Abstract
Fungal exopolysaccharides (EPSs) have been recognized as high value biomacromolecules for the last two decades. These products, including pullulan, scleroglucan, and botryosphaeran, have several applications in industries, pharmaceuticals, medicine, foods etc. Although fungal EPSs are highly relevant, to date information concerning fungal biosynthesis is scarce and an extensive search for new fugal species that can produce novel EPSs is still needed. In most cases, the molecular weight variations and sugar compositions of fungal EPSs are dependent to culture medium composition and different physical conditions provided during fermentation. An inclusive and illustrative review on fungal EPS is presented here. The general outline of the present work includes fungal EPS production, their compositions and applications. An emphasis is also given to listing out different fungal strains that can produce EPSs.
Keywords: application, exopolysaccharide composition, exopolysaccharide production, fungal exopolysaccharide
Introduction
Carbohydrates are naturally occurring and well distributed, and are the most important building blocks of the biosphere. These evolutionary and biologically important organic compounds are present on Earth in different forms. Traditionally, on the basis of the number of sugar units, carbohydrates are classified into three groups: monosaccharides, oligosaccharides, and polysaccharides. The natural macromolecules composed of several monosaccharide units (more than ten) are known as polysaccharides and are synthesized at different stages of life cycle of every living organisms for different purposes. The monosaccharide units of polysaccharides are joined to each other by an acetal linkage. These acetal linkages are formed by the reaction of a hemiacetal hydroxyl group of one unit with an alcohol group of another unit which liberates water to give a glycosidic bond. Polysaccharides not only have different sequences of monomeric units but also have different sequences of glycosidic linkages and different types of branching. They may be amorphous or even insoluble in water. All these factors together give polysaccharides a great diversity of structure, property, and functions. Polysaccharides used at the industrial level are almost all of plant and seaweed origin.1 These long-chain and high molecular weight polymers, such as starch, alginate, Arabic gum, carrageenan, agar, and gaur gum, are widely employed in the food, pharmaceutical, and cosmetic industries.2–4
Microorganisms, however, are known for their ability to synthesize polysaccharides with different structural complexities.5,6 These polysaccharides either remain attached to the cell surface or are found in the extracellular medium.7 Two hundred years ago, Henri Braconnot discovered chitin, the first carbohydrate polymer which is found in edible mushrooms.8,9 The extracellular polysaccharide production by microbes was first reported in 1861 as a “viscous fermentation” by Pasteur. The organism that produces this polysaccharide was a bacterium identified as Leuconostoc mesenteroides by Van Tieghem.10 Among the three major classes of microbial polysaccharides, exopolysaccharides (EPSs) had several advantages over intracellular and cell wall polysaccharides including huge production in short time, easy isolation, and purification. EPSs of microbial origin might represent a valid alternative to plant and algal products considering that their properties are almost identical to those currently used gums.1 In other cases, the microbial products have unusual molecular structures and peculiar conformations, thus conferring unique and potentially interesting properties with potential industrial uses.1,3,5,11 Together with the knowledge of biological properties, the structures and fermentation process of microbial EPSs produced are also very important for understanding their physiological activities and industrial applicability.
Among the microbial EPS producers, bacteria and fungi are most common. Bacterial EPSs have been studied extensively by researchers.6 EPS production from fungi has been studied adequately over the last two decades. Different EPS production by fungi including Ganoderma lucidum, Agaricus blazi, Cordyceps sp., Lentinus edodes, and Grifola frondosa through submerged cultures had been reported, all of which have different and interesting biological activities.12
In the present review, an attempt was taken to recapitulate all the existing literature concerning fungal EPS production, composition, and biological applications in a single frame to allow us to realize the advances and perspectives in the knowledge and applications of fungal EPSs.
Production of Exopolysaccharides from Fungi
EPS production from fungi mainly depends on the type of fungal strain used, physical conditions maintained during fermentation, and type of medium components applied for the production.
Fungal strains producing exopolysaccharides
At present, a considerable number of fungi including higher basidiomycetes, lower filamentous fungi, and yeasts from different ecological niches were known for their ability to synthesize EPSs in laboratory culture systems. However, many still remain uninvestigated or under explored. For better observations at a glance, list of reported fungal EPS producers are represented in Table 1.
Table 1.
List of some fungi, are reported to produce exopolysaccharides in laboratory culture techniques.
| Organism | References |
|---|---|
| Absidia corymbifera | 92 |
| Absidia cylindrospora | 93 |
| Acremonium charticela | 94 |
| Acremonium diospyri | 95 |
| Acremonium persicinum | 96 |
| Agaricus nevoi HAI610 | 39 |
| Agaricus xanthodermus | 96 |
| Agrocybe cylindracea | 18 |
| Agrocybe platensis | 96 |
| Akanthomyces pistillariiformis | 83 |
| Alternaria alternate | 94 |
| Alternaria mellea | 93 |
| Alternaria solani | 93 |
| Antrodia camphorate | 36 |
| Antrodia cinnamomea | 27 |
| Antrodiella ginestae | 96 |
| Armillaria luteo-virens Sacc.QH | 17 |
| Armillaria mellae | 19 |
| Aschersonia samoensis | 83 |
| Aspergillus alliaceus | 97 |
| Aspergillus fumigates | 80 |
| Aspergillus niger | 1 |
| Aspergillus parasiticus | 20 |
| Aspergillus sp. Y16 | 65 |
| Aureobasidium pullulans | 32 |
| Auricularia fuscosuccinea | 96 |
| Beauveria bassiana | 83 |
| Botryosphaeria rhodina | 81,98 |
| Botrytis cinerea | 93,99 |
| Byssochlamys nivea | 100 |
| Calvatia cyathiformis | 96 |
| Candida bogoriensis | 93 |
| Candida boidinii | 75 |
| Cephalosporium serrae Maffei | 95 |
| Cephalosporium stiehmeri | 95 |
| Cephalosporium subverticillatum | 95 |
| Ceratocystis stenoceras | 93 |
| Cerrena maxima IBB681 | 39 |
| Cladosporium herbarum | 94 |
| Cladosporium tricoides | 93 |
| Claviceps purpurea | 19 |
| Climacodon pulcherrimus | 96 |
| Collybia maculate | 26 |
| Cookenia tricholoma | 83 |
| Cordyceps dipterigena | 83 |
| Cordyceps militaris | 34,101 |
| Cordyceps nipponica | 83 |
| Cordyceps sinensis | 34 |
| Cordyceps sphecocephala | 28 |
| Cordyceps taii | 16 |
| Coriolus (Trametes) versicolor | 45,93 |
| Coriolus hirsutus | 93 |
| Cryphonectria parasitica | 55 |
| Cryptococcus albidus 16-1 | 102 |
| Cryptococcus elinorii | 93 |
| Cryptococcus laurentii | 31,75 |
| Cyttaria harioti | 1 |
| Drechslera spicifera | 103 |
| Elsinoe leucospila | 1 |
| Epicoccum nigrum | 99 |
| Flamnulina velutipes | 93 |
| Fomes fomentarius | 29 |
| Fomitopsis pinicola | 104 |
| Fusarium coccophilum | 83 |
| Fusarium oxysporium Dzf 17 | 88 |
| Fusarium solani | 9,94 |
| Ganoderma applanatum | 51 |
| Ganoderma lucidium | 39,105 |
| Ganoderma resinaceum | 64 |
| Gibellula pulchra | 83 |
| Gliomastic gueg | 94 |
| Glomerella cingulata | 106 |
| Gomphidius rutilus | 87 |
| Grifola frondosa | 52,89 |
| Hansenula capsulate | 75 |
| Hansenula holstii | 19 |
| Hansenula minuta | 19 |
| Helotium sp. | 19 |
| Hirsutella sp. | 83 |
| Hymenostilbe sp. | 83 |
| Hypocrella tamurai | 83 |
| Hypsizigus marmoreus | 85 |
| Inonotus levis HAI796 | 39 |
| Isaria farinose BO5 | 84 |
| Lachnum sp. YM261 | 54 |
| Lentinus edodes | 15 |
| Lipomyces starkeyii | 75 |
| Lyophyllus decastes | 35 |
| Melanoporia nigra | 96 |
| Metarhizium anisopliae var. majus | 83 |
| Moniliella pollinis | 107 |
| Morchella crassipes | 108 |
| Mucor circinelloides | 92 |
| Mucor hiemalis | 92 |
| Mucor mucedo | 93 |
| Mucor racemosus | 92,93 |
| Mucor rouxii | 37 |
| Nigrospora oryzae var. glucanicum | 43 |
| Nothopanus hygrophanus | 96 |
| Oligoporus sp. | 96 |
| Oudemansiella canarii | 96 |
| Oudemansiella radicata | 109 |
| Paecilomyces japonica | 110 |
| Paecilomyces lilacinus | 111 |
| Paecilomyces sinclairii | 112 |
| Paecilomyces tenuipes | 44,83 |
| Panaeolus papilionaceus | 96 |
| Penicillium charlesii | 19 |
| Penicillium citrinum | 113 |
| Penicillium islandicum | 19 |
| Penicillium luteum | 114 |
| Penicillium paraphergal | 94 |
| Penicillium varians | 115 |
| Penicillium vermiculatum | 116 |
| Peniophora cinerea | 96 |
| Perenniporia piperis | 96 |
| Pestalotia sp. 815 | 1 |
| Pestalotiopsis sp. KCTC 8637P | 79 |
| Phanerochaete chrysosporium | 117 |
| Phellinus baumii | 30,118 |
| Phellinus gilvus | 33,96 |
| Phellinus igniarus HAI795 | 39 |
| Phellinus robustus HAI531 | 39 |
| Pholiota nameko | 96 |
| Phomopsis foeniculi | 58 |
| Phytocordyceps sp. | 83 |
| Pichia mucosa | 19 |
| Platymonas sp. | 19 |
| Plectania occidentalis | 19 |
| Pleurotus cornucopiae | 63 |
| Pleurotus dryinus IBB903 | 39 |
| Pleurotus eryngii | 63 |
| Pleurotus flabellatus | 96 |
| Pleurotus floridanus | 63 |
| Pleurotus ostreatoroseus | 96 |
| Pleurotus ostreatus | 96,119 |
| Pleurotus pulmonarius | 63 |
| Pleurotus sajor-caju | 63,96 |
| Pleurotus tuber-regium | 49 |
| Polyporus fomentarinus | 93 |
| Polyporus ignarius | 93 |
| Polyporus tuberaster | 93 |
| Psilocybe castanella | 96 |
| Psilocybe subcubensis | 96 |
| Pullularia pullulans | 74 |
| Pycnoporus sanguineus | 96 |
| Rhinocladellia elatior | 75 |
| Rhizomucor pusillus | 92 |
| Rhizopus nigricans | 93 |
| Rhizopus stolonifer | 93 |
| Rhodotorula glutinis | 75 |
| Rigidoporus microporus | 96 |
| Ripartitella cf. brasiliensis | 96 |
| Sarcodon aspratus (Berk) S.lto TG-3 | 120 |
| Schizophyllum commune | 96,99 |
| Sclerotium glucanicum | 25,99 |
| Sclerotium rolfsii | 99 |
| Selenotila peltata | 19 |
| Shiraia bambusicola | 12 |
| Sorangium cellulosum | 46 |
| Sporobolomyces salmonicolor AL1 | 102 |
| Sporothrix schenkii | 93 |
| Stemphylium sp. | 121 |
| Stereum sanguinolentum | 19 |
| Stropharia rugosoannulata | 90 |
| Syncephalastrum racemosum | 92 |
| Thamnidium elegann | 92 |
| Torrubiella tenuis | 83 |
| Torulopsis melibiosum | 19 |
| Torulopsis pinus | 19 |
| Trametes versicolor | 39,96 |
| Trametes villosa | 96 |
| Tremella brasiliensis | 19 |
| Tremella encephala | 19 |
| Tremella foliacea | 19 |
| Tremella fuciformis | 122 |
| Tremella mesenterica | 19 |
| Tremella subanomala | 19 |
| Trichaptum byssogenum | 96 |
| Tricholoma crassum | 96 |
| Trichosporon asahii | 123 |
| Trogia buccinalis | 96 |
| Tuber sinense | 48 |
| Tyromyces pseudolacteus | 96 |
| Ustilago maydis | 124 |
| Zygosporium masonii | 83 |
Parameters affecting exopolysaccharide production
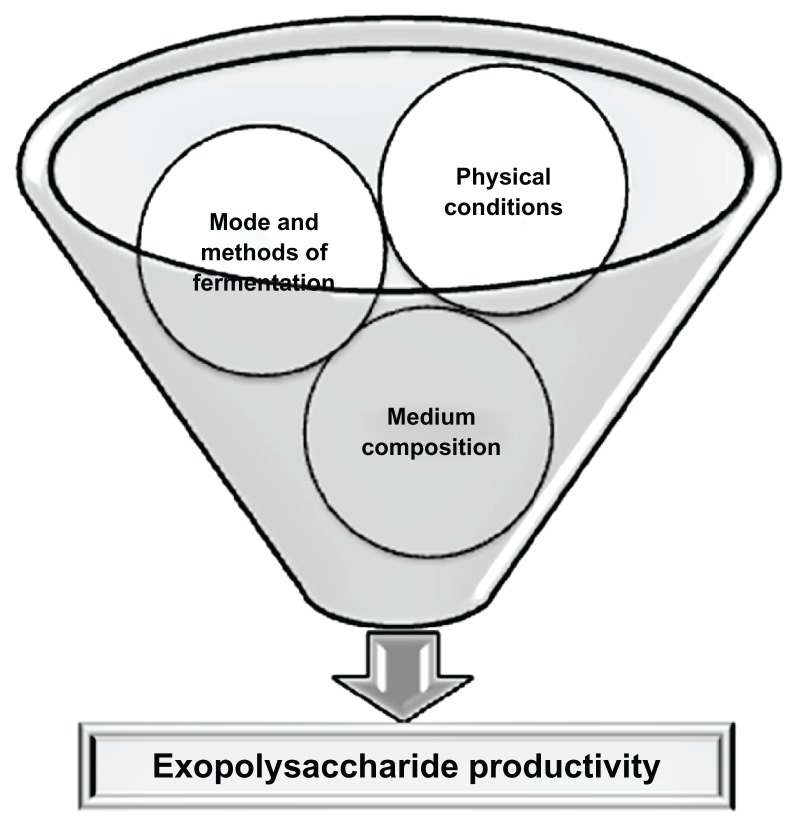
EPSs are generally synthesized intracellularly and secreted to the surroundings. Very little information is available regarding the biosynthesis of EPSs from fungi.1,13 Only a few fungal EPS biosynthesis pathways have been studied, such as EPS biosynthesis by G. lucidum.14 Most researchers have studied the optimization of culture medium and conditions for EPS production from different fungal strains. In this context, Figure 1 recapitulates the main factors upon which fungal EPS production is dependent. The majority of the published papers followed liquid submerged culture techniques for EPS production and some researchers used statistical methods including response surface methodology, orthogonal matrix method using Box-Behnken design, Plackett-Burman design, central composite design, or fractional factorial design for optimization of EPS production in liquid submerged fermentation.15–18 Sandford suggested that most of the EPS producing fungi are aerobic or facultative anaerobic, and oxygen limitation did not support EPS production.19 Like Sandford, published articles have also showed that EPS production by fungi normally reached its optimum level in shaking conditions when oxygen is available in the medium.19 Ruperez and Leal evaluated the EPS production by Aspergillus parasiticus in agitated and static submerged fermentation.20 In agitated culture, this organism produced more EPSs (0.41 gm/L) than static culture (0.18 gm/L). Agitated culture technique for EPS production by this Aspergillus species was also favorable considering the cultivation time. In agitated culture, maximum EPS production was found after 5 days of fermentation whereas in static culture 6 days of cultivation are needed.
Figure 1.
A schematic illustration: main factors on which fungal exopoly-saccharide production depends (Physical conditions: temperature, pH, oxygen level, incubation time etc.; Medium composition: carbon source, nitrogen source, different salts, special additives like vegetative oils, vitamin etc.; Mode and methods of fermentation: agitated culture, static culture, submerged culture using general optimization process or statistical methods like response surface methodology, orthogonal matrix method using Box-Behnken design, Plackett-Burman design etc.).
Roukas and Liakopoulou-Kyriakides examined the production of pullulan by Aureobasidium pullulans P56 and reported that EPS production was higher (23 gm/L) at an aeration rate of 1 vvm, in comparison to cultures with low aeration of 0.5 vvm (14 gm/L) and culture without oxygen supply (12 gm/L).21 Kim et al reported with high aeration rate (3.5 vvm), the increase in the dissolved oxygen is associated with higher EPS production by Paecilomyces sinclairii.22 Xu and Yun examined the influence of aeration on EPS production by Paecilomyces tenuipes C240 in a stirred-tank fermenter and found that at a high aeration rate (3.5 vvm), maximum EPS (2.36 gm/L) was produced.23 Among the three different extracellular proteoglycans produced by this organism, two of them showed variation in their composition when produced in various aeration conditions. Gibbs and Seviour reported that pullulan production from A. pullulans ATTC 9348 was optimally supported up to a certain level of dissolved oxygen in bioreactor, over which EPS production decreased.24 These findings suggest that EPS production by fungi is oxygen dependent but there is a specific limit beyond which production might be reduced.
The pH of culture medium is another reflective factor that persuades the fungal EPS production.25 Generally, fungi favored low pH for EPS production with a range between pH 3.0 to 6.5.15,18,26–32 A few fungi preferred neutral or alkaline pH for maximum EPS production.33–35 In 2004, Shu and Lung examined the effects of pH on EPS production by Antrodia camphorate and reported that variation in medium pH induces A. camphorate to produce EPS with different molecular weight (Mw).36 They noticed that relatively high Mw EPSs in low amount was produced at lower medium pH while low Mw EPS with high yield was recorded at higher medium pH. In 2012, Abdel-Aziz et al reported that acid pH shock induced the EPS production by Mucor rouxii.37 Authors reported that M. rouxii showed normal growth at pH 5.0 to 7.5 but EPS production increased gradually with increase in pH values up to a pH of 10.0. They also noticed that when the initial medium pH adjusted at pH 3.5, it showed maximum flocculating activity.37
Most of the fungal strains produced maximum EPSs within a temperature range 22 ºC to 30 ºC (Table 2). Only a few reports suggested that 20 ºC was most effective for EPS production by fungi.26,34
Table 2.
Optimum culture conditions for maximum exopolysaccharide production by different fungal strains.
| Organism | Different parameters used for EPS production in liquid submerged culture | References | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Carbon (gm/L) | Nitrogen (gm/L) | Salts and phosphates | Additives | pH | Tem. (ºC) | F. Days | EPS (gm/L) | ||
| Agrocybe cylindracea | Maltose (60) | Martone A-1 (6) | CaCl2, MgSO4 · 7 H2O | – | 6.0 | 25 | 10 | 3.0 | 18 |
| Alternaria alternata | Glucose (4%) | Yeast extract (2%) | KH2PO4, MgSO4 · 7 H2O | – | 3.0 | 30 | 9 | 4.5 | 94 |
| Antrodia cinnamomea | Glucose (5%) | Calcium nitrate (0.5%) | FeSO4 | Nicotinic acid (0.1%) | 5.5 | 28 | 14 | 0.49 | 27 |
| Armillaria luteo-virens Sacc. | Glucose (31.26) | Yeast extract (1.06) | KH2PO4, K2HPO4, MgSO4 · 7 H2O | – | – | 23 | 5 | 5.40 | 17 |
| Aureobasidium pullulans CJ001 | Sucrose (50) | Yeast extract (2) | KH2PO4, MgSO4 · 7 H2O, Nacl, (NH4)2SO4 | – | 6.0 | 22 | 4 | 31.25 | 32 |
| Collybia maculate | Glucose (30) | Martone A-1 (20) | K2HPO4, CaCl2 | – | 5.5 | 20 | 5 | 2.4 | 26 |
| Cordyceps militaris NG1 | Sucrose (40) | Corn steep powder (5) | – | – | 8.0 | 30 | 16 | 5.05 | 34 |
| Cordyceps militaris NG3 | Sucrose (30) | Corn steep powder (10) | – | – | 8.0 | 30 | 15 | 3.4 | 101 |
| Cordyceps sinensis | Sucrose (20) | Corn steep powder (25) | CaCl2, MgSO4 · 7 H2O | – | 4.0 | 20 | 16 | 4.15 | 34 |
| Cordyceps sphecocephala | Sucrose (40) | Yeast extract (6) and polypeptone (2) | KH2PO4, K2HPO4, MgSO4 · 7 H2O | – | 4.0 | 25 | 11 | 2.5 | 28 |
| Cordyceps taii | Xylose (31.27) | (NH4)2SO4 (0.15) and soybean steep liquor (4.85) | KH2PO4, FeSO4, MgSO4 · 7 H2O | Vitamin–A and Vitamin–D (0.01 gm/L) | 5.5 | 28 | 6 | 43.87 | 16 |
| Coriolus (Trametes) versicolor | Glucose (15) | Malt extract (3), yeast extract (6) and peptone (5) | – | 5.5 | 28 | 9 | 0.64 | 45 | |
| Cryptococcus laurentii AL100 | Sucrose (40) | Yeast extract (1) | KH2PO4, MgSO4 · 7 H2O, Nacl, CaCl2 | – | 5.3 | 22 | 4 | 6.4 | 31 |
| Fomes fomentarius | Glucose (50) | Yeast extract (3) | CaCl2, MgSO4 · 7 H2O | Silk worm chrysalis (3 gm/L) | 6.0 | 25 | 8 | 3.64 | 29 |
| Fomitopsis pinicola | Glucose (4%) | Yeast extract (0.5%) and malt extract (0.1%) | K2HPO4, MgSO4 · 7 H2O | – | 6.0 | 25 | 11 | 4.4 | 104 |
| Ganoderma applanatum | Glucose (60) also maltose | Yeast extract (2) | KH2PO4, MgSO4 · 7 H2O, FeCl3, MnSO4 | Glutamic acid (1 gm/L), biotin (0.5 mg/L), thiamine (0.1 gm/L) | 4.5 | 25 | 12 | 1.35 | 51 |
| Gomphidius rutilus | Sucrose (30) | Soybean meal (3) | K2HPO4, KH2PO4, MgSO4 · 7 H2O, ZnSO4, FeSO4 | – | 8.0 | 25 | 6 | 0.54 | 87 |
| Lentinus edodes | Glucose (15.78) | Yeast extract (5.86) | KH2PO4, K2HPO4, MgSO4 · 7 H2O | – | 4.48 | 28 | 8 | 0.751 | 15 |
| Lyophyllum decastes | Glucose (3%) | Yeast extract (2%) | – | – | 7.0 | 25 | 10 | 2.46 | 35 |
| Morchella crassipes | Maltose (44.79) | Tryptone (4.21) | – | – | 6.0 | 28 | 7 | 9.67 | 108 |
| Mucor rouxii | Beet-molasses | – | – | – | 3.5 | 28 | 2 | – | 37 |
| Nigrospora oryzae var. glucanicum | Glucose (120) | Yeast extract (1) and urea (0.1) | KH2PO4, MgSO4 · 7 H2O, NaCl | – | 5.6 | 28 | 5 | 4.5–5.3 | 43 |
| Oudemansiella radicata | Sucrose (39.3) | Peptone (3.16) | KH2PO4, MgSO4 · 7 H2O | – | – | 28 | 5 | 2.65 | 109 |
| Paecilomyces sinclairii | Sucrose (60) | Corn steep powder (10) | KH2PO4, K2HPO4 | – | 6.0 | 30 | 9 | 7.4 | 112 |
| Paecilomyces teunuipes C240 | Glucose (3) | KNO3 (0.4) | KH2PO4, MgSO4 · 7 H2O | – | 6.0 | 28 | 8 | 2.36 | 44 |
| Phellinus baumii pilát | Glucose (34.12) | Peptone (4) and yeast extract (5) | KH2PO4, MgSO4 · 7 H2O | Thiamine (0.0075 gm/L) | 6.5 | 28 | 6 | 2.363 | 30 |
| Phellinus gilvus | Glucose (30) | Corn steep powder (5) | KH2PO4, K2HPO4, MgSO4 · 7 H2O, | – | 9.0 | 30 | 11 | 5.3 | 33 |
| Pleurotus tuber-regium | Glucose (30) | Yeast extract (4) | KH2PO4, MgSO4 · 7 H2O | Tween 80 (3 gm/L) | – | 30 | 7 | 1.03 | 49 |
| Rhodotorula glutinins | Glucose (3%) | Yeast extract (0.3%) | – | – | 6.0 | 25 | 7 | 2.06 | 66 |
| Sarcodon aspratus (Berk) S. lto TG-3 | Glucose (30) | Yeast extract (15) | KH2PO4, CaCl2 | – | 5.0 | 25 | 4 | 2.68 | 120 |
| Shiraia bambusicola WZ-003 | Maltose (30) | Yeast extract (3) | KH2PO4, MgSO4 · 7 H2O) | Soybean oil (0.1% | 6.0 | 26 | 5 | 0.53 | 12 |
| Sporobolomyces salmonicolor | Sucrose (5%) | Ammonium sulphate (0.25%) | KH2PO4, MgSO4 · 7 H2O, NaCl, CaCl2 | – | 5.3 | 22 | 5 | 5.63 | 102 |
| Stemphylium sp. | Glucose (30) and Sucrose (3) | Yeast extract (6.3) and peptone (2) | MgSO4 · 7 H2O, K2HPO4 | – | 6.0 | 30 | 7 | – | 121 |
| Tremella fuciformis | Glucose (20) | Tryptone (2) | KH2PO4, K2HPO4, MgSO4 · 7 H2O | – | 8.0 | 28 | 5 | 3.05 | 125 |
Abbreviations: Tem., Incubation temperature; F. Days, Fermentation time in days.
EPS production by fungi at different time intervals was studied by many researchers. Their findings suggested that EPS production could be maximized either at its late exponential stage or its early stationary stages of growth. Unlike bacterial EPS production, fungi needed long incubation time for maximum EPS production. Generally 4 to 15 days of fermentation needed by different fungal strains for optimum EPS production (Table 2). Shu and Lung studied the effect of incubation time on EPS produced by A. camphorate.36 They found that high molecular weight EPS reduced with increase in fermentation time and, that after completion of fermentation, only low molecular weight EPSs were present in the fermented broth. Similar results were also reported by Lee et al when they were studied the pullulan production from A. pullulans.38
Although in most cases composition of EPS is independent to the type of carbohydrate used for the production of that EPS, the production intensity is very much dependant on the carbon source used and its concentration. In general, glucose, sucrose, maltose, lactose, fructose, galactose, xylose, cellobiose, sorbitol, xylitol, mannitol, and different types of agricultural byproducts are used as carbon source in the culture medium. In most of the cases glucose, sucrose, and maltose have been selected as the most influential carbon sources for the production of fungal EPSs (Table 2). These observations indicate that there may be some effects of catabolic repression of different sugars in various EPS synthesis, that different fungal strains have different sugar uptake fascinations, or that these sugars may be easily metabolized by fungi. Actual reasons, however, are not clearly understood as in most cases fungal growth and EPS production are not directly proportional. Elisashvili et al studied eight basidiomycetes for EPS production.39 They reported that G. lucidum, Inonotus levis, and Phellinus robustus produced maximum EPSs in media containing glucose as a carbon source. Cerrena maxima, Phellinus igniarius, Trametes versicolor, on the other hand, favored maltose while Agaricus nevoi favored mannitol as the most supportive carbon source for EPS production. Bae et al evaluated the effect of carbon sources on EPS production by Paecilomyces japonica and reported that maltose is the preferred carbon source and results in higher EPS production (30 gm/L) than sucrose (25 gm/L).40 In an another report, Elisashvili et al reported an interesting observation that L. edodes and Pleurotus spp. strains produced maximum EPSs in culture media containing sodium gluconate as carbon source but the reasons were not clearly understood.41 The concentration of selected carbon source in the culture media is another critical factor for EPS production. In most of the findings, between 30 and 60 gm/L carbon was suggested to best support EPS production from fungi (Table 2), although a few exceptions were also reported by Fariña et al,42 Sudhakaran and Shewale,43 Xu et al,44 Tavares et al.45 Fariña et al studied Sclerotium rolfsii ATCC201126, which produced approximately four times more scleroglucan when grown in 150 gm/L sucrose than 20 gm/L from the same carbon source.42 Sometimes combined carbon sources induced EPS production by fungi, demonstrated in the report of Zhang et al where they observed that 2 gm/L glucose with 30 gm/L starch influenced Sorangium cellulosum for maximum EPS (17.5 gm/L) production.46
Nitrogen supplementation is another variable that is reported to induce EPS production. Both inorganic and organic nitrogen sources were tested by several researchers to find the appropriate one. Among the organic sources peptone, yeast extract poly peptone, Martone A-1, Soybean meal, and corn steep powder were tested mostly. From numerous research findings, it was noticed that yeast extract and corn steep powder are good nitrogen supplements that induce EPS production from different fungal strains (Table 2). Among the various inorganic sources ammonium chloride, ammonium sulfate, sodium nitrate, potassium nitrate, urea, and diammonium oxalate monohydrate are commonly studied by researchers. Many observations suggested that in the presence of inorganic nitrogen sources, fungi produce less EPSs in comparison to organic nitrogen supplements. Among the inorganic nitrogen sources, ammonium salts are frequently more efficient than other inorganic salts.5 In very few studies, other inorganic salts have been found to best provide nitrogen sources for EPS production from fungi. Sodium nitrate had been found most suitable for the production of epiglucan by Epicoccum nigrum and for the production of scleroglucan by S. rolfsii ATCC201126.42,47 Urea was found most effective for EPS production from Nigrospora oryzae var. glucanicum.43 Sutherland reported that EPS production generally occurred in nitrogen limiting conditions.1 Researchers examined and reported that for different fungal strains, different concentrations of selected nitrogen favored maximum EPS production (Table 2). Except for a few reports, scientists found that in comparison to carbon sources very little nitrogen is required by fungi for EPS production and concentrations between 1–10 gm/L is are sufficient (Table 2). In exceptional cases, where more than 10 gm/L nitrogen sources were reported as best EPS production supportive, an interesting observation was recorded by almost all researchers: the concentration of carbon sources is always ≤ 30 gm/L which indicates lower consumption of carbon comparing others.
Many findings indicate that a phosphate source is an important addition needed by fungi for EPS production. Potassium dihydrogen phosphate (KH2PO4) and dipotassium monohydrogen phosphate (K2HPO4) have been reported as the most efficient phosphate supplements (Table 2). Among the different additional ionic salts, many describe magnesium sulphate (MgSO2·7H2O) as most suitable for EPS production by different fugal strains while in some cases calcium chloride (CaCl2), sodium chloride (NaCl) are also needed by the fungi for optimal production (Table 2). Tang et al practiced the one-variable-at-a- time approach and response surface methodology to evaluate the effect of metal ions on EPS (Tuber polysaccharide) production by Tuber sinense.48 From their research they concluded that 30 mM Mg2+ and 5 mM K+ maximized the EPS production (5.86 gm/L) in submerged culture, which was 130.7% higher compared to EPS produced in basal medium without metal ions.
Effects of some other additives including vegetable oils, fatty acids, surfactants, and vitamins were also studied and reported.12,16,49,50 These reports showed that sometimes these additives resulted in maximum EPS production. Lee et al used glutamic acid, biotin, and thiamine in culture medium for maximum EPS production.51 Yang and He reported that addition of 0.1% soybean oil in culture medium influenced EPS production by Shiraia bambusicola WZ-003.12 Yang et al evaluated the effect of additional fatty acids on mycelia growth and EPS production by suspended and immobilized G. lucidum.50 They had reported that in suspension culture, palmetic acid and oleic acid at less than 0.25 gm/L showed incensement in EPS production whereas linoleic acid had a strong reductive effect on EPS production by this organism. In immobilized cultures only palmitic acid showed influential effect on EPS production. Addition of 3 gm/L Tween 80 induced maximum EPS production by Pleurotus tuber-regium.49 Conversely, Hsieh et al reported that surfactants like Tween 80 or Span 80, although induce the cell growth of G. frondosa, significantly reduce the EPS production by the organism.52 The authors also evaluated the effect of plant oils on EPS production and reported that addition of 0.5% olive oil at the stationary phage of fungal growth induced maximum EPS (2.248 gm/L) production while same oil addition in early growth phage induced mycelia growth and reduced EPS production. Xiao et al used vitamin A and D in the culture medium as growth-stimulating factor for maximum EPS production by Oudemansiella radicata.16 Lin and Chen reported that 0.1% (v/v) thiamine induced EPS production in complex medium by Antrodia cinnamomea.53 Use of nicotinic acid (0.1% v/v) in the EPS production medium was reported by Lin and Sung when they evaluated the optimization process for EPS production by A. cinnamomea.27
Compositions of EPS Produced by Different Fungi
The monomeric composition and structure of fungal EPSs were usually evaluated by different experimental analysis of intact EPSs, hydrolyzed or partially hydrolyzed EPSs, or their derivatives. In general, these studies were analyzed through paper chromatography, HPLC, gas-liquid chromatography (GLC), gas-liquid chromatography-mass spectrometry (GLC-MS), and 1D and 2D NMR spectroscopy. The composition of fungal EPSs vary from pure sugars to sugars combined with a second unit such as protein, phosphate, sulfate, or amine. Different types of sugar unites were found in fungal EPSs such as glucose, mannose, galactose, xylose, fucose, and rhamnose. It was also noticed that EPSs composed of the same monosaccharide units that were synthesized by different fungi had different molecular weight. This is caused by differing chain length or branching patterns. To review the composition of EPSs and their corresponding molecular weight, see Table 3.
Table 3.
Compositions and molecular weight (Mw) distributions of some fungal exopolysaccharide.
| Organism | EPS composition | Mw of EPS | Refernences |
|---|---|---|---|
| Acremonium diospyri Crandall | Glucose | – | 126 |
| Antrodia camphorate | – | 2.18 × 105 Da | 36 |
| Aspergillus alliaceus | Galactosamine, galactose, glucose, acetate | – | 97 |
| Aspergillus niger | Glucose | – | 1 |
| Aspergillus parasiticus | Galactosamine, galactose, glucose, acetate, phosphate | – | 20 |
| Aspergillus sp. Y16 | Mannose, galactose | 15 KDa | 65 |
|
Botryosphaeria rhodina RCYU30101 |
Glucose | 1.82 × 106 Da | 82 |
| Candida boidinii | Glucose, mannose | 850 KDa | 75 |
| Cordyceps sphecocephala | Glucose, galactose, mannose, protein | 1st—1831 KDa; 2nd—27 KDa; 3rd—2.2 KDa |
28 |
| Cryphonectria parasitica | 1st type—glucose, galactose, mannose; 2nd type—glucose |
Varied from 20 to 1000 gm/mol/1000 | 55 |
| Cryptococcus laurentii AL100 | Arabinose, mannose, glucose, galactose, rhamnose | 4200 Da | 31 |
| Cryptococcus neoformans | 1st—glucuronic acid, xylose, mannose; 2nd—galactose, xylose, mannose; 3rd—mannose, protein |
1.7 to 7.0 × 106 Da | 86 |
| Cyttaria harioti | Glucose | – | 1 |
| Drechslera spicifera | Glucose | – | 103 |
| Elsinoe leucospila | Glucose | – | 1 |
|
Epicoccum nigrum Ehrenb. ex Schlecht |
Glucose | – | 47 |
| Fusarium solani SD5 | Galactose, rhamnose | 1.87 × 105 Da | 9 |
| Isaria farinose BO5 | Mannose, galactose, glucose, uronic acid, protein (4.40%) | 208 KDa | 84 |
| Lachnum sp. YM261 | Glucose | 21670 Da | 54 |
| Nigrospora oryzae var. glucanicum | Glucose | – | 43 |
| Penicillium varians | Galactose, glucose | – | 115 |
| Pestalotia sp. 815 | Glucose | – | 57 |
| Phanerochaete chrysosporium | Glucose | – | 117 |
| Phomopsis foeniculi | 1st type—rhamnose, mannose, galactose; 2nd type—mannose | 1st—13 to 22 KDa; 2nd—62 to 123 KDa |
58 |
| Pleurotus sp. | Glucose, galactose, mannose, protein | 63 | |
| Pleurotus tuber-regium | Glucose, mannose | 1st—3.18 × 106 Da; 2nd—4.30 × 106 Da |
49 |
| Sporobolomyces sp. | Galactose | – | 102 |
| Stemphylium sp. | Mannose, glucose | – | 9 |
| Tremella fuciformis | Mannose, xylose, fucose, protein | 1.3 to 1.5 × 106 Da | 125 |
| Trichosporon asahii | Mannose, xylose, glucuronic acid | – | 123 |
The linkage patterns and form of the monosaccharide units of fungal EPSs are also very diverse. Lachnum sp. YM261 produces an extracellular glucan with β-(1, 3)-D-pyran glycosidic bonding.54 Ruperez and Leal reported an EPS of A. parasiticus having monosaccharides with α-configuration and also reported that it contained predominantly 1, 6 linkages.20 Forabosco et al reported a novel type of pullulan production by Cryphonectria parasitica.55 Unlike other pullulans which contain α-(1, 6) maltotriose, this one contains high levels of α-(1, 6) maltotetrose subunits. Xu et al reported that in two types of bioreactor EPSs having different molecular weight (total five) were isolated.56 From a stirred-tank reactor, three types of EPSs were isolated among which two had glucose as the major monosaccharide component while the other types were composed majorly of mannose. In an airlift reactor, this organism produced two different EPSs. One showed glucose as its main building block, while the other showed arabinose. Pestolotan, an EPS produced by Pestalotia sp. 815, has three β-(1, 6) branched glucose residues for every five β-(1, 3) residues.57 Corsaro et al reported on two types of EPS production of Phomopsis foeniculi.58 One EPS produced by this organism is a galactan with structure of (-6)-β-D-Galacto furanose (1, 5)-β-D-Galacto furanose (1, 5)-β-D-Galacto furanose (1-)n. The other is a mannan with a backbone of α-(1, 6)-linked mannopyranose units. Leung et al reported an EPS having β-D-glucan backbone produced by Cordyceps sinensis Cs-HK1.59 Wang et al reported another type of EPS production from the same Cs-HK1 strain, where researchers found that the isolated EPS has a chemical composition of α-D glucopyranose, α-D-pyrano glucuronic acid in molar ratio of 8:1 and trace amount of mannose.60 Ichikawa et al reported an extracellular glucuronoxylomannan production from Trichosporon asahii.61 This EPS has a chemical backbone of α-(1, 3)-D-mannan like hexasaccharide substituted with one β-(1, 2)-glucopyranosyluronic acid and six β-D-xylopyranosyl units. Bernabe et al reported an EPS production from Lecanicillium muscarium CBS 413.70C.62 The structural composition study suggests the presence of α-(1, 4)-glucopyranosyl units and α-(1, 6)- glucopyranosyl units. Gutierrez et al reported that six species of Pleurotus genus produced EPSs with the main building block of β-(1, 3)-D-glucose and that 25% of total units are C-6 branched.63 Kim et al reported on the production of an EPS by G. resinaceum where under different culture conditions in 5-l stirred tank bioreactor, composition of EPSs were varied and that the main monomeric units of these EPSs were fucose, xylose, mannose, glucose, galactose as well as some protein moities.64 EPS of P. tuber-regium has a main building block of 1,6 linked mannopyranosyl residues almost all of which are branched at O-2 with a side chain containing two 2,6 linked mannopyranosyl residues and a terminal glucopyranosyl residue.49 The back bone of the EPS produced by endophytic Aspergillus sp. Y16 contains mainly 1, 2 linked α-D-mannopyranose units, substituted at C-6 by 1, 6 linked α-D-mannopyranose, 1 linked β-D-galactofuranose and 1 linked β-D-mannopyranose units.65 Ibrahim et al evaluated the compositions of EPS produced by Rhodotorula glutinins and reported that its EPS is made up of mannose, glucose and arabinose in a molar ratio of 3.2:1.0:0.8.66S. cellulosum produced an EPS containing 38.3% proteins and 58.5% carbohydrates, of which glucose, mannose and glucuronic acid were present at 51.3%, 39.2% and 10.5%, respectively.46 Mahapatra and Banerjee reported a rhamnogalactan production from endophytic fungus Fusarium solani SD5. Structural elucidation of this EPS indicated the presence of terminal α-L-rhamnopyranosyl, (1, 2)-α-L-rhamnopyranosyl, (1, 4)-β-D-galactopyranosyl, (1, 4, 6)-β-D-galactopyranosyl moieties in a molar ratio of 1:1:3:1.9
Applications of Different Fungal EPSs
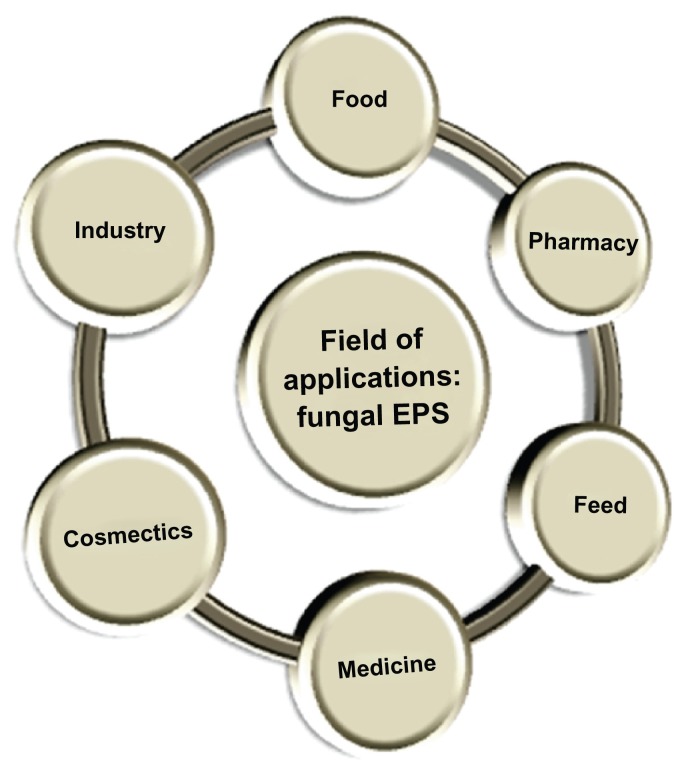
Microbial EPSs, including fungal EPSs, have gained importance from the last few decades as several studies showed different applications that not only indicate the alternative source of marketed plant or seaweed polysaccharides but also have some new and interesting bioapplicability. Furthermore, upstream and downstream processing of these EPSs is easier and one can produce a much larger amount in a shorter time when compared to plant or algal polysaccharide production. Fungal EPSs have several applications in the food and pharmaceutical industries among others. An overview on different fields where fungal EPSs can be applied is presented in Figure 2. Among the different fungal EPSs pullulan, Scleroglucan, and Botryosphaeran are well known for their applications in different fields. Here, some reported applications of different fungal EPSs are listed out for understanding the wide functional scope of these biomacromolecules.
Figure 2.
An overview: field of applications of fungal exopolysaccharides.
Pullulan, an EPS of A. pullulans, can be used as a thickener, a viscosity stabilizer in the food industry, and also for the preparation of nontoxic, biodegradable, edible plastic materials.67
Fungal EPS Scleroglucan is used industrially for the Enhanced Oil Recovery.68 Scleroglucan is sometimes called schizophyllan under the industrial name BIOVIS (produced by Degussa society) with bacterial polysaccharide, xanthan gum applied is used for preparation of a drilling fluids (water based muds) with low mud toxicity.69 Farwick et al reported the high water binding capacity of scleroglucan on epithelial cells.70 Other industrial applications of this EPS were in preparation of adhesives, water colors, printing inks, and animal feed composition.71 It is also used in the manufacturing of cosmetics and in various skin care products, creams and protective lotions.72,73
EPS of Byssochlamy nivea showed Kaolin-flocculating behavior. Gomoiu and Catley suggested that this EPS can be used for sedimentation of fibers in downstream removal of paper fibers from white water effluent in paper industries and thus possesses pollution reducing efficacy in paper industries as well as rivers where industrial effluents were discarded.74
EPSs from five yeast strains and one yeast-like fungus showed drag-reducing activity.75
Exopolymer (glycoprotein) produced by G. lucidum can increase the swimming endurance capacity of mice by about 10 minutes and reduced the muscle and liver glycogen exhaustion by 18.5% and 67.2% respectively.76
Fungal β-glucans (both extracellular and intracellular) are effective in promoting health, protection from mutations and treatment of diseases like cancer, microbial infections, hypercholesterolaemia and diabetes.77,78
Pestan, a fungal EPS produced by Pestalotiopsis sp. KCTC 8637, has applications in wastewater treatment as a biosorbent of lead and zinc. Each gram of pestan absorbed 120 mg lead and 60 mg zinc.79 EPS of Aspergillus fumigatus also showed sorption efficiency of two heavy metal ions, copper and lead.80
Botryosphaeran, a EPS of Botryosphaeria rhodina, was shown to significantly decrease the clastogenic effect of cyclophosphamide- induced micronucleus formation in polychromatic erythrocytes of bone marrow and reticulocytes in peripheral blood in mice.81 Botryosphaeran is also toxicologically accepted and a potent immunomodulator.82
EPSs produced by Akanthomyces pistillariiformis BCC2694, Cordyceps dipterigena BCC2073, P. tenuipes BCC2656, and Phytocordyceps sp. BCC2744 was shown to be biocompatible and have potentiality as a wound dressing material through testing induction in interleukine-8 production in normal human dermal fibroblasts cells.83
EPS of Phellinus baumii Pilát showed direct immune-stimulating activity on splenocyte proliferative response and acid phosphatase activity in peritoneal macrophages of mice.30 Wang et al reported the immunomodulatory activities of EPS produced by C. sinensis Cs-HK1. The activities were evaluated in Raw 264.7 macrophage cell cultures.60
Water soluble extracellular polysaccharides of Isaria farinose BO5 showed antitumor and antioxidant activity in Kunming mice.82 EPS of Hypsizigus marmoreus and Fomes formentarius showed antitumor activity examined on human gastric cancer cells SGC-7901.29,85
Extracellular glucuronoxylomannan of Cryptococcus neoformans inhibited the entry of HIV virus in TZM-bl cell lines.86
An extracellular glucan of Lachnum sp. YM261 showed strong anti-ageing activity tested in D-gal model mice.54
C. sinensis Cs-HK1 produced an EPS which has moderate antioxidant activities.57 EPSs of Ganoderma resinaceum, Gomphidius rutilus and endophytic Aspergillus sp. Y16 showed in vitro anti oxidant activity.64,65,87
Endophytic Fusarium oxysporium DzF17 has been reported as an EPS producer. Its EPS showed elicitor activities on growth and diosgenin production in cell suspension culture of Dioscorea zinbiberensis.88
Endophytic F. solani SD5 was reported to produce an extracellular rhamnogalactan that showed antiinflammatory and anti-allergic activity in vitro. EPS (1000 μg/mL) protects 55% of erythrocytes from hypotonic solution induced membrane lysis. Compound 48/80 induced mast cell degranulation was also protected by 56% with 100 μg/mL EPS.9
The EPS of G. frondosa HB0071 showed inhibitory effect on matrix metallo proteinase-1 expression in UV-irradiated human dermal fibroblasts and thus may contribute to inhibitory action in photo-aging skin by reducing matrix metallo proteinase- 1 related matrix degradation system.89
P. baumii and Stropharia rugosoannulata produce EPS which show hypoglycemic activity in streptozotocin induced diabetic rats.90
Preliminary examinations with EPS produced by R. glutinins are found to have anti-oxidant, antiviral, and antitumor activities.66
Conclusion
In 2001, Hurtley et al despondently stated that the chemistry and biology of carbohydrate research is like a “Cinderella field”, though promising but to date does not get as much attention as genomes and proteins.91 This realization is tinged with realism, although fungi have been extensively applied in industry since the 1940s for diverse bioactive metabolite production. In the case of EPS production, however, the efforts were not satisfactory to quench the thirst for knowledge in this field up until recently. Considerable increases in investigation of fungal EPS production or their physio-chemical characterizations by researchers were noticed only over the last two decades. Nevertheless, the findings are impressive from both the scientific angle and applicability. The hope is that this trend will continue to increase and will enrich both scientific knowledge base and provide better assistance for life in the future.
Footnotes
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest. Provenance: the authors were invited to submit this paper.
Funding
Author(s) disclose no funding sources.
References
- 1.Sutherland IW. Extracellular polysaccharides. In: Rhem HJ, Reed G, editors. Biotechnology. Vol. 6. VCH; Weinheim: 1996. pp. 615–57. [Google Scholar]
- 2.Roller S, Dea ICM. Biotechnology in the production and modification of biopolymers for foods. Crit Rev Biotechnol. 1992;12(3):261–77. [Google Scholar]
- 3.Wang Y, McNeil B. Scleroglucan. Crit Rev Biotechnol. 1996;16(3):185–215. doi: 10.3109/07388559609147421. [DOI] [PubMed] [Google Scholar]
- 4.Manzi P, Pizzoferrato L. Beta-glucans in edible mushrooms. Food Chem. 2000;68(3):315–8. [Google Scholar]
- 5.Seviour RJ, Stasinopoulos SJ, Auer DPF, Gibbs PA. Production of pullulan and other exopolysaccharides by filamentous fungi. Crit Rev Biotechnol. 1992;12(3):279–98. [Google Scholar]
- 6.Sutherland IW. Structure-function relationships in microbial exopolysaccharides. Biotech Adv. 1994;12(2):393–448. doi: 10.1016/0734-9750(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 7.Banik RM, Kanari B, Upadhyay SN. Exopolysaccharide of the gellan family: prospects and potential. World J Microb Biot. 2000;16(5):407–14. [Google Scholar]
- 8.Muzzarelli RRA, Boudrant J, Meyer D, Manno N, DeMarchis M, Paoletti MG. Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohyd Polym. 2012;87(2):995–1012. [Google Scholar]
- 9.Mahapatra S, Banerjee D. Structural elucidation and bioactivity of a novel exopolysaccharide from endophytic Fusarium solani SD5. Carbohyd Polym. 2012;90(1):683–9. doi: 10.1016/j.carbpol.2012.05.097. [DOI] [PubMed] [Google Scholar]
- 10.Robyt JF. Essentials of carbohydrate chemistry. Spinger-Verlag; New York: 1998. [Google Scholar]
- 11.Clementi F. Alginate production by Azotobacter vinelandii. Crit Rev Biotechnol. 1997;17(4):327–61. doi: 10.3109/07388559709146618. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, He G. Influence of nutritional conditions on exopolysaccharide production by submerged cultivation of the medicinal fungus Shiraia bambusicola. World J Microb Biot. 2008;24(12):2903–7. [Google Scholar]
- 13.Donot F, Fontana A, Baccou JC, Schorr-Galindo S. Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohyd Polym. 2012;87(2):951–62. [Google Scholar]
- 14.Tang YJ, Zhong JJ. Exopolysaccharide biosynthesis and related enzyme activities of the medicinal fungus, Ganoderma lucidum, grown on lactose in a bioreactor. Biotechnol Lett. 2002;24(12):1023–6. [Google Scholar]
- 15.Feng YL, Li WQ, Wu XQ, Cheng JW, Ma SY. Statistical optimization of media for mycelial growth and exo-polysaccharide production by Lentinus edodes and a kinetic model study of two growth morphologies. Biochem Eng J. 2010;49(1):104–12. [Google Scholar]
- 16.Xiao JH, Xiao DM, Xiong Q, Liang ZQ, Zhong JJ. Nutritional requirements for the hyperproduction of bioactive exopolysaccharides by submerged fermentation of the edible medicinal fungus Cordyceps taii. Biochem Eng J. 2010;49:241–9. [Google Scholar]
- 17.Jiao Y, Chen Q, Zhou J, Zhang H, Chen H. Improvement of exopolysaccharides production and modeling kinetics by Armillaria luteo-virens Sacc. In submerged cultivation. LWT-Food Sci Technol. 2008;41(9):1694–700. [Google Scholar]
- 18.Kim HO, Lim JM, Joo JH, et al. Optimization of submerged culture condition for the production of mycelial biomass and exopolysaccharides by Agrocybe cylindracea. Bioresource Technol. 2005;96(10):1175–82. doi: 10.1016/j.biortech.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Sandford PA. Advances in Carbohydrate Chemistry and Biochemistry. Vol. 36. Academic press, Inc; 1979. Exocellular, microbial polysaccharides; pp. 265–313. [DOI] [PubMed] [Google Scholar]
- 20.Ruperez P, Leal JA. Extracellular galactosaminogalactan from Aspergillus parasiticus. Trans Br Mycol Soc. 1981;77(3):621–5. [Google Scholar]
- 21.Roukas T, Liakopoulou-Kyriakides M. Production of pullulan from beet molasses by Aureobasidium pullulans in a stirred tank fermenter. J Food Eng. 1999;40(1–2):89–94. [Google Scholar]
- 22.Kim SW, Hwang HJ, Xu CP, Choi JW, Yun JW. Effect of aeration and agitation on the production of mycelial biomass and exopolysaccharides in an enthomopathogenic fungus Paecilomyces sinclairii. Lett Appl Microbiol. 2003;36(5):321–6. doi: 10.1046/j.1472-765x.2003.01318.x. [DOI] [PubMed] [Google Scholar]
- 23.Xu CP, Yun JW. Influence of aeration on the production and the quality of the exopolysaccharides from Paecilomyces tenuipes C240 in a stirred-tank fermenter. Enzyme Microb Tech. 2004;35(1):33–9. [Google Scholar]
- 24.Gibbs PA, Seviour RJ. Does the agitation rate and/or oxygen saturation influence exopolysaccharide production by Aureobasidium pullulans in batch culture? Appl Microbiol Biotechnol. 1996;46(5–6):503–10. [Google Scholar]
- 25.Wang Y, McNeil B. pH effects on exopolysaccharide and oxalic acid production in cultures of Sclerotium glucanicum. Enzyme Microb Tech. 1995;17(2):124–30. [Google Scholar]
- 26.Lim JM, Kim SW, Hwang HJ, et al. Optimization of medium by orthogonal matrix method for submerged mecelial culture and exopolysaccharide production in Collybia maculate. Appl Biochem Biotech. 2004;119(2):159–70. doi: 10.1385/abab:119:2:159. [DOI] [PubMed] [Google Scholar]
- 27.Lin ES, Sung SC. Cultivating conditions influence exopolysaccharide production by the edible Basidiomycete Antrodia cinnamomea in submerged culture. Int J Food Microbiol. 2006;108(2):182–7. doi: 10.1016/j.ijfoodmicro.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Oh JY, Cho EJ, Nam SH, Choi JW, Yun JW. Production of polysaccharide- peptide complexes by submerged mycelial culture of an entomopathogenic fungus Cordyceps sphecocephala. Process Biochem. 2007;42(3):352–62. [Google Scholar]
- 29.Chen W, Zhao Z, Chen SF, Li YQ. Optimization for the production of exopolysaccharide from Fomes fomentarius in submerged culture and its antitumor effect in vitro. Bioresource Technol. 2008;99(8):3187–94. doi: 10.1016/j.biortech.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 30.Luo J, Liu J, Ke C, et al. Optimization of medium composition for the production of exopolysaccharides from Phellinus baumii Pilát in submerged culture and the immuno-stimulating activity of exopolysaccharides. Carbohyd Polym. 2009;78(3):409–15. [Google Scholar]
- 31.Pavlova K, Rusinova-Videva S, Kuncheva M, Kratchanova M, Gocheva M, Dimitrova S. Synthesis and characterization of an exopolysaccharide by Antarctic yeast strain Cryptococcus laurentii AL100. Appl Biochem Biotechnol. 2011;163(8):1038–52. doi: 10.1007/s12010-010-9107-9. [DOI] [PubMed] [Google Scholar]
- 32.Wu S, Chen J, Pan S. Optimization of fermentation conditions for the production of pullulan by a new strain of Aureobasidium pullulans isolated from sea mud and its characterization. Carbohyd Polym. 2012;87(2):1696–700. doi: 10.1016/j.carbpol.2011.08.062. [DOI] [PubMed] [Google Scholar]
- 33.Hwang HJ, Kim SW, Xu CP, Choi JW, Yun JW. Production and molecular characteristics of four groups of exopolysaccharides from submerged culture of Phellinus gilvus. J Appl Microbiol. 2003;94(4):708–19. doi: 10.1046/j.1365-2672.2003.01903.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim HO, Yun JW. A comparative study on the production of exopolysaccharides between two entomopathogenic fungi Cordyceps militaris and Cordyceps sinensis in submerged mycelial cultures. J Appl Microbiol. 2005;99(4):728–38. doi: 10.1111/j.1365-2672.2005.02682.x. [DOI] [PubMed] [Google Scholar]
- 35.Pokhrel CP, Ohga S. Submerged culture conditions for mycelial yield and polysaccharides production by Lyophyllum decastes. Food Chem. 2007;105(2):641–6. [Google Scholar]
- 36.Shu CH, Lung MY. Effect of pH on the production and molecular weight distribution of exopolysaccharide by Antrodia camphorate in batch cultures. Process Biochem. 2004;39(8):931–7. [Google Scholar]
- 37.Abdel-Aziz MS, Hamed HA, Mouafi FE, Gad AS. Acidic pH-shock induces the production of an exopolysaccharide by the fungus Mucor rouxii: Utilization of Beet-molasses. New York Sci J. 2012;5(2):52–61. [Google Scholar]
- 38.Lee JH, Kim JH, Zhu IH, et al. Optimization of conditions for the production of pullulan and high molecular weight pullulan by Aureobasidium pullulans. Biotechnol Lett. 2001;23(10):817–20. [Google Scholar]
- 39.Elisashvili VI, Kachlishvili ET, Wasser SP. Carbon and nitrogen source effects on basidiomycetes exopolysaccharide production. Appl Biochem Micro+ 2009;45(5):531–5. [PubMed] [Google Scholar]
- 40.Bae JT, Park JP, Song CH, Yu CB, Park MK, Yun JW. Effect of carbon sources on the mycelial growth and exo-biopolymer production by submerged culture of Paecilomyces japonica. J Biosci Bioeng. 2001;91(5):522–4. doi: 10.1263/jbb.91.522. [DOI] [PubMed] [Google Scholar]
- 41.Elisashvili V, Wasser S, Tan KK, Chichua D, Kachlishvili E. Extracellular polysaccharide production by culinary-medicinal Shiitake mushroom Lentinus edodes (Berk.) Singer and Pleurotus (Fr.) P. Karst. species depending on carbon and nitrogen source. Int J Med Mushr. 2004;6(2):165–72. [Google Scholar]
- 42.Fariña JI, Siñeriz F, Molina OE, Perotti NI. High scleroglucan production by Sclerotium rolfsii: Influence of medium composition. Biotechnol Lett. 1998;20(9):825–31. [Google Scholar]
- 43.Sudhakaran VK, Shewale JG. Exopolysaccharide production by Nigrospora oryzae var. glucanicum. Enzyme Microb Tech. 1988;10(9):547–51. [Google Scholar]
- 44.Xu CP, Kim SW, Hwang HJ, Choi JW, Yun JW. Optimization of submerged culture conditions for mycelial growth and exo-biopolymer production by Paecilomyces tenuipes C240. Process Biochem. 2003;38:1025–30. [Google Scholar]
- 45.Tavares APM, Agapito MSM, Coelho MAZ, et al. Selection and optimization of culture medium for exopolysaccharide production by Coriolus (Trametes) versicolor. World J Microb Biot. 2005;21(8–9):1499–507. [Google Scholar]
- 46.Zhang J, Wang R, Jiang P, Lui Z. Production of an exopolysaccharide bioflocculant by Sorangium cellulosum. Lett Appl Microbiol. 2002;34(3):178–81. doi: 10.1046/j.1472-765x.2002.01068.x. [DOI] [PubMed] [Google Scholar]
- 47.Schmid F, Stone BA, McDougall M, et al. Structure of epiglucan, a highly side-chain/branched (1-3;16)-β-glucan from the micro fungus Epicoccum nigrum Ehrenb. Ex Schlecht. Carbohyd Res. 2001;331(2):163–71. doi: 10.1016/s0008-6215(01)00023-4. [DOI] [PubMed] [Google Scholar]
- 48.Tang YJ, Zhu LL, Liu RS, Li HM, Li DS, Mi ZY. Quantitative response of cell growth and Tuber polysaccharides biosynthesis by medicinal mushroom Chinese truffle Tuber sinense to metal ion in culture medium. Bioresource Technol. 2008;99(16):7606–15. doi: 10.1016/j.biortech.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Zhang BB, Cheung CK. Use of stimulatory agents to enhance the production of bioactive exopolysaccharide from Pleurotus tuber-regium by submerged fermentation. J Agr Food Chem. 2011;59(4):1210–6. doi: 10.1021/jf104425w. [DOI] [PubMed] [Google Scholar]
- 50.Yang FC, Ke YF, Kuo SS. Effect of fatty acids on the mycelial growth and polysaccharide formation by Ganoderma lucidum in shake flask cultures. Enzyme Microb Tech. 2000;27(3–5):295–301. doi: 10.1016/s0141-0229(00)00213-1. [DOI] [PubMed] [Google Scholar]
- 51.Lee WY, Park Y, Ahn JK, Ka KH, Park SY. Factors influencing the production of endopolysaccharide and exopolysaccharide from Ganoderma applanatum. Enzyme Microb Tech. 2007;40(2):249–54. [Google Scholar]
- 52.Hsieh C, Wang HL, Chen CC, Hsu TH, Tseng MH. Effect of plant oil and surfactant on the production of mycelial biomass and polysaccharides in submerged culture of Grifola frondosa. Biochem Eng J. 2008;38(2):198–205. [Google Scholar]
- 53.Lin ES, Chen YH. Factors affecting mycelial biomass and exopolysaccharide production in submerged cultivation of Antrodia cinnamomea using complex media. Bioresource Technol. 2007;98(13):2511–7. doi: 10.1016/j.biortech.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Ye M, Chen W, Qiu T, Yuan R, Ye Y, Cai J. Structural characterization and anti-ageing activity of extracellular polysaccharide from a strain of Lachnum sp. Food Chem. 2012;132(1):338–43. doi: 10.1016/j.foodchem.2011.10.087. [DOI] [PubMed] [Google Scholar]
- 55.Forabosco A, Bruno G, Sparapano L, Liut G, Marino D, Delben F. Pullulans production by strains of Cryphonectria parasitica-I. Production and characterization of the exopolysaccharides. Carbohyd Polym. 2006;63(4):535–44. [Google Scholar]
- 56.Xu CP, Kim SW, Hwang HJ, Yun JW. Production of exopolysaccharides by submerged culture of an enthomopathogenic fungus, Paecilomyces tenuipes C240 in stirred-tank and airlift reactors. Bioresource Technol. 2006;97(5):770–7. doi: 10.1016/j.biortech.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 57.Misaki A, Kawaguchi K, Miyaji H, et al. Structure of pestalotan, a highly branched (1-3)-β-D-glucan elaborated by Pestalotia sp 815 and the enhancement of its antitumour activity by polyol modification of the side chains. Carbohyd Res. 1984;129:209–27. doi: 10.1016/0008-6215(84)85313-6. [DOI] [PubMed] [Google Scholar]
- 58.Corsaro MM, Castro CD, Evidente A, et al. Chemical structure of two phytotoxic exopolysaccharides produced by Phomopsis foeniculi. Carbohyd Res. 1998;308(3–4):349–57. doi: 10.1016/s0008-6215(98)00085-8. [DOI] [PubMed] [Google Scholar]
- 59.Leung PH, Zhao S, Ho KP, Wu JY. Chemical properties and antioxidant activity of exopolysaccharides from mycelial culture of Cordyceps sinensis fungus Cs-HK1. Food Chem. 2009;114(4):1251–6. [Google Scholar]
- 60.Wang ZM, Peng X, Lee KLD, Tang JC, Cheung PCK, Wu JY. Structural characterization and immunomodulatory property of an acidic polysaccharide from mycelial culture of cordyceps sinensis fungus Cs-HK1. Food Chem. 2011;125(2):637–43. [Google Scholar]
- 61.Ichikawa T, Nishikawa A, Ikeda R, Shinoda T. Structural studies of a cell wall polysaccharide of Trichosporon asahii containing antigen II. Eur J Biochem. 2001;268(19):5098–106. doi: 10.1046/j.0014-2956.2001.02438.x. [DOI] [PubMed] [Google Scholar]
- 62.Bernabe M, Salvachua D, Jimenez-Barbero J, Leal JA, Prieto A. Structures of wall heterogalactomannans isolated from three genera of entomopathogenic fungi. Fungal Biol. 2011;115(9):862–70. doi: 10.1016/j.funbio.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 63.Gutiérrez A, Prieto A, Martínez AT. Structural characterization of extracellular polysaccharides produced by fungi from the genus Pleurotus. Carbohyd Res. 1996;281(1):143–54. doi: 10.1016/0008-6215(95)00342-8. [DOI] [PubMed] [Google Scholar]
- 64.Kim HM, Kim SW, Hwang HJ, et al. Influence of agitation intensity and aeration rate on production of antioxidative exopolysaccharides from submerged mycelial culture of Ganoderma resinaceum. J Microbiol Biotechn. 2006;16(8):1240–7. [Google Scholar]
- 65.Chen Y, Mao W, Tao H, et al. Structural characterization and antioxidant properties of an exopolysaccharide produced by the mangrove endophytic fungus Aspergillus sp. Y16. Bioresource Technol. 2011;102(17):8179–84. doi: 10.1016/j.biortech.2011.06.048. [DOI] [PubMed] [Google Scholar]
- 66.Ibrahim GS, Mahmoud MG, Asker MMS, Ghazy EA. Production and biological evaluation of exopolysaccharide from isolated Rhodotorula Glutinins. Aust J Basic Appl Sci. 2012;6:401–8. [Google Scholar]
- 67.Paul F, Morin A, Monsan P. Microbial polysaccharides with actual potential industrial applications. Biotechnol Adv. 1986;4(2):245–59. doi: 10.1016/0734-9750(86)90311-3. [DOI] [PubMed] [Google Scholar]
- 68.Holzwarth G. Xanthan and scleroglucan: structure and use in enhanced oil recovery. Dev Ind Microbiol. 1985;26:271–80. [Google Scholar]
- 69.Hamed SB, Belhadri M. Rheological properties of biopolymers drilling fluids. J Petrol Sci Eng. 2009;67:84–90. [Google Scholar]
- 70.Farwick M, Lersch P, Schmitz G, Müllner S, Wattenberg A. “Skin-omics”: use of genomics, proteomics and lipidomics to assess effects of low molecular weight scleroglucan. Cos Sci Technol E Indus. 2009:100–5. [Google Scholar]
- 71.Halleck FE. Paint composition containing polysaccharides. 3447940. US Patent. 1969
- 72.Halleck FE. Wave set composition containing a polysaccharides. 3507290. US Patent. 1970
- 73.Halleck FE. Cosmetic composition employing water-soluble polysaccharide. 3659025. US Patent. 1972
- 74.Catley BJ. Role of pH and nitrogen limitation in the elaboration of the extracellular polysaccharide pullulan by Pullularia pullulans. Applied Microbiol. 1971;22(4):650–4. doi: 10.1128/am.22.4.650-654.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petersen GR, Schubert WW, Richards GF, Nelson GA. Yeasts producing exopolysaccharides with drag-reducing activity. Enzyme Microb Tech. 1990;12(4):255–9. [Google Scholar]
- 76.Yang BK, Jeong SC, Park JB, et al. Swimming endurance capacity of mice after administration of exo-polymer produced from submerged mycelial culture of Ganoderma lucidum. J Microbiol Biotechnol. 2001;11(5):902–5. [Google Scholar]
- 77.Chen J, Seviour R. Medicinal importance of fungal β-(1-3), (1-6)-glucans. Mycol Res. 2007;111(pt 6):635–52. doi: 10.1016/j.mycres.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 78.Mantovani MS, Bellini MF, Angeli JPF, Oliveira RJ, Silva AF, Ribeiro LR. β-Glucans in promoting health: Prevention against mutation and cancer. Mutat Res. 2008;658(3):154–61. doi: 10.1016/j.mrrev.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 79.Moon SH, Park CS, Kim YJ, Park YI. Biosorption isotherms of Pb (II) and Zn (II) on Pestan, an extracellular polysaccharide, of Pestalotiopsis sp. KCTC 8637P. Process Biochem. 2006;41(2):312–6. [Google Scholar]
- 80.Yin Y, Hu Y, Xiong F. Sorption of Cu(II) and Cd(II) by extracellular polymeric substances (EPS) from Aspergillus fumigatus. Int Biodeter Biodegr. 2011;65(7):1012–8. [Google Scholar]
- 81.Miranda CCBO, Dekker RFH, Serpeloni JM, Fonseca EAI, Cólus IMS, Barbosa AM. Anticlastogenic activity exhibited by botryosphaeran, a new exopolysaccharide produced by Botryosphaeria rhodina MAMB-05. Int J Biol Macromol. 2008;42(2):172–7. doi: 10.1016/j.ijbiomac.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 82.Weng BBC, Lin YC, Hu CW, et al. Toxicological and immunomodulatory assessments of botryosphaeran (β-glucan) produced by Botryosphaeria rhodina RCYU 30101. Food Chem Toxicol. 2011;49(4):910–6. doi: 10.1016/j.fct.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 83.Madla S, Methacanon P, Prasitsil M, Kirtikara K. Characterization of biocompatible fungi-derived polymers that induce IL-8 production. Carbohyd Polym. 2005;59(3):275–80. [Google Scholar]
- 84.Jiang Y, Jiang X, Wang P, Mou H, Hu X, Liu S. The antitumor and antioxidative activities of polysaccharides isolated from Isaria farinose BO5. Microbiol Res. 2008;163(4):424–30. doi: 10.1016/j.micres.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 85.Zhang B, Yan P, Chen H, He J. Optimization of production conditions for mushroom polysaccharides with high yield and antitumor activity. Carbohyd Polym. 2012;87(4):2569–75. [Google Scholar]
- 86.Yi HA, Panepinto JC, Jacobs A. Inhibition of HIV entry by extracellular glucuronoxylomannan of Cryptococcus neoformans. Microb Pathogenesis. 2012;52(1):25–30. doi: 10.1016/j.micpath.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 87.Gao C, Wang Z, Su T, Zhang J, Yang X. Optimization of exopolysaccharide production by Gomphidius rutilus and its antioxidant activities in vitro. Carbohyd Polym. 2012;87(3):2299–305. [Google Scholar]
- 88.Li P, Mou Y, Shan T, et al. Effects of polysaccharide elicitors from endophytic Fusarium oxysporium Dzf17 on growth and diosgenin production in cell suspension culture of Dioscorea zingiberensis. Molecules. 2011;16(11):9003–16. doi: 10.3390/molecules16119003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bae JT, Sim GS, Lee DH, et al. Production of exopolysaccharide from mycelial culture of Grifola frondosa and its inhibitory effect on matrix metalloproteinase-1 expression in UV-irradiated human dermal fibroblasts. FEMS Microbiol Lett. 2005;251(2):347–54. doi: 10.1016/j.femsle.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 90.Zhai X, Zhao A, Geng L, Xu C. Fermentation characteristics and hypoglycemic activity of an exopolysaccharide produced by submerged culture of Stropharia rugosoannulata#2. Ann Microbiol. 2012 doi: 10.1007/s13213-012-0555-z. [DOI] [Google Scholar]
- 91.Hurtley S, Service R, Szuromi P. Cinderella’s coach is ready. Science. 2001;291(5512):2337. [Google Scholar]
- 92.Ruiter GAD, Bruggen AWV, Lugt VD, et al. 2-O-methyl-D-mannose residues are immunodominant in extracellular polysaccharides of Mucor racemosus and related molds. J Biol Chem. 1994;269(6):4299–306. [PubMed] [Google Scholar]
- 93.Graber M, Morin A, Duchiron F, Monsan PF. Microbial polysaccharides containing 6-deoxysugars. Enzyme Microb Tech. 1988;10(4):198206. [Google Scholar]
- 94.Nehad EA, Shamy ARE. Physiological studies on the production of exopolysaccharide by fungi. Agric Biol J N Am. 2010;1:1303–8. [Google Scholar]
- 95.Stasinopoulos SJ, Seviour RJ. Exopolysaccharide formation by isolates of Cephalosporium and Acremonium. Mycol Res. 1989;92(1):55–60. [Google Scholar]
- 96.Maziero R, Cavazzoni V, Bononi VLR. Screening of basidiomycetes for the production of exopolysaccharide and biomass in submerged culture. Rev Microbiol. 1999;30(1):77–84. [Google Scholar]
- 97.Miranda BG, Leal JA. Extracellular and cell wall polysaccharides of Aspergillus alliaceus. Trans Br Mycol Soc. 1981;76(2):249–53. [Google Scholar]
- 98.Selbmann L, Stingele F, Petruccioli M. Exopolysaccharide production by filamentous fungi: the example of Botryosphaeria rhodina. A van Leeuw. 2003;84(2):135–45. doi: 10.1023/a:1025421401536. [DOI] [PubMed] [Google Scholar]
- 99.Schmid J, Meyer V, Sieber V. Scleroglucan: biosynthesis, production and application of a versatile hydrocolloid. Appl Microbiol Biotechnol. 2011;91(4):937–47. doi: 10.1007/s00253-011-3438-5. [DOI] [PubMed] [Google Scholar]
- 100.Gomoiu I, Catley BJ. Properties of a kaolin-flocculating polymer elaborated by Byssochlamys nivea. Enzyme Microb Tech. 1996;19(1):45–9. [Google Scholar]
- 101.Kim SW, Xu CP, Hwang HJ, Choi JW, Kim CW, Yun JW. Production and characterization of exopolysaccharides from an enthomopathogenic fungus Cordyceps militaris NG3. Biotechnol Progr. 2003;19(2):428–35. doi: 10.1021/bp025644k. [DOI] [PubMed] [Google Scholar]
- 102.Pavlova K, Koleva L, Kratchanova M, Panchev I. Production and characterization of an exopolysaccharide by yeast. World J Microb Biot. 2004;20(4):435–9. [Google Scholar]
- 103.Aouadi S, Heyraud A, Seigle-Murandi F, Steiman R, Fournet B. Structural analysis and rheological behaviour of an extracellular polysaccharide from Drechslera spicifera. Carbohyd Polym. 1992;17(3):177–83. [Google Scholar]
- 104.Choi D, Maeng JM, Ding JL, Cha WS. Exopolysaccharide production and mycelial growth in an air-lift bioreactor using Fomitopsis pinicola. J Microbiol Biotechnol. 2007;17(8):1369–78. [PubMed] [Google Scholar]
- 105.Papinutti L. Effects of nutrients, pH and water potential on exopolysaccharides production by a fungal strain belonging to Ganoderma lucidum complex. Bioresource Technol. 2010;101(6):1941–6. doi: 10.1016/j.biortech.2009.09.076. [DOI] [PubMed] [Google Scholar]
- 106.Sarkar JM, Hennebert GL, Mayaudon J. Optimization and characterization of an extracellular polysaccharide produced by Glomerella cingulata. Biotechnol Lett. 1985;7(9):631–6. [Google Scholar]
- 107.Sarkar JM, Hennebert GL, Mayaudon J. Optimization and characterization of an extracellular polysaccharide produced by Moniliella pollinis. Biotechnol Lett. 1986;8(5):319–22. [Google Scholar]
- 108.He P, Geng L, Mao D, Xu C. Production, characterization and antioxidant activity of exopolysaccharides from submerged culture of Morchella crassipes. Bioprocess Biosyst Eng. 2012;35(8):1325–32. doi: 10.1007/s00449-012-0720-6. [DOI] [PubMed] [Google Scholar]
- 109.Zou X. Optimization of nutritional factors for exopolysaccharide production by submerged cultivation of the medicinal mushroom Oudemansiella radicata. World J Microb Biot. 2005;21(6–7):1267–71. [Google Scholar]
- 110.Sinha J, Bae JT, Park JP, Song CH, Yun JW. Effect of substrate concentration on broth rheology and fungal morphology during exo-biopolymer production by Paecilomyces japonica in a batch bioreactor. Enzyme Microb Tech. 2001;29(6–7):392–9. [Google Scholar]
- 111.Sarkar JM. Optimization and characterization of an extracellular polysaccharide production by Paecilomyces lilacinus. Biotechnol Lett. 1986;8(11):769–70. [Google Scholar]
- 112.Kim SW, Hwang HJ, Xu CP, Na YS, Song SK, Yun JW. Influence of nutritional conditions on the mycelial growth and exopolysaccharide production in Paecilomyces sinclairii. Lett Appl Microbiol. 2002;34(6):389–93. doi: 10.1046/j.1472-765x.2002.01105.x. [DOI] [PubMed] [Google Scholar]
- 113.Kohama K, Fujimoto M, Kuninaka A, Yoshino H. Structure of malonogalactan, an acidic polysaccharide of Penicillium citrinum. Agr Biol Chem. 1974;38:127–34. [Google Scholar]
- 114.Birkinshaw JH, Raistrick H. Studies in the biochemistry of micro-organiss. XXVII. The production of luteic acid from various sources of carbon by Penicillium luteum Zukal. Biochem J. 1933;27(2):370–5. doi: 10.1042/bj0270370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jansson PE, Lindberg B. Structural studies of varianose. Carbohyd Res. 1980;82:97–102. [Google Scholar]
- 116.Kogan G, Matulová M, Michalková E. Extracellular polysaccharides of Penicillium vermiculatum. Z Naturforsch. 2002;57:452–8. doi: 10.1515/znc-2002-5-609. [DOI] [PubMed] [Google Scholar]
- 117.Buchala AJ, Leisola M. Structure of the β-glucan secreted by Phanerochaete chrysosporium in continuous culture. Carbohyd Res. 1987;165:146–9. [Google Scholar]
- 118.Hwang HJ, Kim SW, Lim JM, et al. Hypoglycemic effect of crude exopolysaccharides produced by a medicinal mushroom Phellinus baumii in streptozotocin-induced diabetic rats. Life Sci. 2005;76(26):3069–80. doi: 10.1016/j.lfs.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 119.Papaspyridi LM, Katapodis P, Gonou-Zagou Z, Kapsanaki-Gotsi E, Christakopoulos P. Optimization of biomass production with enhanced glucan and dietary fibres content by Pleurotus ostreatus ATHUM 4438 under submerged culture. Biochem Eng J. 2010;50(3):131–8. [Google Scholar]
- 120.Joo JH, Lim JM, Kim HO, et al. Optimization of submerged culture conditions for exopolysaccharide production in Sarcodon aspratus (Berk) S.lto TG-3. World J Microb Biot. 2004;20(7):767–73. [Google Scholar]
- 121.Banerjee D, Jana M, Mahapatra S. Production of exopolysaccharideby endophytic Stemphylium sp. Micol Apl Inter. 2009;21(2):57–62. [Google Scholar]
- 122.Zhu H, Cao C, Zhang S, Zhang Y, Zou W. pH-control modes in a 5-L stirred-tank bioreactor for cell biomass and exopolysaccharide production by Tremella fuciformis spore. Bioresource Technol. 2011;102(19):9175–8. doi: 10.1016/j.biortech.2011.06.086. [DOI] [PubMed] [Google Scholar]
- 123.Fonseca FL, Frases S, Casadevall A, Gompertz OF, Nimrichter L, Rodrigues ML. Structural and functional properties of the Trichosporon asahii glucuronoxylomannan. Fungal Genet Biol. 2009;46(6–7):496–505. doi: 10.1016/j.fgb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maribel CM, Humberto HS, Gustavo FGL, et al. Production and partial characterization of an exopolysaccharide from Ustilago maydis in submerged culture. African J Biotechnol. 2012;11:7079–87. [Google Scholar]
- 125.Cho EJ, Oh JY, Chang HY, Yun JW. Production of exopolysaccharides by submerged mycelial culture of a mushroom Tremella fuciformis. J Biotechnol. 2006;127(1):129–40. doi: 10.1016/j.jbiotec.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 126.Seviour RJ, Hensgen K. Exocellular glucan production by Acremonium diospyri. FEMS Microbiol Lett. 1983;16(2–3):343–7. [Google Scholar]