Abstract
Adding proton pump inhibitors (PPIs) to endoscopic therapy has become the mainstay of treatment for peptic ulcer bleeding, with current consensus guidelines recommending high-dose intravenous (IV) PPI therapy (IV bolus followed by continuous therapy). However, whether or not high-dose PPI therapy is more effective than low-dose PPI therapy is still debated. Furthermore, maintaining pH ≥ 4 appears to prevent mucosal bleeding in patients with acute stress ulcers; thus, stress ulcer prophylaxis with acid-suppressing therapy has been increasingly recommended in intensive care units (ICUs). This review evaluates the evidence for the efficacy of IV pantoprazole, a PPI, in preventing ulcer rebleeding after endoscopic hemostasis, and in controlling gastric pH and protecting against upper gastrointestinal (GI) bleeding in high-risk ICU patients. The review concludes that IV pantoprazole provides an effective option in the treatment of upper GI bleeding, the prevention of rebleeding, and for the prophylaxis of acute bleeding stress ulcers.
Keywords: pantoprazole, peptic ulcer bleeding (PUB), pharmacological treatment, proton pump inhibitor (PPI)
Introduction
Peptic ulcer bleeding (PUB) is the most common cause of acute hemorrhage in the upper gastrointestinal (GI) tract. It affects 48–160 people per 100,000 adults every year.1–3 Significant blood loss resulting from impaired blood clotting in the acidic environment of the GI tract may cause death in up to 14% of patients with an acute bleed, with the risk of death increasing 3-fold in patients experiencing rebleeding.1,4–7
Endoscopic therapy significantly reduces rebleeding rates, the need for surgery, and mortality in patients with PUB,8 and guidelines recommend endoscopic therapy within 24 hours in most patients with upper GI bleeding.9 However, despite the high hemostatic rate achieved, rebleeding, with an increased risk of death, can occur in approximately 10%–30% of patients, usually within 3 days of treatment.10–13 Therefore, further medical therapy may be required to support and maintain hemostasis. Data suggest that intravenous (IV) proton pump inhibitors (PPIs) decrease the recurrence of ulcer rebleeding and the need for surgery in patients with PUB;14–16 however, their effect on mortality is less clear.14,15,17–21 Nonetheless, in current practice, the addition of acid suppression medication to endoscopic therapy is the mainstay of treatment for PUB.
GI lesions are also common in critically ill patients, with an incidence close to 100% in patients with serious trauma. GI bleeding from stress ulcers, along with an increased risk of death, occurs in approximately 20% of these patients who have not received prophylactic treatment.22 Therefore, stress ulcer prophylaxis with acid-suppressing therapy has been increasingly recommended in intensive care units (ICUs) to prevent further damage in critical patients, reduce time spent in the ICU, and limit treatment costs.
Pantoprazole, a substituted benzimidazole derivative, is a PPI that decreases acid secretion from gastric parietal cells.23 IV pantoprazole is currently available in more than 80 countries worldwide, where it is indicated for the management of gastroesophageal reflux disease (GERD), gastric and duodenal ulcers, and Zollinger—Ellison syndrome. IV pantoprazole has more recently been indicated for the treatment of bleeding peptic ulcer and the prevention of rebleeding, along with the prophylaxis for acute bleeding stress ulcers, in several countries. In this review, the evidence for the use of IV pantoprazole in the treatment of PUB, prevention of rebleeding, and prophylaxis for acute bleeding stress ulcers, is critically evaluated.
Rationale for the use of PPIs in PUB
A platelet clot will maintain hemostasis for several hours but will disintegrate unless reinforced by a fibrin clot. In vitro studies demonstrate that a neutral pH is required for optimal platelet aggregation. However, in a slightly acidic environment, platelet aggregation is impaired, and at pH < 6, it is virtually abolished.24–28 Although platelet aggregation is important in the initial steps of hemostasis, it may only play a small role. Additionally, coagulation processes appear to be largely responsible for the arrest of gastric hemorrhage.29 In an acidic environment, pepsinogen is activated to pepsin, which can easily digest freshly formed blood clots within minutes.25 Furthermore, plasmin-mediated fibrinolysis is also increased, which may impair reinforcement of the initial platelet clot by a fibrin clot.28 Thus, profound acid suppression may provide therapeutic benefit for patients with bleeding peptic ulcers.25,27–33
Pharmacotherapy can be added to the endoscopic procedure to alter the gastric pH to ≥6 at the site of bleeding, thereby stabilizing the clotting process.24,34 To this end, histamine-2 receptor antagonists (H2RAs), somatostatin, and octreotide, all of which regulate acid secretion by receptor blockage, have been evaluated for the treatment of PUB. However, there is no convincing evidence that any of these drugs are beneficial for this indication.35 These drugs are therefore not recommended in guidelines for non-variceal upper GI bleeding.9
In contrast, PPIs, which directly block the proton pump, have a higher antisecretory potential than H2RAs. Importantly, PPIs have been shown to maintain intragastric pH above 6 for 84%–99% of a 24-hour period.34 Several meta-analyses have demonstrated that IV PPIs (usually administered as a bolus plus continuous infusion for 72 hours) are effective in decreasing the recurrence of ulcer rebleeding and the need for surgery compared with placebo or H2RAs.14–16 The overall effect of PPIs on mortality, however, is contentious. Most meta-analyses report no evidence for an effect of PPIs on all-cause mortality compared with placebo or H2RAs.14,15,17–21 Nonetheless, recent data indicate that, compared with placebo or H2RAs, PPIs significantly reduce mortality in some patient subgroups, including Asian populations and patients with high-risk endoscopic findings.16 In addition, the association of IV PPIs with a reduction in rebleeding in all patients (odds ratio [OR]: 0.53, 95% confidence interval [CI]: 0.37–0.77) and lower mortality rates in patients with high-risk stigmata (OR: 0.18, 95% CI: 0.04–0.80) has been demonstrated in a real-life setting in an observational study of 1869 patients in the Canadian Registry of Patients with Upper Gastrointestinal Bleeding and Endoscopy (RUGBE).36 Reflecting these combined results, consensus guidelines recommend an IV bolus followed by continuous-infusion PPI therapy to decrease rebleeding and mortality in patients with high-risk stigmata who have undergone successful endoscopic therapy.9
Clinical Pharmacology
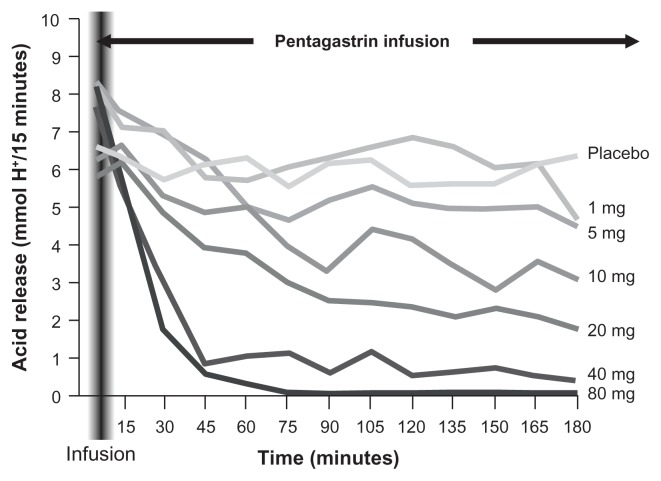
IV pantoprazole is equivalent to oral pantoprazole in the suppression of gastric acid output,37,38 providing fast and sustained acid suppression within 1 hour of a bolus dose. In healthy volunteers (n = 12), the IV formulation produced rapid dose-dependent acid inhibition in pentagastrin-stimulated acid secretion, with the 40 mg and 80 mg doses inhibiting acid secretion within 1 hour of administration (Fig. 1).39 Similar rapid (ie, 1-hour) control of acid output was also reported with higher doses of IV pantoprazole (160–240 mg/day, given as divided doses by 15-minute infusion) in 21 patients with Zollinger—Ellison syndrome.40 Control was maintained for up to 7 days in all patients.
Figure 1.
Dose-dependent acid inhibition with pantoprazole.39
In several studies, optimal pH control was achieved with an 80 mg bolus dose of pantoprazole plus an 8 mg/hour infusion. This dosing regimen resulted in an intragastric pH of 7 within 20 minutes of administration in 8 healthy volunteers.41 In this study, pH was maintained above 6 for approximately 84% of the 24-hour period (Table 1). Similar levels of acid suppression were reported in an open-label trial in 20 patients with bleeding peptic ulcer. These patients received an 80 mg IV bolus dose of pantoprazole followed by a continuous infusion of 8 mg/hour for 3 days, as well as a 40 mg bolus dose every 12 hours for 4–7 days following successful endoscopic hemostasis.42 For the first 24 hours, 85% of patients (17 cases) had a median pH of 6.1. In addition, pantoprazole administered at this dose resulted in lower interindividual variability of intragastric pH and a greater median percentage of time during which pH was ≥6 than that observed for an initial 80-mg bolus injection of pantoprazole followed by a 6-mg/hour continuous infusion (64% vs. 47%).42 However, Choi and colleagues43 found no significant difference between high-dose pantoprazole (80 mg, 8 mg/hour) and low-dose pantoprazole (40 mg, 4 mg/hour) in the length of time intragastric pH was above 6 in 61 patients with bleeding ulcers in Korea.
Table 1.
Percentage of time for intragastric pH levels using pantoprazole 80 mg bolus followed by IV infusion at the rate of 8 mg/hour.41
| pH level | ||||
|---|---|---|---|---|
|
|
||||
| >3 | >4 | >5 | >6 | |
| Time (%) | 99 | 99 | 94 | 84 |
In a comparative study, single-dose IV pantoprazole produced longer-lasting acid suppression of pentagastrin-induced gastric acid hypersecretion than single-dose famotidine (P < 0.02) in healthy volunteers.44 In addition, a continuous IV infusion of pantoprazole was equally effective to somatostatin in achieving acid suppression, as assessed by the amount of time pH was >6 (81.5% vs. 82.9%), in 60 patients with PUB.45
Pharmacokinetics
The pharmacokinetics of pantoprazole have been reviewed extensively elsewhere,23 and are summarized in Table 2. In short, pantoprazole is rapidly absorbed and achieves a maximum plasma concentration (Cmax) 2–3 hours after a single dose (Table 2).46 The drug is subject to low first-pass hepatic metabolism, reflected in a bioavailability of 77%.47,48 This is not affected by the ingestion of food.47 The pharmacokinetics of pantoprazole are linear after both oral and IV administration, with area under the curve (AUC) and Cmax increasing in proportion to IV doses up to 240 mg.49
Table 2.
Summary of the pharmacokinetic profile of pantoprazole.
| Parameter | Findings |
|---|---|
| Absorption | |
| Distribution | |
| Metabolism | |
| Elimination |
Abbreviations: AUC, area under the curve; Cmax, maximum plasma concentration; tmax, time to Cmax; t1/2, elimination half-life.
Unlike other PPIs, pantoprazole does not accumulate in the body after repeat administration. In healthy volunteers, pharmacokinetic parameters following IV pantoprazole 30 mg once daily for 5 days were similar to those after a single IV 30 mg dose (after multiple doses, AUC was 5.35 mg · h/L, Cmax was 5.26 mg/L, and elimination half-life [t1/2] was 1.11 hours).46 Despite rapid elimination (Table 2), pantoprazole’s long duration of acid inhibition may be a result of its strong and prolonged binding to the proton pump.50,51 Pantoprazole is eliminated primarily by hepatic metabolism via cytochrome P450 (CYP450) isoenzymes CYP2C1952 and CYP3A4.53,54 Metabolism is independent of the route of administration.
Pantoprazole has a low potential for drug–drug interactions mediated by CYP450, with multiple studies in healthy volunteers showing no clinically significant interactions with a range of other drugs.55 The antiplatelet agent clopidogrel is metabolized into the active form by the liver enzyme CYP2C19, which also metabolizes PPIs. Recent studies have not shown any effect of pantoprazole on the pharmacological activity of clopidogrel; this contrasts to results for omeprazole and esomeprazole, which have resulted in a respective label in the Plavix® SmPC to “avoid concomitant use of Plavix with omeprazole or esomeprazole because both significantly reduce the antiplatelet activity of Plavix”.56–58
In addition, there were no clinically significant changes in the pharmacokinetics of pantoprazole in elderly individuals or in patients with severe renal impairment, allowing administration of pantoprazole without dose adjustment in these patients.46 However, the metabolism of pantoprazole is impaired in patients with severe hepatic cirrhosis.59 AUC and t1/2 values were increased in patients with hepatic impairment (Child class A or B) compared with values in healthy volunteers after oral or IV pantoprazole, although volume of distribution and bioavailability remained constant.46 Thus, in Europe, but not the USA, dose adjustments are recommended in those with hepatic cirrhosis; however, dose adjustments are not required in patients with mild or moderate hepatic impairment.23
Pantoprazole in the Treatment of Bleeding Peptic Ulcer and the Prevention of Rebleeding
IV pantoprazole has been shown to be significantly more effective than H2RAs (ranitidine and famotidine) in preventing ulcer rebleeding after endoscopic hemostasis in prospective studies of varying designs (Table 3).60–63 In studies comparing pantoprazole with ranitidine, pantoprazole was administered in the following dosages: 40 mg IV initial bolus, plus 40 mg IV twice daily (bid) for 3 days, then 40 mg oral once daily (od);60 40 mg bolus following 3 × 40 mg during 72 hours;62 or 80 mg bolus followed by 8 mg/hour infusion.61 The respective ranitidine dosages were: 50 mg IV initial bolus, plus 50 mg IV three times daily (tid) for 3 days, then 150 mg oral bid;60 4 × 150 mg during 72 hours;62 and 50 mg bolus followed by 13 mg/hour infusion.61 Current guidelines advocate combined endoscopic hemostatic treatment modalities (eg, injection plus a mechanical intervention); this was not a requirement for entry into these studies, and should be considered when interpreting results.
Table 3.
Rebleeding rates for pantoprazole IV compared with ranitidine IV in patients with peptic ulcer bleeding.
| Reference | Rebleeding rates (% of patients) | P Value | |
|---|---|---|---|
|
|
|||
| Pantoprazole | Ranitidine | ||
| van Rensburg et al61 | |||
| Gastric ulcer | 6.7 | 14.3 | 0.006 |
| Arterial spurting | 13.9 | 33.9 | 0.01 |
| Hsu et al60 | 3.8 | 16.0 | 0.04 |
| Duvnjak et al62 | 3.2 | 12.9 | NR |
Abbreviation: NR, not reported.
Following endoscopic hemostasis in patients with gastroduodenal ulcer (n = 62–1244), rebleeding rates for patients receiving IV pantoprazole (3.2%–6.7%) were generally significantly lower than those for patients receiving IV ranitidine (12.9%–16.0%; P < 0.05; Table 3).60–62 In the largest of the three studies, IV pantoprazole (80 mg bolus followed by 8 mg/hour infusion; n = 618) significantly reduced rebleeding rates in patients with gastric ulcers but not duodenal ulcers compared with IV ranitidine (50 mg bolus followed by 13 mg/hour infusion; n = 626).61 The authors suggested that greater acid suppression might be needed in patients with gastric bleeding than in those with duodenal ulcer bleeding. Furthermore, there was a general overall low rate of rebleeding in this trial, which may be accounted for by the inclusion of patients with Forrest Ib ulcers (which may not exhibit high rates of bleeding as was once thought) and the inadvertent inclusion of patients with Forrest IIb and III lesions.61 The statistical power of the study may have been further reduced by the large number of centers and endoscopists involved in the trial, which may have impacted standardization of assessment. In addition, the ranitidine dose given was greater than that in other studies, and the rate of rebleeding in the ranitidine group was lower than previously reported (3.2% vs. 13–16%).60–62,64 Of significance, IV pantoprazole was more effective than IV ranitidine at reducing rebleeding in those with arterial spurting, ie, in those at high-risk for rebleeding (Table 3).61 In addition, Jensen and colleagues64 found no significant difference in 3-day or 30-day rebleeding rates with IV pantoprazole (80 mg plus 8 mg/hour for 72 hours) compared with IV ranitidine (50 mg plus 6.25 mg/hour for 72 hours; 3-day: 4.2% vs. 6.9%; 30-day: 6.9% vs. 14.3%), although the study was stopped prematurely and only 149 patients were enrolled. Therefore, these results are likely explained by the small sample size that was not powered to meet the primary endpoint. In another study in patients with acute esophageal variceal bleeding, the combination of sclerotherapy plus IV pantoprazole (3 × 40 mg; n = 35) was more effective than sclerotherapy plus IV ranitidine (3 × 50 mg; n = 35). Rebleeding occurred in 12% and 26% of patients, respectively, during the first 6 days, and patients receiving pantoprazole had fewer days in the ICU (4.8 vs. 8.5 days; P < 0.001), required fewer units of blood during transfusion and had lower mortality rates (9% vs. 21%) than patients receiving ranitidine.65
In a single prospective, randomized, single-blind study in 164 patients undergoing endoscopic submucosal dissection for gastric neoplasm, IV pantoprazole delayed bleeding compared with IV famotidine.63 Two hours before endoscopic mucosal dissection, patients received either IV pantoprazole 80 mg bolus followed by 8 mg/hour continuous infusion for the first 24 hours, 2 × 40 mg IV pantoprazole on the second day and then 40 mg oral pantoprazole for 8 weeks (n = 85) or IV famotidine 2 × 20 mg for 2 days, then 20 mg oral for 8 weeks (n = 79). Following treatment, bleeding rates were significantly lower in patients receiving pantoprazole than in those receiving famotidine (3.5% vs. 12.7%; P = 0.031).63
In a single study evaluating the efficacy of IV pantoprazole compared with IV omeprazole (both administered as an 80 mg bolus followed by continuous infusion 8 mg/hour for 3 days) in the prevention of rebleeding after successful endoscopic hemostasis of upper non-variceal bleeding in 164 patients, pantoprazole was significantly more effective than omeprazole.66 Rebleeding rates were significantly lower in patients treated with pantoprazole than in those receiving omeprazole (3.7% vs. 10.2%; P = 0.022). In addition, pantoprazole significantly reduced the need for blood transfusion (P < 0.001) and the length of hospital stay (P < 0.001) compared with omeprazole.
In patients with bleeding peptic ulcers, the use of high-dose pantoprazole (80 mg IV bolus followed by an infusion at a rate of 8 mg/hour for 72 hours) following successful endoscopic therapy was effective in reducing rebleeding, transfusion requirements, and hospital stay in a double-blind, placebo-controlled, prospective trial.67 Rebleeding rates were lower in the pantoprazole group than in the placebo group (7.8% vs. 19.8%; P = 0.01), and patients receiving pantoprazole required significantly fewer transfusions (1 ± 2.5 vs. 2 ± 3.3; P = 0.003), fewer days of hospitalization (5.6 ± 5.3 vs. 7.7 ± 7.3; P = 0.0003), and less frequent rescue therapy (19.8% vs. 7.8%; P = 0.01). Similar results were observed in a study in Asian patients, where high-dose IV pantoprazole (80 mg IV bolus followed by continuous infusion of 8 mg/hour for 3 days) resulted in fewer incidences of rebleeding (3.7% vs. 16.0%; P = 0.034), less frequent need for surgery (0% vs. 8.0%; P = 0.034), and shorter hospital stays (6.4 vs. 8.2 days; P = 0.040) than did no treatment.68
Data on levels of acid suppression suggest that high-dose IV pantoprazole will be more effective than low dose pantoprazole in the prevention of ulcer rebleeding. Furthermore, data from 72 patients with ulcer bleeding following endoscopic hemostasis demonstrated that high-dose pantoprazole (initial dose of 80 mg followed by continuous IV infusion of 8 mg/hour for 48 hours) resulted in higher endoscopic stabilization (86% vs. 61%; P = 0.012), lower rebleeding (13% vs. 38%; P < 0.001), shorter stay in the ICU (4.8 vs. 7.9 days; P < 0.001) and lower transfusion requirements (1.8 vs. 3.1 units; P < 0.001) than low-dose pantoprazole (initial dose of 80 mg followed with standard dose of 2 × 40 mg daily for 48 hours).69 However, several studies have shown that there are no significant differences in the efficacy between high-dose IV pantoprazole (80 mg IV bolus followed by continuous infusion of 8 mg/hour for 3 days) and low-dose pantoprazole (80 mg oral dose bid for 3 days or 80 mg IV bolus followed by 40 mg IV bolus every 12 hours for 3 days).68,70,71 Furthermore, a meta-analysis that included these trials, along with those for other PPIs, showed no significant differences between high-dose PPI therapy and non-high-dose PPI therapy in rates of rebleeding, surgical intervention, or mortality after endoscopic treatment in patients with PUB.72 These results may be accounted for, in part, by ethnic differences, as some studies, including that by Hung and colleagues,68 were conducted in Asian populations. It is possible that high-dose and low-dose PPIs have similar abilities to saturate parietal cells in Chinese populations who have been identified as having smaller parietal cell mass than European populations.72 In addition, the enrolment of patients with lower potential for rebleeding (eg, Forrest class IIc and III, or seriously ill patients) may have diluted the effect of high-dose PPI treatment. For example, the study by Bajaj and colleagues70 excluded patients unable to take oral medication, which might have excluded seriously ill patients.
Cost-Effectiveness of Pantoprazole Treatment for PUB and the Prevention of Rebleeding
Data suggest that initiation of IV pantoprazole is cost-effective in patients with a high risk of ulcer bleeding following urgent endoscopic therapy.73 IV pantoprazole (80 mg bolus followed by 8 mg/hour for 3 days) demonstrated higher effectiveness (a 17% decrease in rebleeding) at lower cost (CAN$67 less per hospitalized patient) than no treatment. In addition, data from the USA and Canada suggest that administering high-dose IV PPI for 3 days is cost-effective compared with not doing so in patients with bleeding ulcers after successful endoscopic hemostasis.74 The cost-effectiveness ratios for high-dose and non-high-dose IV PPIs were $9112 USD and $3293 CAD versus $11,819 USD and $4284 CAD in this study.
Pantoprazole in the Prophylaxis of Acute Stress Ulcer Bleeding in Critical Care Patients
Evidence suggests that maintaining pH ≥ 4 is sufficient to prevent mucosal bleeding in patients with acute stress ulcers. In a pilot study, intermittent pantoprazole IV effectively controlled gastric pH and protected against upper GI bleeding in high-risk ICU patients without the development of tolerance.75 IV pantoprazole (40 mg od or bid and 80 mg od, bid, or tid) was more effective than IV cimetidine (300 mg bolus then 50 mg/hour) in maintaining pH > 4 in 200 enterally fed patients; pH was maintained above 4 for 89% versus 49% of the time, respectively. From day 1 to day 2 the amount of time pH was ≥4 increased for all doses of pantoprazole, but decreased for cimetidine because of the rapid development of tolerance.
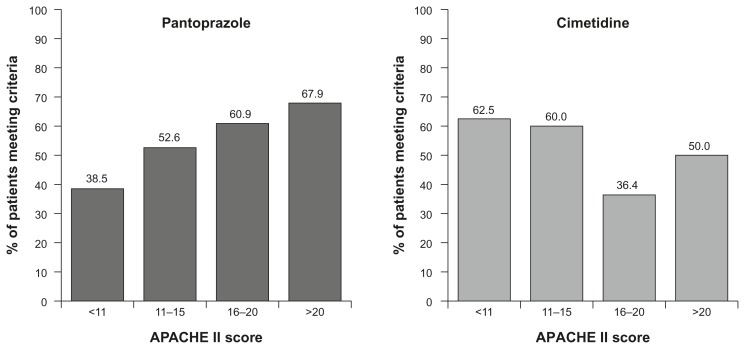
IV pantoprazole may be particularly useful in raising gastric pH in more seriously ill ICU patients.76 In this study, as the baseline Acute Physiology and Chronic Health Evaluation II (APACHE II) score increased, there was an increase in the percentage of patients with pH > 4 in more than 80% of aspirates with pantoprazole (40 mg od or bid, 80 mg od, bid, or tid; Fig. 2); the opposite was seen for cimetidine (300 mg bolus + 50 mg/hour).76
Figure 2.
Patients with pH > 4 80% of the time following administration on pantoprazole IV or cimetidine IV.
Finally, in a prospective, single-blind, randomized trial in 35 critically ill patients on mechanical ventilation, there were no significant differences in control of gastric pH > 4, GI bleeding, or mortality among IV pantoprazole 40 mg od, IV ranitidine 50 mg every 8 hours, and esomeprazole via nasogastric tube 40 mg od.77 The authors concluded that all three medications were effective at controlling gastric pH in the management of stress ulceration prophylaxis in critically ill patients.
Safety and Drug Interactions
Pantoprazole IV is well-tolerated even at high daily doses (272 mg/day).78 The most frequently reported adverse events are headache (0.7%) and diarrhea (0.3%), with thrombophlebitis at the injection site and other GI disturbances being reported less frequently.42,78 There were no clinically relevant changes in laboratory parameters with pantoprazole treatment in clinical trials.78
Extensive data show that there are no metabolic drug interactions when pantoprazole is used in combination with several other medications (eg, antacids, phenazone [antipyrine], caffeine, carbamazepine, cinacalcet, clarithromycin, ciclosporin, diazepam, diclofenac, β-acetyldigoxin, ethanol, glibenclamide, levothyroxine sodium, metoprolol, naproxen, sustained-release nifedipine, oral contraceptives, phenprocoumon, phenytoin, piroxicam, tacrolimus, theophylline, or warfarin).55 Pantoprazole’s low potential for these CYP-mediated drug–drug interactions may be of particular importance in the management of patients receiving multiple drugs, such as those with a stress ulcer.55
Dosing
The recommended dose of IV pantoprazole is 40 mg once daily by IV injection for the treatment of GERD or gastric or duodenal ulcer in patients for whom oral administration is not possible.79 Current consensus guidelines recommend high-dose IV PPI therapy in patients with PUB who have undergone successful endoscopic therapy, stating that an IV bolus followed by continuous infusion PPI therapy is effective at decreasing rebleeding in these patients.80 Data presented in the current review suggest that pantoprazole given as an IV 80 mg bolus dose followed by an 8 mg/hour infusion for 72 hours is effective at controlling intragastric pH and preventing rebleeding following endoscopic therapy, although whether this is more effective than lower doses remains under debate, and issues of study design and sample size must be considered. Initial treatment should be followed by oral pantoprazole 40 mg once a day for 4–8 weeks, depending on the clinical case.
Pantoprazole 40 mg or 80 mg once or twice a day is likely to be appropriate for the prophylaxis of acute stress ulcer bleeding in critical care patients.
Conclusion
The efficacy of IV pantoprazole in the treatment of bleeding peptic ulcers and the prevention of rebleeding has been shown in several active-comparator and placebo-controlled studies. IV pantoprazole also appears to be effective as a prophylaxis of acute stress ulcer bleeding in critical care patients. In those patients who will require a relatively high IV dose and who may be using other concomitant medications, pantoprazole has the benefit of a low potential to interact with the hepatic CYP450 enzyme system in humans, thereby reducing the potential for drug–drug interactions.55 IV pantoprazole is well tolerated even at high daily doses,42,78 and there is no need for dose adjustment for patients who are elderly, those who have renal insufficiency, or patients with mild or moderate hepatic insufficiency. In conclusion, IV pantoprazole provides an effective option in the treatment of upper GI bleeding and the prevention of rebleeding, and for the prophylaxis of acute bleeding stress ulcers.
Footnotes
Authors Contributions
CJvR and SC contributed to the writing of the first draft of this manuscript, made critical revisions throughout manuscript development and approved the final version.
Competing Interests
SC received payment for writing or reviewing this manuscript from Nycomed. CJvR discloses no competing interests.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
Funding Sources
CJvR did not receive any funding for the development of this manuscript. SC was supported by funding from Nycomed GmbH, a Takeda Company.
References
- 1.Lassen A, Hallas J, Schaffalitzky de Muckadell OB. Complicated and uncomplicated peptic ulcers in a Danish county 1993–2002: a population-based cohort study. Am J Gastroenterol. 2006;101(5):945–53. doi: 10.1111/j.1572-0241.2006.00518.x. [DOI] [PubMed] [Google Scholar]
- 2.Lewis JD, Bilker WB, Brensinger C, Farrar JT, Strom BL. Hospitalization and mortality rates from peptic ulcer disease and GI bleeding in the 1990s: relationship to sales of nonsteroidal anti-inflammatory drugs and acid suppression medications. Am J Gastroenterol. 2002;97(10):2540–9. doi: 10.1111/j.1572-0241.2002.06037.x. [DOI] [PubMed] [Google Scholar]
- 3.Targownik LE, Nabalamba A. Trends in management and outcomes of acute nonvariceal upper gastrointestinal bleeding: 1993–2003. Clin Gastroenterol Hepatol. 2006;4(12):1459–66. doi: 10.1016/j.cgh.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Kang JY, Elders A, Majeed A, Maxwell JD, Bardhan KD. Recent trends in hospital admissions and mortality rates for peptic ulcer in Scotland 1982–2002. Aliment Pharmacol Ther. 2006;24(1):65–79. doi: 10.1111/j.1365-2036.2006.02960.x. [DOI] [PubMed] [Google Scholar]
- 5.Soplepmann J, Peetsalu A, Peetsalu M, Tein A, Juhola M. Peptic ulcer haemorrhage in Tartu County, Estonia: epidemiology and mortality risk factors. Scand J Gastroenterol. 1997;32(12):1195–200. doi: 10.3109/00365529709028146. [DOI] [PubMed] [Google Scholar]
- 6.Thomsen RW, Riis A, Christensen S, Nørgaard M, Sørensen HT. Diabetes and 30-day mortality from peptic ulcer bleeding and perforation: a Danish population-based cohort study. Diabetes Care. 2006;29(4):805–10. doi: 10.2337/diacare.29.04.06.dc05-1748. [DOI] [PubMed] [Google Scholar]
- 7.Mose H, Larsen M, Riis A, Johnsen SP, Thomsen RW, Sørensen HT. Thirty-day mortality after peptic ulcer bleeding in hospitalized patients receiving low-dose aspirin at time of admission. Am J Geriatr Pharmacother. 2006;4(3):244–50. doi: 10.1016/j.amjopharm.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Cook DJ, Guyatt GH, Salena BJ, Laine LA. Endoscopic therapy for acute nonvariceal upper gastrointestinal hemorrhage: a meta-analysis. Gastroenterology. 1992;102(1):139–48. doi: 10.1016/0016-5085(92)91793-4. [DOI] [PubMed] [Google Scholar]
- 9.Barkun AN, Bardou M, Kuipers EJ, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152(2):101–13. doi: 10.7326/0003-4819-152-2-201001190-00009. [DOI] [PubMed] [Google Scholar]
- 10.Lau JY, Sung JJ, Lee KK, et al. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med. 2000;343(5):310–6. doi: 10.1056/NEJM200008033430501. [DOI] [PubMed] [Google Scholar]
- 11.Kovacs TO, Jensen DM. The short-term medical management of non-variceal upper gastrointestinal bleeding. Drugs. 2008;68(15):2105–11. doi: 10.2165/00003495-200868150-00003. [DOI] [PubMed] [Google Scholar]
- 12.Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med. 2008;359(9):928–37. doi: 10.1056/NEJMra0706113. [DOI] [PubMed] [Google Scholar]
- 13.Elmunzer BJ, Young SD, Inadomi JM, Schoenfeld P, Laine L. Systematic review of the predictors of recurrent hemorrhage after endoscopic hemostatic therapy for bleeding peptic ulcers. Am J Gastroenterol. 2008;103(10):2625–32. doi: 10.1111/j.1572-0241.2008.02070.x. [DOI] [PubMed] [Google Scholar]
- 14.Leontiadis GI, Sharma VK, Howden CW. Systematic review and meta-analysis of proton pump inhibitor therapy in peptic ulcer bleeding. BMJ. 2005;330(7491):568. doi: 10.1136/bmj.38356.641134.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor therapy for peptic ulcer bleeding: Cochrane collaboration meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82(3):286–96. doi: 10.4065/82.3.286. [DOI] [PubMed] [Google Scholar]
- 16.Leontiadis GI, Sreedharan A, Dorward S, et al. Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding. Health Technol Assess. 2007;11(51):iii–iv. 1–164. doi: 10.3310/hta11510. [DOI] [PubMed] [Google Scholar]
- 17.Leontiadis GI, McIntyre L, Sharma VK, Howden CW. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev. 2004;1(3):CD002094. doi: 10.1002/14651858.CD002094.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Khuroo MS, Farahat KL, Kagevi IE. Treatment with proton pump inhibitors in acute non-variceal upper gastrointestinal bleeding: a meta-analysis. J Gastroenterol Hepatol. 2005;20(1):11–25. doi: 10.1111/j.1440-1746.2004.03441.x. [DOI] [PubMed] [Google Scholar]
- 19.Bardou M, Toubouti Y, Benhaberou-Brun D, Rahme E, Barkun AN. Meta-analysis: proton-pump inhibition in high-risk patients with acute peptic ulcer bleeding. Aliment Pharmacol Ther. 2005;21(6):677–86. doi: 10.1111/j.1365-2036.2005.02391.x. [DOI] [PubMed] [Google Scholar]
- 20.Andriulli A, Annese V, Caruso N, et al. Proton-pump inhibitors and outcome of endoscopic hemostasis in bleeding peptic ulcers: a series of meta-analyses. Am J Gastroenterol. 2005;100(1):207–19. doi: 10.1111/j.1572-0241.2005.40636.x. [DOI] [PubMed] [Google Scholar]
- 21.Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev. 2006;1:CD002094. doi: 10.1002/14651858.CD002094.pub3. [DOI] [PubMed] [Google Scholar]
- 22.Metz DC. Potential uses of intravenous proton pump inhibitors to control gastric acid secretion. Digestion. 2000;62(2–3):73–81. doi: 10.1159/000007798. [DOI] [PubMed] [Google Scholar]
- 23.Cheer SM, Prakash A, Faulds D, Lamb HM. Pantoprazole: an update of its pharmacological properties and therapeutic use in the management of acid-related disorders. Drugs. 2003;63(1):101–33. doi: 10.2165/00003495-200363010-00006. [DOI] [PubMed] [Google Scholar]
- 24.Green FW, Jr, Kaplan MM, Curtis LE, Levine PH. Effect of acid and pepsin on blood coagulation and platelet aggregation. A possible contributor prolonged gastroduodenal mucosal hemorrhage. Gastroenterology. 1978;74(1):38–43. [PubMed] [Google Scholar]
- 25.Berstad A. Does profound acid inhibition improve haemostasis in peptic ulcer bleeding? Scand J Gastroenterol. 1997;32(4):396–8. doi: 10.3109/00365529709007691. [DOI] [PubMed] [Google Scholar]
- 26.Berstad A. Antacids and pepsin. Scand J Gastroenterol Suppl. 1982;75:13–5. [PubMed] [Google Scholar]
- 27.Berstad A. Antacids, pepsin inhibitors, and gastric cooling in the management of massive upper gastrointestinal haemorrhage. Scand J Gastroenterol Suppl. 1987;137:33–8. doi: 10.3109/00365528709089759. [DOI] [PubMed] [Google Scholar]
- 28.Patchett SE, Enright H, Afdhal N, O’Connell W, O’Donoghue DP. Clot lysis by gastric juice: an in vitro study. Gut. 1989;30(12):1704–7. doi: 10.1136/gut.30.12.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whittle BJ, Kauffman GL, Jr, Moncada S. Hemostatic mechanisms, independent of platelet aggregation, arrest gastric mucosal bleeding. Proc Natl Acad Sci U S A. 1986;83(15):5683–7. doi: 10.1073/pnas.83.15.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etherington DJ, Taylor WH. The pepsins from human gastric mucosal extracts. Biochem J. 1970;118(4):587–94. doi: 10.1042/bj1180587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor WH. Pepsins of patients with peptic ulcer. Nature. 1970;227(5253):76–7. doi: 10.1038/227076a0. [DOI] [PubMed] [Google Scholar]
- 32.Walker V, Taylor WH. Pepsin 1 secretion in chronic peptic ulceration. Gut. 1980;21(9):766–71. doi: 10.1136/gut.21.9.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pearson JP, Ward R, Allen A, Roberts NB, Taylor WH. Mucus degradation by pepsin: comparison of mucolytic activity of human pepsin 1 and pepsin 3: implications in peptic ulceration. Gut. 1986;27(3):243–8. doi: 10.1136/gut.27.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin HJ, Lo WC, Lee FY, Perng CL, Tseng GY. A prospective randomized comparative trial showing that omeprazole prevents rebleeding in patients with bleeding peptic ulcer after successful endoscopic therapy. Arch Intern Med. 1998;158(1):54–8. doi: 10.1001/archinte.158.1.54. [DOI] [PubMed] [Google Scholar]
- 35.Leontiadis GI, Howden CW. Pharmacologic treatment of peptic ulcer bleeding. Curr Treat Options Gastroenterol. 2007;10(2):134–42. doi: 10.1007/s11938-007-0065-4. [DOI] [PubMed] [Google Scholar]
- 36.Barkun A, Sabbah S, Enns R, et al. The Canadian Registry on Nonvariceal Upper Gastrointestinal Bleeding and Endoscopy (RUGBE): Endoscopic hemostasis and proton pump inhibition are associated with improved outcomes in a real-life setting. Am J Gastroenterol. 2004;99(7):1238–46. doi: 10.1111/j.1572-0241.2004.30272.x. [DOI] [PubMed] [Google Scholar]
- 37.Hartmann M, Ehrlich A, Fuder H, et al. Equipotent inhibition of gastric acid secretion by equal doses of oral or intravenous pantoprazole. Aliment Pharmacol Ther. 1998;12(10):1027–32. doi: 10.1046/j.1365-2036.1998.00406.x. [DOI] [PubMed] [Google Scholar]
- 38.Metz DC, Forsmark C, Lew EA, et al. Replacement of oral proton pump inhibitors with intravenous pantoprazole to effectively control gastric acid hypersecretion in patients with Zollinger-Ellison syndrome. Am J Gastroenterol. 2001;96(12):3274–80. doi: 10.1111/j.1572-0241.2001.05325.x. [DOI] [PubMed] [Google Scholar]
- 39.Simon B, Müller P, Bliesath H, et al. Single intravenous administration of the H+, K(+)-ATPase inhibitor BY 1023/SK&F 96022—inhibition of pentagastrin-stimulated gastric acid secretion and pharmacokinetics in man. Aliment Pharmacol Ther. 1990;4(3):239–45. doi: 10.1111/j.1365-2036.1990.tb00468.x. [DOI] [PubMed] [Google Scholar]
- 40.Lew EA, Pisegna JR, Starr JA, et al. Intravenous pantoprazole rapidly controls gastric acid hypersecretion in patients with Zollinger-Ellison syndrome. Gastroenterology. 2000;118(4):696–704. doi: 10.1016/s0016-5085(00)70139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunner G, Luna P, Hartmann M, Wurst W. Optimizing the intragastric pH as a supportive therapy in upper GI bleeding. Yale J Biol Med. 1996;69(3):225–31. [PMC free article] [PubMed] [Google Scholar]
- 42.van Rensburg CJ, Hartmann M, Thorpe A, et al. Intragastric pH during continuous infusion with pantoprazole in patients with bleeding peptic ulcer. Am J Gastroenterol. 2003;98(12):2635–41. doi: 10.1111/j.1572-0241.2003.08723.x. [DOI] [PubMed] [Google Scholar]
- 43.Choi KD, Kim N, Jang IJ, et al. Optimal dose of intravenous pantoprazole in patients with peptic ulcer bleeding requiring endoscopic hemostasis in Korea. J Gastroenterol Hepatol. 2009;24(10):1617–24. doi: 10.1111/j.1440-1746.2009.05939.x. [DOI] [PubMed] [Google Scholar]
- 44.Pisegna JR, Huang M, Asvar C, et al. Inhibition of pentagastrin-induced gastric acid hypersecretion by single-dose intravenous pantoprazole compared with single-dose intravenous famotidine and placebo in healthy subjects. Gastroenterology. 1998;114(Suppl 1):A259. [Google Scholar]
- 45.Tsibouris P, Tsianos G, Vassilopoulos A, Lapas C, Babanis A, Galeas T. High dose pantoprazole and somatostatin continuous iv infusion are equally effective to suppress intragastric pH after endoscopic haemostasis in patients with a peptic ulcer bleeding. Gut. 2005;54(Suppl VII):A-218. [Google Scholar]
- 46.Huber R, Hartmann M, Bliesath H, Lühmann R, Steinijans VW, Zech K. Pharmacokinetics of pantoprazole in man. Int J Clin Pharmacol Ther. 1996;34(5):185–94. [PubMed] [Google Scholar]
- 47.Radhofer-Welte S. Pharmacokinetics and metabolism of the proton pump inhibitor pantoprazole in man. Drugs Today (Barc) 1999;35(10):765–72. doi: 10.1358/dot.1999.35.10.561695. [DOI] [PubMed] [Google Scholar]
- 48.Parsons ME. Pantoprazole, a new proton-pump inhibitor, has a precise and predictable profile of activity. Eur J Gastroenterol Hepatol. 1996;8(Suppl 1):S15–20. doi: 10.1097/00042737-199610001-00004. [DOI] [PubMed] [Google Scholar]
- 49.Bliesath H, Huber R, Hartmann M, Luhmann R, Wurst W. Dose linearity of the pharmacokinetics of the new H+/K(+)-ATPase inhibitor pantoprazole after single intravenous administration. Int J Clin Pharmacol Ther. 1996;34(Suppl 1):S18–24. [PubMed] [Google Scholar]
- 50.Shin JM, Sachs G. Restoration of acid secretion following treatment with proton pump inhibitors. Gastroenterology. 2002;123(5):1588–97. doi: 10.1053/gast.2002.36593. [DOI] [PubMed] [Google Scholar]
- 51.Shin JM, Vagin O, Munson K, Kidd M, Modlin IM, Sachs G. Molecular mechanisms in therapy of acid-related diseases. Cell Mol Life Sci. 2008;65(2):264–81. doi: 10.1007/s00018-007-7249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka M, Ohkubo T, Otani K, et al. Metabolic disposition of pantoprazole, a proton pump inhibitor, in relation to S-mephenytoin 4′-hydroxylation phenotype and genotype. Clin Pharmacol Ther. 1997;62(6):619–28. doi: 10.1016/S0009-9236(97)90081-3. [DOI] [PubMed] [Google Scholar]
- 53.Meyer UA. Metabolic interactions of the proton-pump inhibitors lansoprazole, omeprazole and pantoprazole with other drugs. Eur J Gastroenterol Hepatol. 1996;8(Suppl 1):S21–5. doi: 10.1097/00042737-199610001-00005. [DOI] [PubMed] [Google Scholar]
- 54.Andersson T. Pharmacokinetics, metabolism and interactions of acid pump inhibitors. Focus on omeprazole, lansoprazole and pantoprazole. Clin Pharmacokinet. 1996;31(1):9–28. doi: 10.2165/00003088-199631010-00002. [DOI] [PubMed] [Google Scholar]
- 55.Blume H, Donath F, Warnke A, Schug BS. Pharmacokinetic drug interaction profiles of proton pump inhibitors. Drug Saf. 2006;29(9):769–84. doi: 10.2165/00002018-200629090-00002. [DOI] [PubMed] [Google Scholar]
- 56.Angiolillo DJ, Gibson CM, Cheng S, et al. Differential effects of omeprazole and pantoprazole on the pharmacodynamics and pharmacokinetics of clopidogrel in healthy subjects: randomized, placebo-controlled, crossover comparison studies. Clin Pharmacol Ther. 2011;89(1):65–74. doi: 10.1038/clpt.2010.219. [DOI] [PubMed] [Google Scholar]
- 57.Ferreiro JL, Ueno M, Tomasello SD, et al. Pharmacodynamic evaluation of pantoprazole therapy on clopidogrel effects: results of a prospective, randomized, crossover study. Circ Cardiovasc Interv. 2011;4(3):273–9. doi: 10.1161/CIRCINTERVENTIONS.110.960997. [DOI] [PubMed] [Google Scholar]
- 58.BMS. Plavix prescribing information. 2012. [Accessed Apr 23, 2012]. Available at http://products.sanofi.us/plavix/plavix.html.
- 59.Fitton A, Wiseman L. Pantoprazole. A review of its pharmacological properties and therapeutic use in acid-related disorders. Drugs. 1996;51(3):460–82. doi: 10.2165/00003495-199651030-00012. [DOI] [PubMed] [Google Scholar]
- 60.Hsu PI, Lo GH, Lo CC, et al. Intravenous pantoprazole versus ranitidine for prevention of rebleeding after endoscopic hemostasis of bleeding peptic ulcers. World J Gastroenterol. 2004;10(24):3666–9. doi: 10.3748/wjg.v10.i24.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Rensburg C, Barkun AN, Racz I, et al. Clinical trial: intravenous pantoprazole vs. ranitidine for the prevention of peptic ulcer rebleeding: a multicentre, multinational, randomized trial. Aliment Pharmacol Ther. 2009;29(5):497–507. doi: 10.1111/j.1365-2036.2008.03904.x. [DOI] [PubMed] [Google Scholar]
- 62.Duvnjak M, Supanc V, Troskot B, et al. Comparison of intravenous pantoprazole with intravenous ranitidine in prevention of rebleeding from gastroduodenal ulcers. Gut. 2001;49(Suppl III):2379. [Google Scholar]
- 63.Jeong HK, Park CH, Jun CH, et al. A prospective randomized trial of either famotidine or pantoprazole for the prevention of bleeding after endoscopic submucosal dissection. J Korean Med Sci. 2007;22(6):1055–9. doi: 10.3346/jkms.2007.22.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jensen DM, Pace SC, Soffer E, Comer GM. Continuous infusion of pantoprazole versus ranitidine for prevention of ulcer rebleeding: a US multicenter randomized, double-blind study. Am J Gastroenterol. 2006;101(9):1991–9. doi: 10.1111/j.1572-0241.2006.00773.x. [DOI] [PubMed] [Google Scholar]
- 65.Naumovski-Mihalic S, Katicic M, Colic-Cvrlje V, et al. Intravenous proton-pump inhibitors versus H2 antagonists for treatment of acute esophageal variceal bleeding. Gut. 2003;52(Suppl IV):A98. [Google Scholar]
- 66.Chahin NJ, Meli M, Zaca F. Endoscopic injection plus continuous intravenous pantoprazole vs. endoscopic injection plus continuous intravenous omeprazole for the treatment of upper nonvariceal bleeding. Can J Gastroenterol. 2006;20(Suppl A):112. [Google Scholar]
- 67.Zargar SA, Javid G, Khan BA, et al. Pantoprazole infusion as adjuvant therapy to endoscopic treatment in patients with peptic ulcer bleeding: prospective randomized controlled trial. J Gastroenterol Hepatol. 2006;21(4):716–21. doi: 10.1111/j.1440-1746.2006.04292.x. [DOI] [PubMed] [Google Scholar]
- 68.Hung WK, Li VK, Chung CK, et al. Randomized trial comparing pantoprazole infusion, bolus and no treatment on gastric pH and recurrent bleeding in peptic ulcers. ANZ J Surg. 2007;77(8):677–81. doi: 10.1111/j.1445-2197.2007.04185.x. [DOI] [PubMed] [Google Scholar]
- 69.Naumovski-Mihalic S, Katicic M, Cavric G, et al. High dose proton pump inhibitors in the treatment of intensive setting patients with peptic ulcer bleeding: fact or fictions. Gut. 2010;59(Suppl III):A334. [Google Scholar]
- 70.Bajaj JS, Dua KS, Hanson K, Presberg K. Prospective, randomized trial comparing effect of oral versus intravenous pantoprazole on rebleeding after nonvariceal upper gastrointestinal bleeding: a pilot study. Dig Dis Sci. 2007;52(9):2190–4. doi: 10.1007/s10620-006-9282-2. [DOI] [PubMed] [Google Scholar]
- 71.Yüksel I, Ataseven H, Köklü S, et al. Intermittent versus continuous pantoprazole infusion in peptic ulcer bleeding: a prospective randomized study. Digestion. 2008;78(1):39–43. doi: 10.1159/000158227. [DOI] [PubMed] [Google Scholar]
- 72.Wang CH, Ma MH, Chou HC, et al. High-dose vs. non-high-dose proton pump inhibitors after endoscopic treatment in patients with bleeding peptic ulcer: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2010;170(9):751–8. doi: 10.1001/archinternmed.2010.100. [DOI] [PubMed] [Google Scholar]
- 73.Herba K, Kennedy W, Barkun A, Fallone C. Cost effectiveness of IV PPI’s in the treatment of non-variceal upper GI bleeding following urgent endoscopy. Gut. 2002;51(Suppl III):A105. [Google Scholar]
- 74.Barkun AN, Herba K, Adam V, Kennedy W, Fallone CA, Bardou M. High-dose intravenous proton pump inhibition following endoscopic therapy in the acute management of patients with bleeding peptic ulcers in the USA and Canada: a cost-effectiveness analysis. Aliment Pharmacol Ther. 2004;19(5):591–600. doi: 10.1046/j.1365-2036.2004.01808.x. [DOI] [PubMed] [Google Scholar]
- 75.Somberg L, Morris J, Jr, Fantus R, et al. Intermittent intravenous pantoprazole and continuous cimetidine infusion: effect on gastric pH control in critically ill patients at risk of developing stress-related mucosal disease. J Trauma. 2008;64(5):1202–10. doi: 10.1097/TA.0b013e31815e40b5. [DOI] [PubMed] [Google Scholar]
- 76.Wright M, Karlstadt RG. Intravenous pantoprazole (IVP) demonstrates a greater increase in gastric aspirates with a pH ≥ 4.0 than continuously infused cimetidine (C) in ICU (Intensive care unit) patients with high baseline APACHE II (APSII) Gastroenterology. 2003;124(4 Suppl 1):A830. [Google Scholar]
- 77.Kalaghchi B, Ghandi M, Hoque ME, et al. Randomized comparison of gastric pH control with ranitidine, esomeprazole and pantoprazole for stress ulcer prophylaxis in critically ill patients. Gastroenterology. 2004;126(4 Suppl 2):A445. [Google Scholar]
- 78.Trepanier EF. Intravenous pantoprazole: a new tool for acutely ill patients who require acid suppression. Can J Gastroenterol. 2000;(Suppl 14D):D11–20. doi: 10.1155/2000/608413. [DOI] [PubMed] [Google Scholar]
- 79.Nycomed. Summary of Product Characteristics. Protium 40 mg i.v. Powder for Solution for Injection. 2010. [Accessed Apr 23, 2012]. Available at http://www.medicines.org.uk/emc/medicine/13058.
- 80.Barkun A, Bardou M, Marshall JK. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003;139(10):843–57. doi: 10.7326/0003-4819-139-10-200311180-00012. [DOI] [PubMed] [Google Scholar]