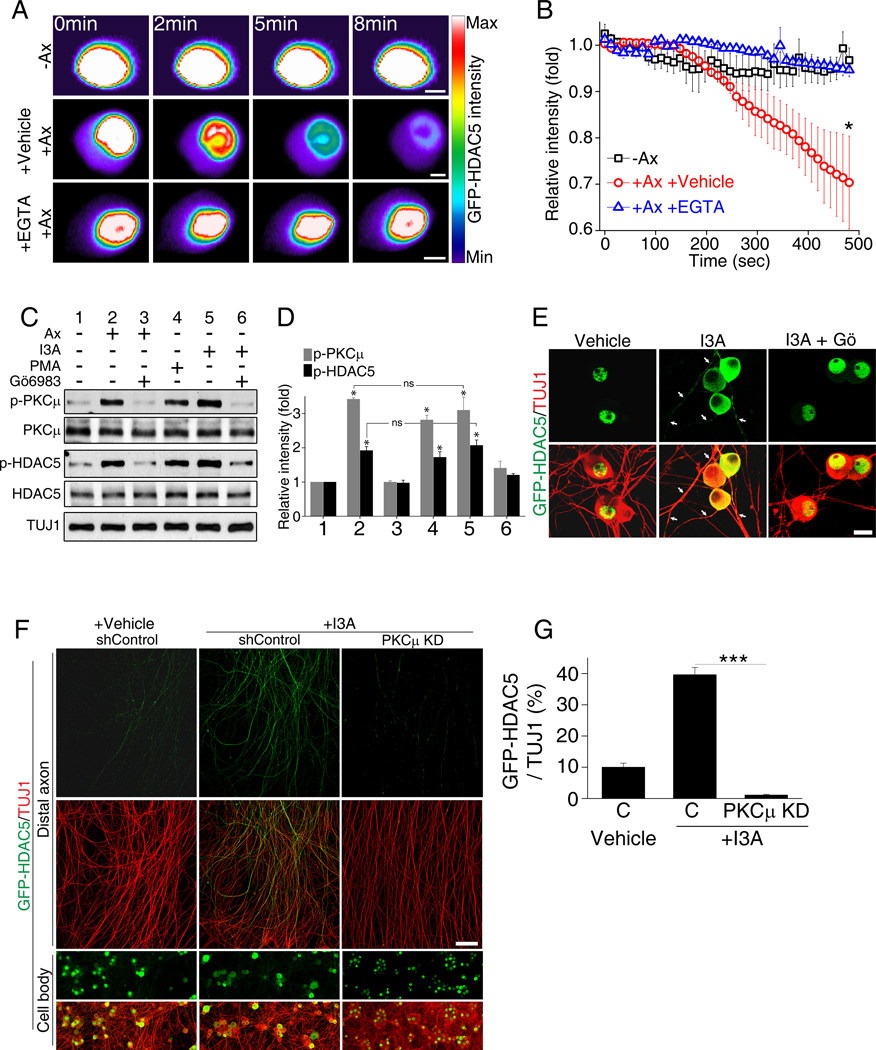
Calcium influx and PKCµ are known to promote nuclear export of the class II histone deacetylase HDAC5 in cardiomyocytes (Vega et al, 2004) and hippocampal neurons (Chawla et al., 2003) and we have shown that PKC phosphorylates HDAC5 locally in injured axons (Cho and Cavalli, 2012). We thus examined whether axotomy in DRG neurons induces HDAC5 nuclear export. Cultured DRG neurons expressing GFP-HDAC5 were axotomized and fluorescence intensity in the nucleus visualized over time. Control uninjured DRG nuclei displayed a stable level of fluorescence intensity, whereas axotomy induced a dramatic decrease in GFP-HDAC5 intensity in the nucleus (Figures 2A and 2B and Movie S6). This axotomy-induced effect was blocked by minimizing the increase in somatic calcium levels with EGTA (Figures 2A and 2B and Movie S6). This experiment reveals that axotomy leads to HDAC5 nuclear export in a calcium-dependent manner.
Figure 2. Axotomy induces HDAC5 nuclear export through PKCµ activation.
(A) Intensity profiles of GFP-HDAC5-expressing DRG cell bodies. GFP-HDAC5 intensity in DRG cell body was monitored over time with or without laser axotomy in the presence or absence of 1mM EGTA. Scale, 5µm. (B) Average intensity plot of nuclear GFP-HDAC5 (−Ax, without axotomy; +Ax +Vehicle, with axotomy treated with vehicle; +Ax +EGTA, with axotomy treated with EGTA; n=4, 5 and 4 for each; *p<0.05). (C) Cultured DRG neurons were treated as indicated for 1 hour and analyzed by western blot. (I3A, ingenol 3-angelate, 50 nM; PMA, phorbol 12-myristate 13-acetate, 10 µM). (D) Quantification of (C) (n=5; *p<0.05; ns, not significant). (E) DRG neurons expressing GFP-HDAC5 were treated with vehicle, 50nM I3A or I3A with 10µM Gö6983 for 1 hour and stained for TUJ1 antibody. Scale, 50µm. Arrowheads: TUJ1 positive axons. (F) DRG spot cultures expressing GFP-HDAC5 were infected with control shRNA or PKCµ shRNA and treated with vehicle or 5 nM I3A, as indicated. Cell body and axon areas were analyzed. Scale,100µm. (G) Quantification of GFP-HDAC5 intensity normalized to TUJ1 intensity (C, shControl; PKCµ KD, PKCµ shRNA; n=15, 12 and 10 for each; ***p<0.001). Data are represented as mean±SEM. See also Figure S1.
We next tested whether PKCµ activation promotes HDAC5 nuclear export in DRG neurons. To achieve this, we first established a way to activate PKCµ pharmacologically. We tested whether phorbol myristate acetate (PMA) or the small molecule activator of PKC, ingenol 3-angelate (I3A) (Kedei et al., 2004), leads to PKCµ activation. Cultured DRG neurons were treated for 1 hour with PMA or I3A and the level of phosphorylation of PKCµ and HDAC5 was determined. Compared to uninjured neurons, I3A and PMA induced PKCµ and HDAC5 phosphorylation similarly to axotomy (Figures 2C and 2D). The presence of the PKC inhibitor Gö6983 prevented both I3A- and axotomy-induced phosphorylation of PKCµ and HDAC5 (Figures 2C and 2D). Because I3A is a more selective activator of PKC (Kedei et al., 2004), we continued to use I3A in our experiments.
To test whether I3A promotes HDAC5 nuclear export, cultured DRG neurons expressing GFP-HDAC5 were treated with I3A for 1 hour in the presence or absence of Gö6983 and GFP-HDAC5 localization was analyzed. In vehicle-treated neurons, GFP-HDAC5 localized mostly to the nucleus, whereas following I3A treatment, GFP-HDAC5 localized mostly to the cytoplasm and also to the axon (Figures 2E and S1). I3A’s effect depended on PKC since neurons pre-treated with Gö6983 displayed mostly nuclear GFP-HDAC5 following I3A treatment (Figure 2E). Furthermore, knock down of PKCµ (performed as described in (Cho and Cavalli, 2012), prevented GFP-HDAC5 localization to axons following I3A treatment (Figures 2F, and 2G). These experiments demonstrate that PKCµ activation causes HDAC5 nuclear export and enhances HDAC5 levels in cytoplasm and axon compartments.
HDAC5 is transported from the soma to the axon tip following injury
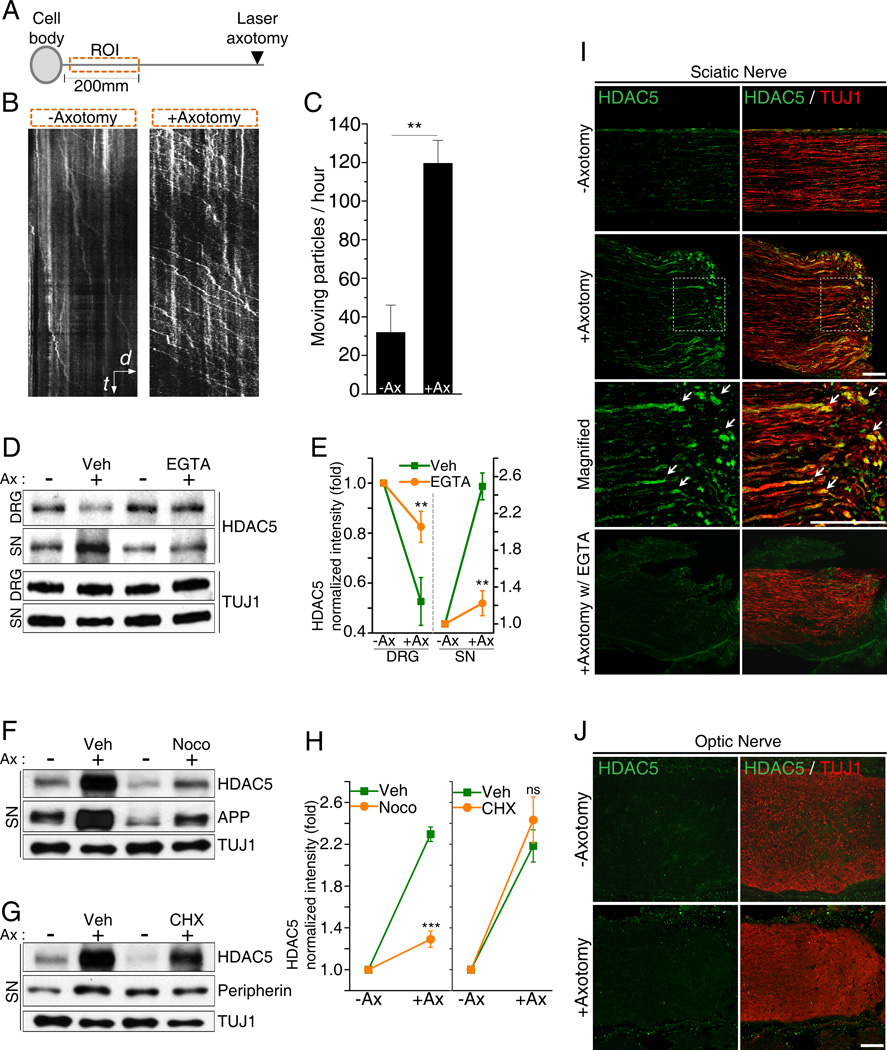
We have previously shown that HDAC5 accumulates at the site of axon injury, where it deacetylates tubulin to promote growth cone dynamics and axon regeneration (Cho and Cavalli, 2012). To determine whether the accumulation of HDAC5 in axon tips results from transport from the soma to the axon, we monitored GFP-HDAC5 movement in cultured DRG neurons in an axon segment immediately adjacent to the cell body (Figure 3A). While in uninjured axons, most of GFP-HDAC5 puncta were stationary; laser axotomy greatly increased the number of mobile GFP-HDAC5 puncta (Figures 3B and 3C, Movies S7 and S8). These puncta displayed unidirectional movement towards the tip of the axon, with velocities of 1.04±0.22 µm/sec (n=9), consistent with the interaction between HDAC5 and the anterograde molecular motor kinesin-1 we reported (Cho and Cavalli, 2012).
Figure 3. Injury induces HDAC5 anterograde transport from DRG cell bodies to distal axons.
(A) Scheme of the experimental setting indicating the region of interest (ROI) located in an axonal segment immediately proximal to the cell body. (B) GFP-HDAC5 transport following distal laser axotomy was monitored.in the ROI. Representative Kymograph is shown. t, time, 60sec; d, distance, 10µm. (C) Quantification of the number of moving GFP-HDAC5 particles per hour along the axons (−Ax, without axotomy; +Ax, with axotomy; n=5 for each; **p<0.01). (D) The sciatic nerve was axotomized in the presence of vehicle or 10mM EGTA. DRG and a 1cm proximal segment of sciatic nerve was analyzed by western blot 24 hours later. (E) Quantification of (D) (n=4 for each; **p<0.01). (F and G) As in (D) but the nerve was injured in the presence of 330µM nocodazole or 1mM cycloheximide. (H) Quantification of (F and G) (n=4 for each; *** p<0.001; ns, not significant). (I and J) Longitudinal sections of sciatic nerves (I) and optic nerves (J) with or without axotomy were stained for HDAC5 and tubulin after 24 hours. Scale, 100µm. Data are represented as mean±SEM.
To demonstrate that injury increases HDAC5 transport from the soma to the axon in vivo, we determined whether increased HDAC5 levels in injured axons correlate with a reduction of HDAC5 levels in DRG cell bodies. Sciatic nerves were axotomized and HDAC5 levels analyzed in a nerve segment proximal to the injury site, as well as in DRG cell bodies. This experiment revealed that,the accumulation of HDAC5 in injured peripheral nerves is paralleled with a decrease in HDAC5 levels in DRG cell bodies (Figures 3D and 3E). The presence of EGTA at the site of nerve injury reduced HDAC5 accumulation in the nerve, indicating that HDAC5 transport requires injury-induced calcium influx. HDAC5 accumulation in the nerve was also reduced by the presence of microtubule-destabilizing agent nocodazole, but not by the protein synthesis inhibitor cycloheximide (Figures 3F, 3G and 3H), revealing that HDAC5 accumulation in the nerve is due to anterograde axonal transport. APP, a known anterogradely transported protein (Koo et al., 1990), and peripherin, a known locally synthesized protein (Perlson et al., 2005) were used as positive controls for the effect of nocodazole and cycloheximide, respectively (Figures 3F, 3G and 3H). Immunofluorescence of sciatic nerve sections further confirmed that the calcium-dependent HDAC5 accumulation at the site of injury occurs in axons, which were co-labeled with the axon marker TUJ1 (Figure 3I). In contrast, optic nerve injury failed to increase HDAC5 levels at the tip of injured RGC axons (Figure 3J), consistent with the failed tubulin deacetylation we observed there (Cho and Cavalli, 2012). These results suggest that nerve injury in DRG neurons leads to HDAC5 anterograde transport and accumulation at the injured axon tips, but HDAC5 accumulation fails to occur at the tips of RGC axons following optic nerve injury.
Axon injury increases acetylated histone H3 levels
Histone acetylation is controlled by the activities of histone acetyltransferases (HATs) and HDACs. Injury-induced HDAC5 nuclear export may correlate with an increase in histone acetylation levels. We thus analyzed nuclear and cytoplasmic fractions prepared from naive or axotomized cultured DRG neurons for the levels of HDAC5 and acetylation of the core histone H3. Axotomy decreased HDAC5 levels in the nucleus, with a respective increase in cytoplasmic levels (Figure 4A). The decrease of nuclear HDAC5 in axotomized DRG correlated with an increase in the acetylated histone H3 (Ac-H3) levels, whereas total histone H3 levels remained constant (Figure 4A). In vivo, nerve injury also induced an increase in Ac-H3 levels, which was blocked by the presence of locally applied EGTA (Figures 4B, 4C) and decreased nuclear HDAC5 (Figure S1). To assess whether the injury-induced increase in Ac-H3 levels occurred specifically in neurons, we quantified the intensity of Ac-H3 in TUJ1 positive neurons in naive and injured conditions and confirmed a DRG specific increase in Ac-H3 levels (Figures 4D and 4E). Because HDAC5 has a low basal deacetylase activity (Lahm et al., 2007) and becomes associated with deacetylase activity when bound to HDAC3 (Fischle et al., 2002), we next examined the distribution of HDAC3 in naive and injured DRG. We observed that nerve injury triggered HDAC3 re-localization to the cytoplasm (Figures 4F and 4G). To determine if HDAC5 nuclear export is required for HDAC3 cytoplasmic re-localization, we performed these experiments using HDAC5 KO mice (Chang et al., 2004). HDAC3 cytoplasmic localization was increased in HDAC5 KO TUJ1 positive DRG neurons compared to WT (Figure 4G and S1). Nerve injury enhanced nuclear HDAC3 localization in HDAC5 KO mice, contrasting with the decreased HDAC3 nuclear localization in WT (Figure 4G and S1). Whereas the levels of Ac-H3 were higher in uninjured HDAC5 KO TUJ1 positive DRG compared to WT (Figure 4E and S1), nerve injury induced a decrease in Ac-H3 in TUJ1 positive DRG HDAC5 KO neurons relative to WT (Figure 4E and S1), consistent with increased nuclear HDAC3 (Figures 4G and S1). These results suggest that nuclear export of HDAC5 may be coupled to nuclear export of HDAC3 in injured DRG neurons to increase histone H3 acetylation levels and that compensatory mechanisms may regulate HDAC3 localization and Ac-H3 in HDAC5 KO mice following injury.
Figure 4. Axotomy increases acetylation of histone H3.
(A) Cytoplasmic and nuclear fraction were prepared from axotomized (+Ax) or naive (−Ax) cultured DRG neurons and analyzed by western blot (N, nuclear fraction; C, cytoplasm fraction; H3, histone H3; Ac-H3, acetylated histone H3). (B) DRG were collected 24 hours after sciatic nerve surgery in the presence of vehicle (+V) or 10mM EGTA (+E) and analyzed by western blot. (C) Quantification of (B) (n=3; ***p<0.001; ns, not significant). (D) DRGs collected 24 hours after sciatic nerve axotomy were stained with Ac-H3 and TUJ1. Scale, 50µm. (E) Average intensity of Ac-H3 from (D) and from sections collected from HDAC5 KO mice (n=6 for each; ***p<0.001, *p<0.05). (F) DRG sections stained with HDAC3 and TUJ1. Scale, 50µm. (G) Average of relative intensity of nuclear HDAC3 over cytoplasmic HDAC3 in WT and HDAC5 KO mice (n=13 for each; ***p<0.001, *p<0.05). (H) The RGC layer of retina that received or not a prior (24 hours) optic nerve axotomy were stained for Ac-H3 and DAPI. Scale, 10µm. Data are represented as mean±SEM. See also Figure S1.
Considering that HDAC3 moves into the nucleus in RGC following optic nerve injury (Pelzel et al., 2010), this data further support the notion that DRG and RGC neurons display different responses to injury. We thus tested whether the lack of PKCµ phosphorylation we observed in injured RGC neurons (Figure 1H) corresponds to a failure to increase the level of Ac-H3. Indeed, rather than an increase, we observed a decrease in the level of Ac-H3 in RGC nuclei following optic nerve injury, with some nuclei completely lacking Ac-H3 (Figure 4H). Together, these experiments indicate that axon injury increases Ac-H3 levels mainly via HDAC5 nuclear export in DRG neurons, but Ac-H3 levels decrease in RGC neurons following injury.
HDAC5 nuclear export is required for axon regeneration
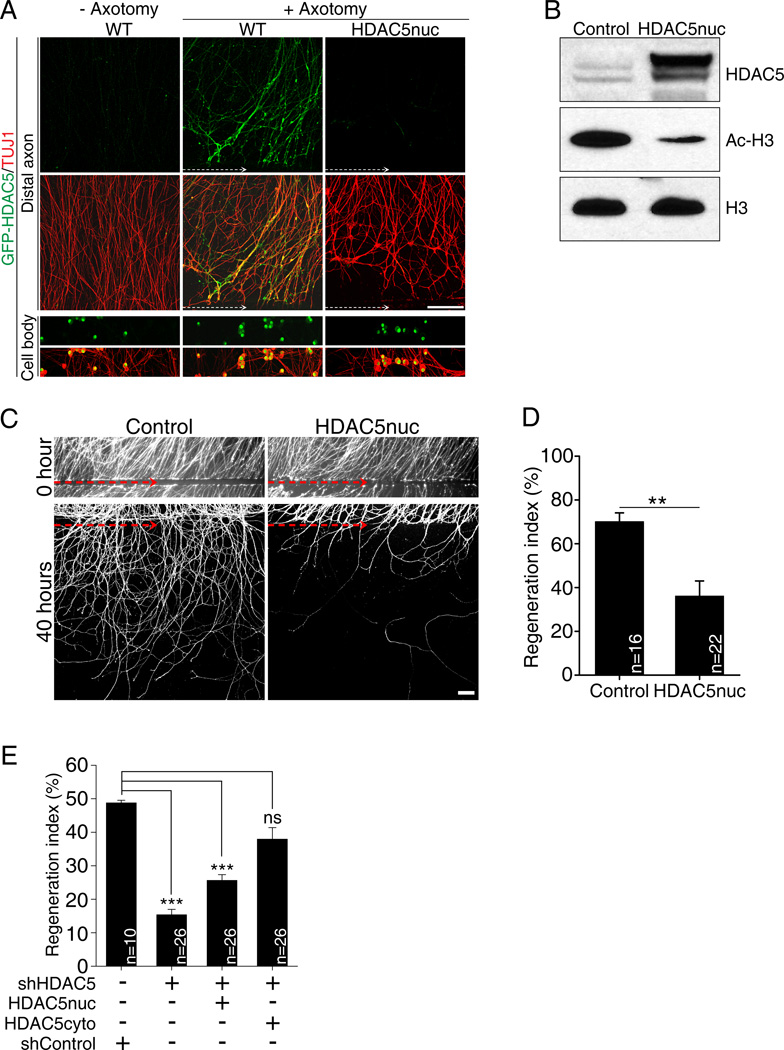
Peripheral nerve injury activates a pro-regenerative gene expression program that is essential to promote axon regeneration (Smith and Skene, 1997). If HDAC5 nuclear export is required to activate such pro-regenerative gene expression program, then preventing HDAC5 nuclear export should limit axon regeneration. We tested this possibility by engineering an HDAC5 mutant that is trapped in the nucleus and unable to be exported to the cytoplasm of DRG neurons. Based on previous studies (Vega et al., 2004) (Ha et al., 2010), we mutated serine residues 259, 280 and 498 to aspartic acids (GFP-HDAC5nuc). In contrast to GFP-HDAC5, which reaches injured axon tips, GFP-HDAC5nuc was trapped in DRG nuclei and failed to reach axons following axotomy (Figure 5A and S2). DRG expressing GFP-HDAC5nuc also displayed decreased levels of Ac-H3 compared to DRG expressing GFP as a control (Figure 5B), indicating that mutation of these serine residues affects GFP-HDAC5nuc localization, but not its ability to regulate H3 deacetylation.
Figure 5. Expression of a nuclear HDAC5 mutant decreases the level of histone H3 acetylation and impairs axon regeneration in vitro.
(A) DRG spot cultures overexpressing GFP-HDAC5 (WT) or GFP-HDAC5nuc mutant were axotomized and stained with TUJ1. White dotted arrow, axotomy line. Scale, 100µm. (B) DRG neurons expressing GFP (control) or GFP-HDAC5nuc were analyzed by western blot. (C) DRG spot cultures expressing GFP only (control) or GFP together with GFP-HDAC5nuc were axotomized and fixed 40 hours after axotomy. Axons were visualized by GFP live imaging. Red dotted arrow indicates the axotomy site. Scale, 100µm. (D) In vitro regeneration index calculated from images in (C) (**p<0.01). (E) In vitro regeneration index calculated as in (D) in control (shControl), HDAC5 knock-down (shHDAC5), HDAC5 knock-down plus GFP-HDAC5nuc and HDAC5 knock-down plus GFP-HDAC5cyto (***p<0.001; ns, not significant). Data are represented as mean±SEM. See also Figure S2.
We then monitored axon regeneration in vitro in DRG neurons expressing GFP-HDAC5nuc. We visualized axon regrowth by live-cell fluorescence imaging after in vitro axotomy in DRG expressing GFP only or GFP together with GFP-HDAC5nuc and measured the regenerative capacity of injured axons after axotomy, as described (Cho and Cavalli, 2012). Axotomized control axons displayed robust regeneration, with a regeneration index of 70.1±4.1%, whereas GFP-HDAC5nuc expression strongly suppressed axon regeneration to 36.1±6.9% (Figures 5C and 5D). These results indicate that the shuttling of HDAC5 out of the nucleus following axon injury is required to stimulate axon regeneration. Furthermore, because these experiments were performed in the presence of endogenous HDAC5, which is transported to the axon after injury (Cho and Cavalli, 2012), this data indicates that axonal HDAC5 is not sufficient to enhance regeneration when HDAC5 is also in the nucleus. To further test nuclear vs. axonal role of HDAC5, we knocked down HDAC5 and expressed GFP-HDAC5nuc or a cytosolic-trapped HDAC5 (GFP-HDAC5cyto). We note that we chose a knock down approach due to the compensatory mechanisms regulating histone acetylation observed in HDAC5 KO mice (Figures 4E, 4G and S1). Mutation of serine residues 259 and 498 to aspartic acid resulted in a mostly cytoplasmic HDAC5 (GFP-HDAC5tcyto) that localized to axons (Figure S2). Consistent with our previous work (Cho and Cavalli, 2012), HDAC5 knock down impaired axon re-growth (Figure 5E). We found that GFP-HDAC5tcyto, but not GFP-HDAC5nuc rescued axon re-growth to control levels (Figure 5E). These results indicate that in the absence of nuclear HDAC5, axonal HDAC5 is sufficient to promote axon regeneration.
Consistent with the idea that cytoplasmic localization of HDAC5 correlates with axon growth capacity, we observed that in freshly dissociated embryonic DRG neurons, which display high growth capacity, HDAC5 was mainly found in the cytoplasm whereas, after 15 days in vitro, HDAC5 was mostly in the nucleus (Figure S2). Together these experiments point to a critical role of HDAC5 subcellular localization in the control of axon growth capacity.
HDAC5 nuclear export activates a pro-regenerative transcriptional program
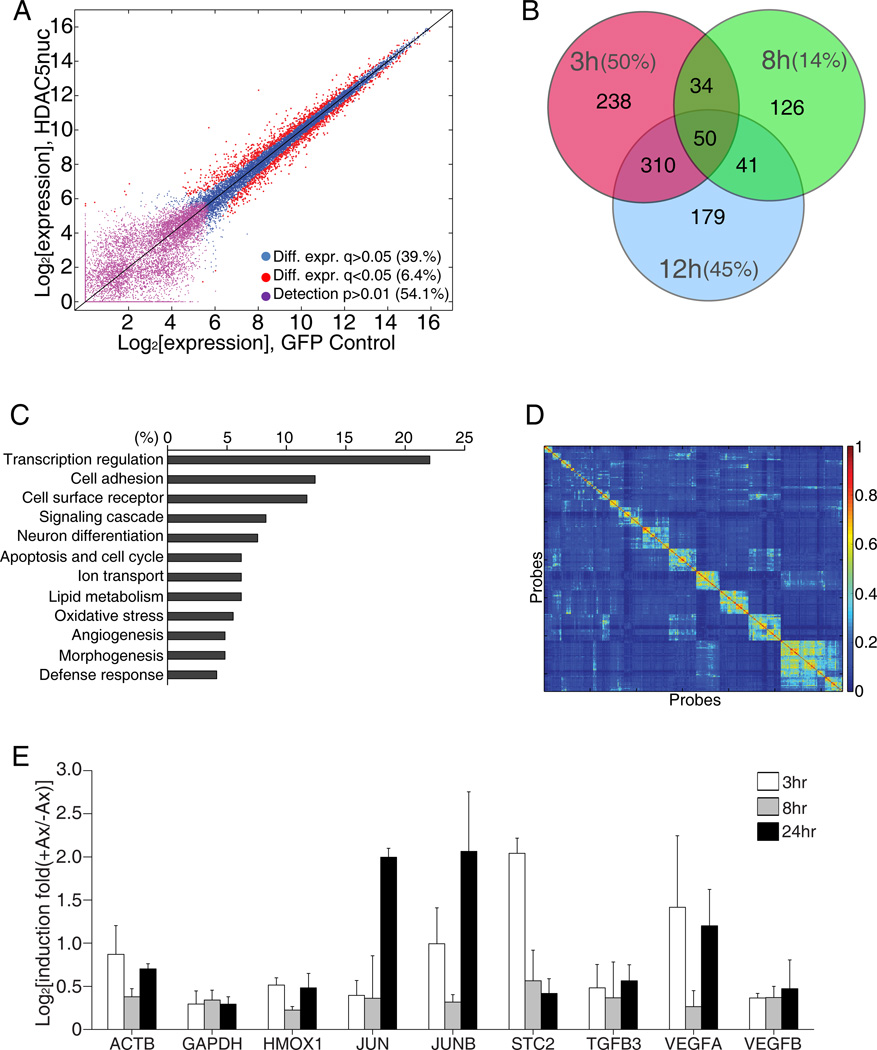
To further determine the function of HDAC5 nuclear export in the expression of pro-regenerative genes following axon injury, we examined changes in gene expression in cultured DRG by microarray analysis, comparing DRG expressing GFP or GFP-HDAC5nuc at 0, 3, 8, 12 and 40 hours after in vitro axotomy. Comparison of pre-axotomy time points using differential fold-change analyses revealed that global expression differences were similar to those seen between replicates indicating that gene expression was not generally affected by the expression of GFP-HDAC5nuc (Figure 6A). We used a fold change in expression of at least 1.3 relative to the 0 hour time point to select for injury-responsive genes and of 1.5 relative to GFP neurons to select for HDAC5-dependent genes (Table S1). Whereas the majority of changes in gene expression in GFP neurons occurred at 8 hours (Table S1 and Figure S3), the majority of gene expression changes in GFP-HDAC5nuc neurons occurred at 3 hour (Table S1 and Figure 6B). According to Gene Ontology terms, these HDAC5-dependent genes have prominent roles in the regulation of transcription (Figure 6C). Indeed, several transcription factors, previously identified to play important roles in neuronal injury response and axon growth were identified as HDAC5-dependent genes. These include jun (Broude et al., 1997) (Leah et al., 1993), KLF4 and KLF5 (Cayrou et al., 2002; Moore et al., 2009), Fos (Buschmann et al., 1998) (Xiong et al., 2010) and Gadd45a (Befort et al., 2003) (Table S1) and their expression was modulated by GFP-HDAC5nuc at one or more time points examined (Table S1). This analysis suggests that injury-induced HDAC5 nuclear export plays an important role in the regulation of regeneration-associated genes.
Figure 6. HDAC5 regulates transcriptional activation after injury.
(A) Dependence of basal gene expression on GFP-HDAC5nuc expression. Red dots: differentially expressed probes with FDR-corrected q-value<0.05 (1,637 probes, 6.4%), violet dots: probes below level of detection in both conditions (13,902, 54.1%), blue dots: remaining probes (10,158, 39.5%). (B) Venn diagram of HDAC5-dependent genes at the indicate time points after axotomy. The percentage of all genes at each time point that are HDAC5-dependent is indicated. (C) GeneOntology analysis of the HDAC5-depdendent genes shown in (B) at the 3 hours time point. (D) Heat map representation of the pairwise co-clustering frequency matrix of 646 expression vectors corresponding to 323 probes in GFP and GFP-HDAC5nuc expressing DRG neurons. Ordering of probes along horizontal and vertical axis based on hierarchical clustering. (E) Quantitative PCR analysis of mRNA samples extracted from mouse DRG that received a sciatic nerve axotomy 3, 8 or 24 hours prior to DRG dissection (n=3). See also Figure S3 and Table S1, S2 and S3.
To uncover dynamic temporal patterns of genes regulated by HDAC5 we conducted a robust cluster analysis on the 323 genes that exhibited a strong response to injury in either GFP or GFP-HDAC5nuc expressing neurons (Table S2). A robustness metric for the similarity of temporal profiles was calculated by counting the number of times a pair of gene expression profiles co-clustered across all clustering sets (Figure 6D). Since we treated the temporal vectors of the GFP and GFP-HDAC5nuc conditions separately, but in the same clustering analysis, we were able to explore specific dynamics of patterns that were similar in the two conditions, versus different according to their co-occurrence in clusters (Figure S3 and Table S3). The patterns of genes uncovered in response to injury had varying temporal responses, consistent with previous reports (Blesch et al., 2012; Michaelevski et al., 2010).
To validate our in vitro microarray analysis, we examined the expression levels of genes identified as injury-regulated using real-time quantitative PCR (qPCR) analysis of mRNA extracted from DRG following sciatic nerve injury. This analysis confirmed that a set of genes, which includes jun, were regulated by both in vitro and in vivo injuries (Figure 6E). Together, these analyses support the notion that injury-induced HDAC5 nuclear export regulates the expression of genes important for axon regeneration.
Promoting HDAC5 nuclear export mimics the conditioning injury response
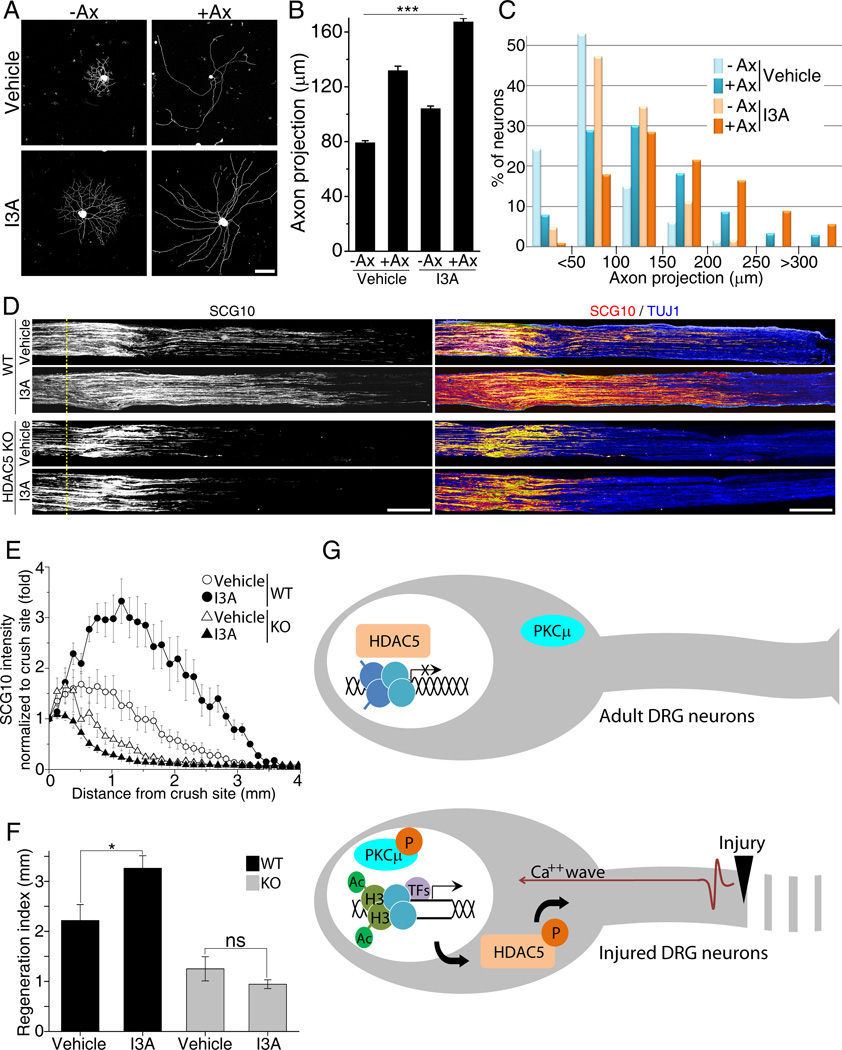
DRG neurons exposed to a prior conditioning lesion exhibit a dramatic improvement in axon regeneration compared to that of a naive neuron, which is attributed to expression of pro-regenerative genes (Smith and Skene, 1997). To test whether injury-induced HDAC5 nuclear export contributes to the priming effect of a conditioning lesion, we enhanced HDAC5 nuclear export by administering I3A subcutaneously directly above L4, L5 DRG in mice that received or not a simultaneous sciatic nerve crush injury. DRG neurons were cultured 3 days later and axon growth projection length analyzed as described (Abe et al., 2010). As expected, naive DRG neurons grow short axons, whereas a prior nerve injury enhances growth capacity leading to longer, less arborized axon (Figure 7A) (Smith and Skene, 1997). We found that I3A treatment partially mimicked the conditioning injury effect, enhancing the growth capacity of naive DRG neurons and also potentiating the effect of a conditioning injury (Figures 7A, 7B and 7C). I3A treatment increased the average projection length compared to vehicle treatment (Figure 7B), and also increased the percentage of neurons bearing long axons (Figure 7C).
Figure 7. PKCµ activation by I3A enhances axon regeneration.
(A) Adult DRG neurons were dissected from mice that received or not a prior (3 days) sciatic nerve injury and were treated with vehicle or I3A; cultured for 16 hours and stained with TUJ1. Scale, 100µm. (B) Average axon projection length was calculated from images in (A) (n=447 for −Ax, 518 for +Ax with vehicle, 4 mice; n=457 for −Ax, 548 for +Ax with I3A, 4 mice; ***p<0.001). (C) Histogram of the distribution in projection lengths of DRGs. (D) Representative longitudinal sections of sciatic nerve of wild type or HDAC5 KO mice three days after crush injury stained with SCG10 and TUJ1. Scale, 500µm. (E) Normalized SCG10 intensity plotted in function of the distance from crush line (yellow dotted line; n=6 mice for each condition;. (F) Regeneration index from (E) (*p<0.05; ns, not significant). (G) Proposed model for the role of HDAC5 in activation of a pro-regenerative program. In uninjured adult DRG neurons, HDAC5 is mostly nuclear and repress expression of regeneration-associated genes via histone deacetylation (top panel). Axon injury induces a calcium wave back propagating to the cell body, causing PKCµ activation and nuclear translocation followed by HDAC5 nuclear export, thereby relieving the inhibition of gene transcription via increased histone acetylation and enhancing axon regeneration (bottom panel). Data are represented as mean±SEM. See also Figure S3.
To determine if I3A treatment also increase DRG axon growth capacity in vivo, we administered I3A subcutaneously directly above L4, L5 DRG in mice that received a simultaneous sciatic nerve crush injury and quantified regenerating axons, labeled for SCG10 (Shin et al., 2012) three days later.. I3A treatment increased dramatically the number of axons that regenerated past the crush site (Figures 7D, 7E and 7F). We calculated a regeneration index by measuring the distance away from the crush site in which the average SCG10 intensity is half that observed at the crush site (Abe et al., 2010). The regeneration index was significantly higher in nerves treated with I3A compared with vehicle treated nerves (Figure 7F). This experiment suggests that I3A treatment primes DRG neurons for accelerated growth after injury. To determine whether the effects of I3A depend on HDAC5, we performed these experiments using HDAC5 KO mice. We observed that I3A failed to stimulate axon regeneration in HDAC5 KO mice (Figures 7D, 7E and 7F), suggesting that I3A acts mainly through HDAC5. We note that although our model would predict an increased axon growth capacity in HDAC5 KO mice (Figure 7G), we observed instead decreased axon regeneration in these mice (Figures 7D, 7E and 7F). This likely reflects the requirement for HDAC5 in the axon as shown in Figure 5E and as we previously reported (Cho and Cavalli, 2012). In addition, the decreased rather than increased levels of Ac-H3 in injured HDAC5 KO DRG neurons (Figure 4E and S1) suggests that compensatory mechanisms negatively impact axon re-growth ability in HDAC5 KO mice. Together, these experiments suggest that HDAC5 nuclear export underlies in part the priming effects of a conditioning injury and that enhancing HDAC5 nuclear export accelerates axon regeneration in sensory neurons.
To determine if I3A treatment increases the growth capacity of other types of neurons, we measured reinnervation of the neuromuscular junction in the extensor hallucis longus muscle 14 days following sciatic nerve injury. I3A was injected subcutaneously in the lumbar region in YFP-16 mice (Feng et al., 2000), which express YFP in motor neurons, and reinnervation quantified by measuring the percentage of NMJ boutons that co-localized with YFP. We observed accelerated regeneration promoted by I3A treatment in motor axons re-innervating the NMJ (Figure S3). Although the observed accelerated motor neuron regeneration may result from non-cell autonomous or secondary effects, this result suggests that the I3A-dependent mechanisms described for DRG neurons may also increase growth capacity of motor neurons to promote long distance regeneration and target re-innervation.
DISCUSSION
Injured adult peripheral neurons successfully re-gain growth competence via changes in gene expression to promote successful regeneration (Smith and Skene, 1997; Tedeschi, 2011). Yet little is known about the mechanisms by which injury signals emanating at the distantly located axonal injury site unlock a silent pro-regenerative transcriptional program. Our study defines a calcium-initiated signaling mechanism from the axon injury site back to the cell body that elicits epigenetic changes via HDAC5 nuclear export. The observation that several components of this HDAC5-dependent pathway fail to be activated in injured RGC neurons suggests that it may play a role in their inability to regenerate following injury.
Calcium- and PKC- dependent signaling mechanism for the control of axon regeneration
Studies in Aplysia and C. elegans have shown that local calcium entry at the site of injury is necessary for the formation of a new growth cone, a prerequisite for subsequent axon regeneration (Bradke et al., 2012). Axotomy also triggers membrane depolarization that is sufficient to further promote local calcium influx leading to the initiation of a back-propagating calcium wave (Ghosh-Roy et al., 2010; Mandolesi et al., 2004; Ziv and Spira, 1993). Our results indicate that in DRG neurons, the mechanism by which this calcium signal reaches the cell body involves calcium release from internal stores and VGCCs. In cortical neurons, the calcium back-propagation requires the activation of VGSCs, since TTX was shown to strongly reduce calcium elevation in the cell body following axotomy (Mandolesi et al., 2004). In contrast, we observed that in DRG neurons, blocking TTX-sensitive or TTX-resistant sodium channels did not block calcium wave propagation and had no effect on calcium levels in the cell body following injury. Another striking difference between the response of cortical and DRG neurons to axotomy is the magnitude of cell body calcium changes, which are much larger both in amplitude and duration in DRG than in cortical neurons (Mandolesi et al., 2004). While prolonged elevated somatic calcium levels have been shown to be damaging to CNS neurons (Zundorf and Reiser, 2011), DRG neurons appear to be able to survive prolonged period of elevated calcium levels, which may be essential to trigger downstream regenerative signaling cascades. This back-propagating calcium signaling is inherently faster than the positive injury signaling system, which requires microtubule retrograde transport along the axon (Abe and Cavalli, 2008; Rishal and Fainzilber, 2009). It is tempting to speculate that the early calcium-dependent phase of injury signaling could prime the neuronal cell body to the positive injury signals it will receive with a certain delay.
One consequence of increased intracellular calcium is activation of calcium-dependent enzymes such as PKC. Our results suggest that activation of PKCµ in DRG cell bodies depends on a back-propagating calcium wave and fails to occur in RGCs following optic nerve injury. Consistently, earlier studies have shown that neurite outgrowth was enhanced in RGCs cultured in the presence of PMA to activate PKC (Wu et al., 2003). The pro-growth role of PKC has also been reported in other types of neurons in vitro (Campenot et al., 1994) (Tsai et al., 2007), whereas others reported a growth inhibitory role for PKC in CGN and cerebellar neurons (Sivasankaran et al., 2004) (Domeniconi et al., 2005). The precise role of PKC in axon growth and regeneration may thus depend on the specific isoforms involved, their subcellular location, the type of neuron, and its environment.
Histone modifications and the tuning of transcriptional regenerative pathways
The modification of histones by HATs and HDACs shapes chromatin to finely tune transcriptional profiles. Recent observations point to a role for histone modifications in the response of neurons to injury. Increasing histone acetylation promotes axon regeneration in CNS neurons, including cerebellar and retinal neurons (Gaub et al., 2011; Gaub et al., 2010). In agreement with these studies, our results revealed that enhancing HDAC5 nuclear export via PKCµ activation in sensory neurons accelerates axon regeneration. The decrease in Ac-H3 and lack of PKCµ activation we observed in RGCs after optic nerve crush suggest that failure to export HDAC5 in RGCs limits their regenerative ability. Since HDAC5 deacetylase activity requires an association with HDAC3 (Fischle et al., 2002), the reported nuclear accumulation of HDAC3 in injured RGCs (Pelzel et al., 2010), which contrasts with the increased cytoplasmic HDAC3 we observed in injured DRG neurons, may contribute to decreased histone acetylation and repress gene transcription in RGCs. Using a specific antibody against one lysine residue (K18), Gaub et al. reported no changes in Ac-H3 levels in RGCs after optic nerve injury (Gaub et al., 2011), whereas using an antibody recognizing lysines K9 and K14, a chromatin mark enriched in promoter regions of actively transcribed genes, we observed decreased Ac-H3 in injured RGCs. This raises the possibility that a selection of histone acetylation sites within histone H3 N-terminus domain is regulated by injury to control gene expression. Future studies will be needed to explore the roles of nucleo-cytoplasmic cycling of HATs and HDACs in neuronal response to injury and their roles in the differential regenerative ability of central and peripheral neurons.
The developmental silencing of growth capacity appears to correlate with developmental regulation of chromatin modifying enzymes. Indeed, the acetyltransferase p300, which when overexpressed in RGC promotes regeneration, is developmentally regulated (Gaub et al., 2011). In at least two types of CNS neurons, cortical and cerebellar granule neurons, Ac-H3 (K9/14) acetylation decreases developmentally (Gaub et al., 2010). Furthermore, we found that the subcellular localization of HDAC5 correlates with growth capacity, with increased nuclear HDAC5 in mature neurons. Increased nuclear HDAC5 may mark the developmental switch correlating with loss of growth capacity, which is reversible in peripheral neurons, but may not be reversible in RGC neurons.
HDAC5 nuclear export may also have a direct role in transcriptional regulation. Indeed, HDACs can deacetylate transcription factors in addition to histones and inhibit transcription via interaction with co-repressors (Riccio, 2010). Given that HDAC5 also functions as a repressor of the myocyte enhancer factor-2 (MEF2) transcription factor (McKinsey et al., 2000), injury-induced HDAC5 nuclear export may also regulate a pro-regenerative transcriptional program via transcriptional mechanisms. Similarly, overexpression of p300 can promote axon regeneration in the optic nerve via both acetylation of histone H3 and of pro-regenerative transcription factors p53 and C/EBP (Gaub et al., 2011).
HDAC5 nuclear export likely represents a part of an epigenetic response to injury. Indeed the role of DNA methylation has been suggested to regulate axon regeneration in the CNS (Iskandar et al., 2010). Since chromatin remodeling plays an important role in neuronal function (Riccio, 2010), future studies are needed to understand the epigenetic mechanisms induced by injury that promote axon regeneration in the adult nervous system.
Dual role of HDAC5 in axon regeneration
We have previously shown that injury to peripheral neurons leads to HDAC5 accumulation at the tip of injured axons and local tubulin deacetylation, a process required for growth cone dynamics and axon regeneration (Cho and Cavalli, 2012). Here we present evidence that axon injury leads to export of HDAC5 from the nucleus to the cytoplasm and subsequent HDAC5 anterograde transport into the axon. Our results strongly suggest that HDAC5 plays a dual role in peripheral axon regeneration: its exit from the nucleus permits activation of pro-regenerative transcriptional program, and its transport in axons modulates growth cone dynamics to sustain axon regeneration. This dual function of HDAC5 likely explains the decreased axon regeneration in HDAC5 KO compared to WT mice. Furthermore HDAC5 KO mice displayed an unexpected reduced histone H3 acetylation after injury, suggesting decreased transcriptional activity, possibly via compensatory mechanisms. The complexity of roles of HDAC in neuronal development, function, and maintenance is rapidly coming to light and future studies are needed to elucidate the multiple roles of distinct HDACs in axon growth and regeneration.
EXPERIMENTAL PROCEDURES
Surgeries, Chemical Treatments and Sample Preparations
All surgical procedures were approved by Washington University in St. Louis, School of Medicine Animal Studies Committee. Sciatic nerve and optic nerve injury experiments were performed as described previously (Cho and Cavalli, 2012). Ingenol 3-angelate (Adipogen), EGTA, cycloheximide and nocodazole (Sigma) were applied using Surgifoam (Johnson and Johnson). For immunohistochemistry and western blot analysis, tissue was dissected and prepared as described previously (Cho and Cavalli, 2012).
DRG Culture, in vitro Axotomy and Regeneration Assays
Mouse embryonic DRG spot culture, in vitro axotomy and regeneration assay were performed as described (Cho and Cavalli, 2012). For in vitro regeneration assay, GFP expressing DRG neurons were fixed at the indicated time after axotomy and axons visualized by fluorescence microscopy.
Real time imaging and Single Axons Laser Axotomy
Single DRG axons were axotomized using a laser (Mai-Tai, Spectra-Physics, 800nm) in dissociated culture. Calcium imaging was performed as described (Cho and Cavalli, 2012) using Fluo-4AM (Invitrogen). 1µM TTX (Tocris) or 1mM CdCl2 (Sigma) was added 30min prior to laser axotomy. 1µM Thapsigargin (Sigma) or 1µM A887826 (Tocris) was added 20min prior to laser axotomy. To visualize HDAC5 axonal transport, GFP-HDAC5 was expressed in DRG neurons and live imaging was performed at DIV4 following laser axotomy. Kymographs were generated using the ImageJ plug-in Kymograph.
Adult DRG Cultures and In vivo axon Regeneration Assay
For pre-conditioning injury, the sciatic nerves of 4 months old mice were axotomized or not. L4 and L5 DRGs were dissected three days later, cultured for 8 hours, immunostained with TUJ1 and axon projection length calculated as described (Abe et al., 2010). To test for axon regeneration in vivo, sciatic nerves were dissected 3 days after a crush injury. Longitudinal sections of fixed sciatic nerves were stained with SCG10 and TUJ1, as described (Shin et al., 2012). SCG10 fluorescence intensity was measured along the length of the nerve using ImageJ and a regeneration index calculated by measuring the distance away from the crush site in which the average SCG10 intensity is half that observed at the crush site.
RNA Preparation, qPCR, Microarray and Data Analysis of Time-Course Dynamics
DRG spot cultures were axotomized at DIV7, RNA extracted at 0, 3, 8, 12 and 40 hours after axotomy on duplicate samples using PureLink RNA extraction kit (Invitrogen). MouseRef-8 v2.0 BeadChips were used from GTAC at Washington University. Data quality assessment and normalization were performed using GenomeStudio (Illumina). Detailed methods of array analysis and qPCR can be found in Extended Experimental Procedures.
Statistics
Western blot were scanned and quantified by ImageJ. ImageJ macro was used to measure fluorescence intensity in confocal images. For multiple comparisons ANOVA followed by Tukey tests and t-tests were used.
Supplementary Material
Acknowledgments
We thank Dr. Vitaly Klyachko for critical reading of the manuscript. We thank Eric Olson for the generous gift of the HDAC5 KO mice. We thank Domini Montgomery for technical support, Dennis Oakley for assistance with imaging, Ernie Gonzales and the Animal Surgery Core of the Hope Center for Neurological Disorders for assistance with optic nerve surgeries, and the GTAC at Washington University for microarray analysis. This work was supported in part by grants from NIH (DE022000 and NS082446), the McDonnell Center for Cellular and Molecular Neurobiology, and a Hope Center for Neurological Disorders (to VC), and the National Research Foundation of Korea (NRF-2012R1A6A3A03039290) (to YC).
References
- Abe N, Borson SH, Gambello MJ, Wang F, Cavalli V. Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves. J Biol Chem. 2010;285:28034–28043. doi: 10.1074/jbc.M110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe N, Cavalli V. Nerve injury signaling. Current opinion in neurobiology. 2008 doi: 10.1016/j.conb.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambron RT, Walters ET. Priming events and retrograde injury signals. A new perspective on the cellular and molecular biology of nerve regeneration. Molecular neurobiology. 1996;13:61–79. doi: 10.1007/BF02740752. [DOI] [PubMed] [Google Scholar]
- Befort K, Karchewski L, Lanoue C, Woolf CJ. Selective up-regulation of the growth arrest DNA damage-inducible gene Gadd45 alpha in sensory and motor neurons after peripheral nerve injury. The European journal of neuroscience. 2003;18:911–922. doi: 10.1046/j.1460-9568.2003.02827.x. [DOI] [PubMed] [Google Scholar]
- Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE, Yudin D, Rishal I, Rother F, Bader M, et al. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J. 2012;31:1350–1363. doi: 10.1038/emboj.2011.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsland LG, Sahai E, Kelly G, Golding M, Greensmith L, Schiavo G. Deficits in axonal transport precede ALS symptoms in vivo. Proc Natl Acad Sci U S A. 2010;107:20523–20528. doi: 10.1073/pnas.1006869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesch A, Lu P, Tsukada S, Alto LT, Roet K, Coppola G, Geschwind D, Tuszynski MH. Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: superiority to camp-mediated effects. Exp Neurol. 2012;235:162–173. doi: 10.1016/j.expneurol.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F, Fawcett JW, Spira ME. Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat Rev Neurosci. 2012;13:183–193. doi: 10.1038/nrn3176. [DOI] [PubMed] [Google Scholar]
- Broude E, McAtee M, Kelley MS, Bregman BS. c-Jun expression in adult rat dorsal root ganglion neurons: differential response after central or peripheral axotomy. Exp Neurol. 1997;148:367–377. doi: 10.1006/exnr.1997.6665. [DOI] [PubMed] [Google Scholar]
- Buschmann T, Martin-Villalba A, Kocsis JD, Waxman SG, Zimmermann M, Herdegen T. Expression of Jun, Fos, and ATF-2 proteins in axotomized explanted and cultured adult rat dorsal root ganglia. Neuroscience. 1998;84:163–176. doi: 10.1016/s0306-4522(97)00487-9. [DOI] [PubMed] [Google Scholar]
- Campenot RB, Draker DD, Senger DL. Evidence that protein kinase C activities involved in regulating neurite growth are localized to distal neurites. J Neurochem. 1994;63:868–878. doi: 10.1046/j.1471-4159.1994.63030868.x. [DOI] [PubMed] [Google Scholar]
- Cayrou C, Denver RJ, Puymirat J. Suppression of the basic transcription element-binding protein in brain neuronal cultures inhibits thyroid hormone-induced neurite branching. Endocrinology. 2002;143:2242–2249. doi: 10.1210/endo.143.6.8856. [DOI] [PubMed] [Google Scholar]
- Chang S, McKinsey TA, Zhang CL, Richardson JA, Hill JA, Olson EN. Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Molecular and cellular biology. 2004;24:8467–8476. doi: 10.1128/MCB.24.19.8467-8476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla S, Vanhoutte P, Arnold FJ, Huang CL, Bading H. Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J Neurochem. 2003;85:151–159. doi: 10.1046/j.1471-4159.2003.01648.x. [DOI] [PubMed] [Google Scholar]
- Cho Y, Cavalli V. HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. EMBO J. 2012;31:3063–3078. doi: 10.1038/emboj.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeniconi M, Zampieri N, Spencer T, Hilaire M, Mellado W, Chao MV, Filbin MT. MAG induces regulated intramembrane proteolysis of the p75 neurotrophin receptor to inhibit neurite outgrowth. Neuron. 2005;46:849–855. doi: 10.1016/j.neuron.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Fischle W, Dequiedt F, Hendzel MJ, Guenther MG, Lazar MA, Voelter W, Verdin E. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell. 2002;9:45–57. doi: 10.1016/s1097-2765(01)00429-4. [DOI] [PubMed] [Google Scholar]
- Gaub P, Joshi Y, Wuttke A, Naumann U, Schnichels S, Heiduschka P, Di Giovanni S. The histone acetyltransferase p300 promotes intrinsic axonal regeneration. Brain. 2011;134:2134–2148. doi: 10.1093/brain/awr142. [DOI] [PubMed] [Google Scholar]
- Gaub P, Tedeschi A, Puttagunta R, Nguyen T, Schmandke A, Di Giovanni S. HDAC inhibition promotes neuronal outgrowth and counteracts growth cone collapse through CBP/p300 and P/CAF-dependent p53 acetylation. Cell Death Differ. 2010;17:1392–1408. doi: 10.1038/cdd.2009.216. [DOI] [PubMed] [Google Scholar]
- Ghosh-Roy A, Wu Z, Goncharov A, Jin Y, Chisholm AD. Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci. 2010;30:3175–3183. doi: 10.1523/JNEUROSCI.5464-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha CH, Kim JY, Zhao J, Wang W, Jhun BS, Wong C, Jin ZG. PKA phosphorylates histone deacetylase 5 and prevents its nuclear export, leading to the inhibition of gene transcription and cardiomyocyte hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15467–15472. doi: 10.1073/pnas.1000462107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskandar BJ, Rizk E, Meier B, Hariharan N, Bottiglieri T, Finnell RH, Jarrard DF, Banerjee RV, Skene JH, Nelson A, et al. Folate regulation of axonal regeneration in the rodent central nervous system through DNA methylation. J Clin Invest. 2010;120:1603–1616. doi: 10.1172/JCI40000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamber D, Erez H, Spira ME. Local calcium-dependent mechanisms determine whether a cut axonal end assembles a retarded endbulb or competent growth cone. Exp Neurol. 2009;219:112–125. doi: 10.1016/j.expneurol.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Kedei N, Lundberg DJ, Toth A, Welburn P, Garfield SH, Blumberg PM. Characterization of the interaction of ingenol 3-angelate with protein kinase C. Cancer Res. 2004;64:3243–3255. doi: 10.1158/0008-5472.can-03-3403. [DOI] [PubMed] [Google Scholar]
- Koo EH, Sisodia SS, Archer DR, Martin LJ, Weidemann A, Beyreuther K, Fischer P, Masters CL, Price DL. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990;87:1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahm A, Paolini C, Pallaoro M, Nardi MC, Jones P, Neddermann P, Sambucini S, Bottomley MJ, Lo Surdo P, Carfi A, et al. Unraveling the hidden catalytic activity of vertebrate class IIa histone deacetylases. Proc Natl Acad Sci U S A. 2007;104:17335–17340. doi: 10.1073/pnas.0706487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leah JD, Herdegen T, Murashov A, Dragunow M, Bravo R. Expression of immediate early gene proteins following axotomy and inhibition of axonal transport in the rat central nervous system. Neuroscience. 1993;57:53–66. doi: 10.1016/0306-4522(93)90111-r. [DOI] [PubMed] [Google Scholar]
- Liu K, Tedeschi A, Park KK, He Z. Neuronal Intrinsic Mechanisms of Axon Regeneration. Annual review of neuroscience. 2011 doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- Mandolesi G, Madeddu F, Bozzi Y, Maffei L, Ratto GM. Acute physiological response of mammalian central neurons to axotomy: ionic regulation and electrical activity. FASEB J. 2004;18:1934–1936. doi: 10.1096/fj.04-1805fje. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Grafstein B. Axon outgrowth enhanced by a previous nerve injury. Arch Neurol. 1973;29:53–55. doi: 10.1001/archneur.1973.00490250071008. [DOI] [PubMed] [Google Scholar]
- Michaelevski I, Segal-Ruder Y, Rozenbaum M, Medzihradszky KF, Shalem O, Coppola G, Horn-Saban S, Ben-Yaakov K, Dagan SY, Rishal I, et al. Signaling to transcription networks in the neuronal retrograde injury response. Sci Signal. 2010;3:ra53. doi: 10.1126/scisignal.2000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzel HR, Schlamp CL, Nickells RW. Histone H4 deacetylation plays a critical role in early gene silencing during neuronal apoptosis. BMC Neurosci. 2010;11:62. doi: 10.1186/1471-2202-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Perry RB, Doron-Mandel E, Iavnilovitch E, Rishal I, Dagan SY, Tsoory M, Coppola G, McDonald MK, Gomes C, Geschwind DH, et al. Subcellular knockout of importin beta1 perturbs axonal retrograde signaling. Neuron. 2012;75:294–305. doi: 10.1016/j.neuron.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A. Dynamic epigenetic regulation in neurons: enzymes, stimuli and signaling pathways. Nat Neurosci. 2010;13:1330–1337. doi: 10.1038/nn.2671. [DOI] [PubMed] [Google Scholar]
- Richardson PM, Issa VM. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309:791–793. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- Rishal I, Fainzilber M. Retrograde signaling in axonal regeneration. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Shadiack AM, Sun Y, Zigmond RE. Nerve growth factor antiserum induces axotomy-like changes in neuropeptide expression in intact sympathetic and sensory neurons. J Neurosci. 2001;21:363–371. doi: 10.1523/JNEUROSCI.21-02-00363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields SD, Ahn HS, Yang Y, Han C, Seal RP, Wood JN, Waxman SG, Dib-Hajj SD. Nav1.8 expression is not restricted to nociceptors in mouse peripheral nervous system. Pain. 2012;153:2017–2030. doi: 10.1016/j.pain.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Shin J, Cho Y, Beirowski B, Milbrandt J, Cavalli V, DiAntonio A. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron. 2012;74:1015–1022. doi: 10.1016/j.neuron.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasankaran R, Pei J, Wang KC, Zhang YP, Shields CB, Xu XM, He Z. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci. 2004;7:261–268. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- Smith DS, Skene JH. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci. 1997;17:646–658. doi: 10.1523/JNEUROSCI.17-02-00646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi A. Tuning the orchestra: transcriptional pathways controlling axon regeneration. Front Mol Neurosci. 2011;4:60. doi: 10.3389/fnmol.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Yang LY, Wu CH, Chang SF, Hsu CY, Wei CP, Leu SJ, Liaw J, Lee YH, Tsai MD. Injury-induced Janus kinase/protein kinase C-dependent phosphorylation of growth-associated protein 43 and signal transducer and activator of transcription 3 for neurite growth in dorsal root ganglion. J Neurosci Res. 2007;85:321–331. doi: 10.1002/jnr.21119. [DOI] [PubMed] [Google Scholar]
- Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Molecular and cellular biology. 2004;24:8374–8385. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QJ. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol Sci. 2006;27:317–323. doi: 10.1016/j.tips.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Wu DY, Zheng JQ, McDonald MA, Chang B, Twiss JL. PKC isozymes in the enhanced regrowth of retinal neurites after optic nerve injury. Invest Ophthalmol Vis Sci. 2003;44:2783–2790. doi: 10.1167/iovs.02-0715. [DOI] [PubMed] [Google Scholar]
- Xiong X, Wang X, Ewanek R, Bhat P, Diantonio A, Collins CA. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J Cell Biol. 2010;191:211–223. doi: 10.1083/jcb.201006039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Shieh CC, Chapman ML, Matulenko MA, Hakeem AH, Atkinson RN, Kort ME, Marron BE, Joshi S, Honore P, et al. A-887826 is a structurally novel, potent and voltage-dependent Na(v)1.8 sodium channel blocker that attenuates neuropathic tactile allodynia in rats. Neuropharmacology. 2010;59:201–207. doi: 10.1016/j.neuropharm.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Spira ME. Spatiotemporal distribution of Ca2+ following axotomy and throughout the recovery process of cultured Aplysia neurons. The European journal of neuroscience. 1993;5:657–668. doi: 10.1111/j.1460-9568.1993.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Zundorf G, Reiser G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid Redox Signal. 2011;14:1275–1288. doi: 10.1089/ars.2010.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.