Abstract
Bastadin-5, a brominated macro-dilactam from the marine sponge Ianthella basta, enhances release of Ca2+ from stores within the sarcoplasmic reticulum (SR) of muscle and non-muscle cells by modulating RyR1/FKBP12 complex. Analogs of bastadin-5 present desirable targets for SAR studies to shed light on the gating mechanism and locus of bastadin-5 binding on these heteromeric channels that mediate essential steps in early coupling of membrane excitation to Ca2+ signaling cascades. Simple, ring-constrained analogs of bastadin-5 were synthesized from substituted benzaldehydes in a convergent manner, featuring an efficient SNAr macroetherification, and evaluated in an assay that measures [3H]-ryanodine that is known to correlate with the functional open state of the Ca2+ channel. The simplified 14-membered ring, atropisomeric analog (±)-7, like bastadin-5, enhanced ryanodine binding to the RyR1/FKBP12 complex (EC50 11 μM), however, unexpectedly, the corresponding achiral 18-membered ring analog 14 potently inhibited binding (IC50 6 μM) under the same conditions. Structure-activity relationships of both families of cyclic analogs showed activity in a ryanodine binding assay that varied with substitutions of the Br atom on the trisubstituted aryl ring by various functional groups. The most active analogs were those that conserved the dibromocatechol ether moiety that corresponds to the ‘western edge’ of the bastadin-5 structure. These data suggest that cyclic analogs of bastadin-5 interact with the channel complex in a complex manner that can either enhance or inhibit channel activity.
Introduction
Natural products have played a major role in our current understanding of how FKBP12, an immunophilin, regulates both cellular signal processing and Ca2+ efflux within the junctional sarcoplasmic reticulum (JSR) of skeletal muscle tissue. Regulated Ca2+ release from the SR is critical for normal striated muscle contractility and dysfunctional Ca2+ channel conductance carries important consequences in disease states of skeletal and cardiac muscle. The immunophillins rapamycin (1) and FK506 (2) are two macrolide immunosuppressant compounds that bind to FKPB12 with high affinity and have been used to explore the RyR1/FKBP12 complex.1-8 Bastadins are known compounds possessing newly identified properties – allosteric interactions with the RyR1/FKBP12 complex.9-13
Bastadin-5 (3) is one member of a family of over 20 bromotyrosine-derived macrolactams that have been isolated from the Verongid marine sponges Ianthella basta (Pallas), I. flabeliformis, I. quadrangulata, I. sp. and Psammaplysilla purpurea.12,14-24 Our previous studies show that several, but not all, bastadins stimulate Ca2+ release from stores in the JSR by binding to the RyR1/FKBP12 channel in skeletal muscle. The most active member of the family is bastadin-5 (3, EC50= 2.2 μM), while a constitutional isomer of 3, bastadin-19 (3a), does not mobilize Ca2+ from the channel (EC50 >100 μM), but competes for the binding site of bastadin-5.13 Bastadin-5 also facilitates FK506-induced release of FKBP12 from RyR1, indicating the former may influence stability of the RyR1/FKBP12 complex.13 Although these effects are concentration-dependent and follow saturable sigmoidal binding isotherms, the locus of binding of bastadin-5 to the RyR1/FKBP12 complex is not yet known.
The structure of 3 is comprised of four brominated, modified tyrosines and tyramines arrayed within a 28-membered macrodilactam. Biosynthetically, each half of the 28-membered ring macrocycle that is common to most bastadins appears to derive from oxidative coupling of one unit each of bromotyramine and bromotyrosine. The two halves are united through two amide bonds to give a pseudo-tetrameric macrodilactam. Each of the 21 bastadins has an α-ketoxime that appears to derive from α-oxidation of the bromotyrosine. Oxidative modification may also occur at C5/C6 to introduce a vinyl bond (e.g. bastadin-4) or a C6 hydroxyl group (e.g. bastadin-7). The most active member of this family, bastadin-5 (3), stimulates release of Ca2+ from stores in the SR by binding to an as-yet unidentified site of the intact RyR1/FKBP12 complex in skeletal muscle tissues.11,13 The RyR1/FKBP12 complex is a ~2.2 MDalton heterotetrameric protein anchored within the junctional sarcoplasmic reticulum (JSR) and spans the 100 nm gap between the JSR and transverse tubule membranes. The RyR1 complex is therefore essential for orchestrating physiological Ca2+ release during excitation contraction coupling of skeletal muscle. Each monomeric polypeptide is comprised of ~5000 amino acid residues folded into several transmembrane domains. Competition studies have revealed that the binding site of 3 is distinct from sites on the channel surface that are recognized as other SR Ca2+ channel effectors, such as Ca2+, Mg2+, ATP, caffeine or the plant alkaloid ryanodine.13 Bastadins mediate their effects upon the Ca2+ channel without compromising the RyR1/FKBP12 association, unlike FK506 which promotes the dissociation of FKBP12 from RyR1. While the macrolide FK506 was useful in revealing the nature of interaction of the large tetrameric channel complex with FKBP12, the bastadins have provided information on the intact RyR1/FKBP12 Ca2+ channel complex. For example, addition of 3 to Ca2+ channel preparations revealed the function of the RyR1/FKBP12 complexes as a regulator in the filling capacity of the Ca2+ stores by influencing the “leak state” conformation of RyR1.10
Members of the bastadin family show a range of potency (EC50 2.3 to >100 μM) for binding to the RyR1/FKBP12 complex. Since the functional groups present in each bastadin are essentially the same, we hypothesize that this structural activity relationship (SAR) is attributable to different preferred solution conformers of bastadins as a result of differences in non-bonded interactions (e.g. Br-steric interactions) in the ‘western’ and ‘eastern’ parts of the bastadins. Consequently, the altered torsional angles and even the overall shape of the molecule, may affect the receptor binding energy. We proposed the synthesis of simple rationally designed cyclic analogs of 3 (e.g. 4-15), which embody the substituted catechol ethers of the ‘western’ hemisphere of 3 within two families of macrocycles bearing 14- and 18-membered ring sizes. Ring-constrained macrocycles with constrained aryl C-O-C-C torsional angles may reveal preferential ryanodine-binding agonism, which in turn may shed light on the minimum pharmacophore of 3. We report here the results of a study which shows the minimal analog 7 (EC50= 11 μM), which embodies the bromocatechol ether unit found in 3, has comparable activity to the natural product 3 in the ryanodine binding assay. Unexpectedly, compound 14, a synthetic 18-membered ring analog of 3, was a potent antagonist in the same binding assay (IC50= 6 μM) which suggests brominated catechol ethers exhibit a bi-modal activity that varies with non-bonded interactions. Interestingly, the ketoximino group found in the natural products appears not to be required for activity. Our studies suggest a complex interplay of stereoelectronic factors in binding of bastadin-5 analogs to the RyR1/FKBP12 receptor, which suggests a bi-modal model for Ca2+ gating, which may be tunable by a selection of properly designed ‘second generation’ analogs.
Synthesis
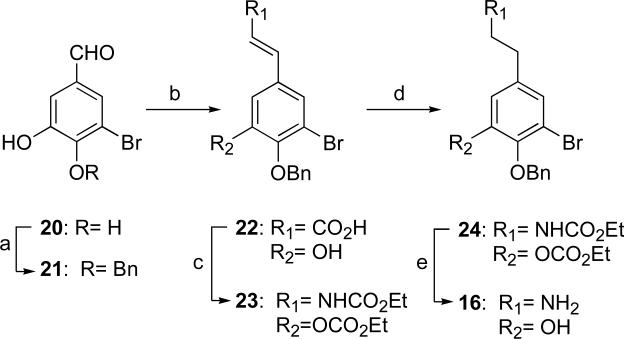
The retro-synthetic analysis shown in Scheme 1 reveals the design for preparation of cyclic analogs. We envisioned the 14-membered cyclic analogs, a smaller, ring-constrained macrocycle embodying a single amide bond, would derive from common intermediates 16 and 17. The larger 18-membered analogs would include an additional β-alanine as a spacer. The units would be assembled by amide bond formation and the pivotal macrocyclization would be accomplished by SNAr coupling according to Zhu and coworkers.25,26 The nitro group in compounds 18 and 19 would activate the leaving group and serve as a handle to introduce variable functionality at this position through diazonium salt displacements.
Scheme 1.
Retrosynthetic analysis for 14- and 18-membered ring analogs of bastadin-5.
Simple Dreiding molecular models predicted that the 14-membered analogs would be locked into fixed conformations as atropisomers due to severe torsional strain that arises from rotation about the long axis of the para-O-aryl linkage, while the 18-membered ring analogs should retain relative conformational mobility.
The synthesis of common intermediate 16 is illustrated in Scheme 2. Key considerations in the synthesis included management of the reductively-sensitive aryl Br groups and O-benzyl protecting groups. Synthesis of substituted phenethylamine 16 was carried out using methodology we had optimized earlier to satisfy these criteria.27
Scheme 2.
Synthesis of Substituted Phenethylamine 16
Key: (a) i. Li2CO3, DMF, 45 °C, 1h; ii. BnBr, 88% (b) i. malonic acid, pyridine, piperidine (cat.), toluene, reflux, 12 h; ii. 1 N HCl 86%; (c) ethyl-chloroformate, Hünig's base, acetone, 0 °C, 2 h; ii. NaN3, H2O, 0 °C; iii. EtOH, toluene, reflux, 12 h, 65%; (d) i. TFA, Et3SiH, -10 °C, 0.5 h; ii. NaHCO3 (aq), 94%; (e) hydrazine, KOH, 1,4-dioxane, reflux, 1h, 92%
The method is amenable to radio-labeling of analogs by insertion of tritium.28 Selective benzylation of the more acidic hydroxyl of 20 was performed through a modification of a published procedure.29 Pretreatment of catechol 20 with base (DMF, Li2CO3, 45 °C, 1 h) prior to the addition of benzyl bromide achieved selective benzylation of the para-OH group to provide 21 in good yield (88%). The position of benzylation was confirmed by nOe experiments. A Doebner-modified Knoevenagel reaction of 21 led to the requisite α,β-unsaturated acid 22 in 86% yield which was converted to enamide 23 by a Curtius rearrangement of the corresponding acyl azide (65% over 3 steps).30 Cationic hydrogenation of enamide 23 gave carbamate 24 (94%).27 Standard conditions for hydrolysis of methyl/ethyl carbamates (e.g. (KOH, H2O, EtOH, reflux) were ineffective when applied to 24 – either starting material or the corresponding oxazolidone, formed by cyclization/elimination of MeOH/EtOH, were returned. More forcing conditions (NH2NH2, KOH, 1,4-dioxane, 80 °C) gave phenethylamine 16 in good yield (92%).
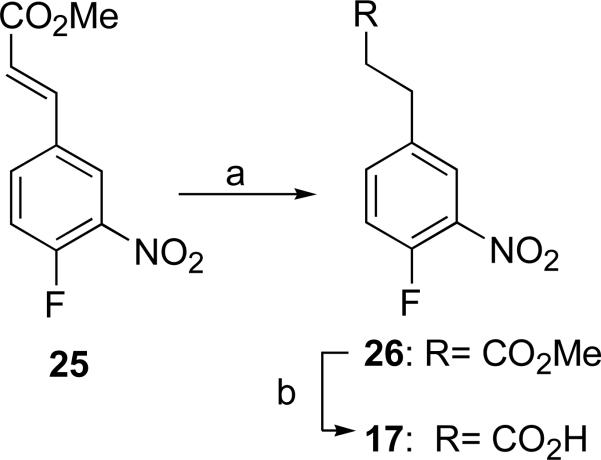
Hydrogenation of methyl 4-fluoro-3-nitrocinnamate (25) in the presence of Wilkinson's catalyst31 with careful control of H2 pressure and temperature gave methyl ester 26 in excellent yield (95%)32 without reduction of the nitro functionality. Saponification of ester 26 completed the synthesis of the dihydrocinnamic acid 17 (86%).
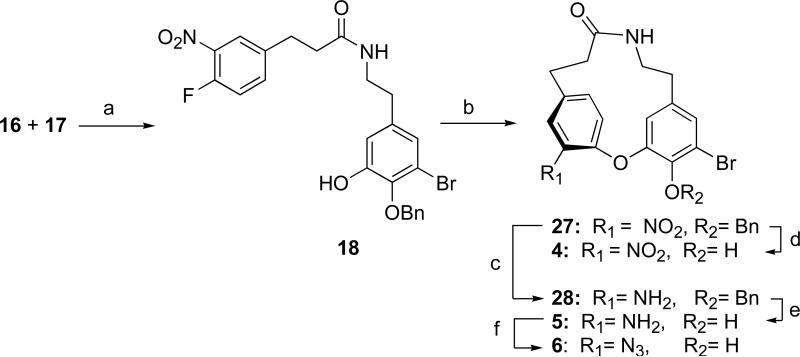
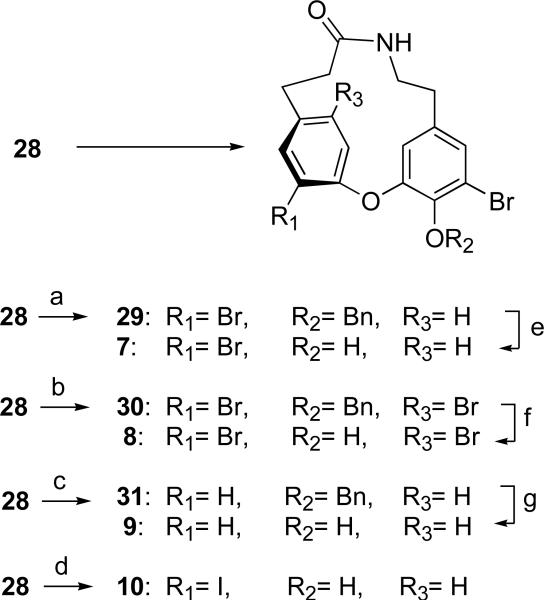
Two possible routes to the cyclic intermediates were considered; SNAr coupling of a suitable aryl fluoride with a phenol followed by macrolactamization, or the reverse sequence of reactions. In the event, the latter method proved to be the most efficient (Scheme 4) whereas the former resulted in uniformly poor yields of cyclic product (<10%). Amide coupling of intermediates 16 and 17 afforded the macroetherification precursor 18 in 72% yield. Macroetherification of 18 under dilute conditions (2mM, K2CO3, 4Å sieves, DMSO, RT) smoothly converted 18 to lactam 27 in high yield (85%). Removal of the benzyl group provided the 14-membered ring-constrained analog 4. Reduction of lactam 27 to the aniline derivative 28 under conditions that preserved the reductively sensitive aryl Br groups (CrCl2, DMF, room temperature, 73%) and set the stage for the synthesis of several analogs via diazonium salts (Scheme 5). Removal of the benzyl group from 28 gave aniline 5. The diazonium salt prepared from 5 was quenched with sodium azide to give the azido analog 6 in 66% yield.33 The dibromo or tribromide compounds 29 and 30 were procured in acceptable yields by Sandmeyer-type reaction of the corresponding diazonium salts prepared from 28 (tert-BuNO2, CH3CN) and use of carefully controlled amounts of CuBr2 (0.8 or 10 equiv; respectively). The use of sub-stoichiometric CuBr2 (0.8 equiv) resulted in formation of 29, however, excess CuBr2 (10 equiv) gave the brominated product 30.34 Compound 28 suffered unexpected reductive deamination when the corresponding diazonium salt was prepared with tert-BuNO2 in the presence of THF to give compound 31, presumably by hydride abstraction from the solvent.34b Quenching the latter diazonium salt potassium iodide resulted in aryl iodide 10. Removal of the benzyl protecting group in 29, 30, and 31, as before, yielded the corresponding phenolic 14-membered analogs 7, 8, and 9, respectively.
Scheme 4.
Synthesis of 14-membered analogs 4, 5 and 6 via macroetherification.
Key: (a) i. EDCI, HOBT, CH2Cl2, RT, 12 h; ii. 0.5 N HCl, 72%; (b) i. K2CO3, DMSO (2 mM), 4 Å sieves, 2 h; ii. 1N HCl, 85%; (c) i. CrCl2, DMF, RT, 12 h; ii. 1 N HCl, 73%; (d) BBr3, –78 °C, 1 h, 91%; (e) BBr3 –78 °C, 1h, 58%; (f) i. AcOH/H2O, 9:1, NaNO2, 0 °C; ii. NaN3, 0 °C, 66%
Scheme 5.
Preparation of C-aryl-substituted 14-membered ring analogs.
Key: (a) i. CuBr2 (0.8 equiv), tBuONO, CH3CN, 0 °C, 1h; ii. 27 in portions, 0 °C, 2h then warmed to rt; iii. 1N HCl, 66% (b) i. CuBr2 (10 eq), tBuONO, CH3CN, 0 °C, 1h; ii. 28 in portions, 0 °C, 2h then warmed to rt; iii. 1N HCl, 32%; (c) i. tBuONO, THF, 0 °C, 1h; ii. 28 in portions, 0 °C, 2h then warmed to rt; iii. 1N HCl, 39%; (d) i. H2SO4, AcOH, NaNO2, 0 °C, 0.5 h; ii. 28 in portions, 0 °C, 1 h then warmed to rt, 20 min; iii. KI, H2O, rt, 15 min then warmed to 70 °C, 15 min, 16%; (e) BBr3, –78 ° C, 1h, 91%; (f) TFA, rt, 24h, 70%; (g) TFA, rt, 24h, 75%.
Synthesis of the 18-membered ring analogs is illustrated in Scheme 6. Phenol 32 was protected as a triisopropylsilyl ether (95%) followed by removal of the N-Boc under standard conditions to provide amine 34 (97%), which was coupled, in turn, with N-Boc-β-alanine to afford amide 35 in 92% yield. Removal of the Boc group from 35 as before (97%) followed by coupling of the resultant free-amine 36 to acid 17 (EDCI, HOBt, CH2Cl2) provided the precursor for cyclization 19 in 92% yield. One-pot, tandem removal of the TIPS protecting group and macroetherification of 19 with CsF smoothly produced the 18-membered cyclic product 37 in excellent yield (84%). The nitro group in lactam 37 was reduced as before to provide the aniline 38 (58%) which was deprotected to provide phenolic amine 12 (54%). Diazotization, substitution and deprotection reactions were carried out using similar sequences to those described above to provide azido analog 13 (69%), dibromo analog 14 and tribromide 15.
Scheme 6.
Synthesis of 18-membered analogs by SNAr macroetherification
Key: (a) i. Boc2O, CH3CN, rt, 12 h; ii. Na2CO3, MeOH, H2O (4:1), rt, 76%; (b) TIPSCl, DMF, rt, 24 h, 95%; (c) i. TFA, CH2Cl2, (1:1), rt, 1h; ii. NaHCO3 (aq), 95%; (d) i. N-Boc-β-alanine, EDCI, HOBt, CH2Cl2, rt, 12 h; ii. 0.5 N HCl, 92%; (e) i. TFA, CH2Cl2, (1:1), rt, 1 h; ii. NaHCO3 (aq), 97%; (f) i. 17, EDCI, HOBt, CH2Cl2, rt, 12 h; ii. 0.5 N HCl; 93%; (g) CsF, DMSO (2mM), 4 Å sieves, rt, 20 h, 84%; (h) BBr3, CH2Cl2, rt, 8h, 82%; (i) CrCl2, DMF, rt, 12h, 55%; (j) BBr3, CH2Cl2, rt, 8h, 54%; (k) i. AcOH/H2O, 9:1, NaNO2 0 °C; ii. NaN3, 0 °C, 69%; (l) i. CuBr2 (0.8 equiv), tBuONO, CH3CN, 0 °C, 1h; ii. 40 in portions, 0 °C, 2h then warmed to rt; iii. 1N HCl, 25%; (m) BBr3, CH2Cl2, rt, 8h, 94%; (l) i. CuBr2 (3.0 equiv), tBuONO, CH3CN, 0 °C, 1h; ii. 40 in portions, 0 °C, 2h then warmed to rt; iii. 1N HCl, 29%; (m) BBr3, CH2Cl2, rt, 8h, 72%.
Structure and Biological Evaluation
For the purpose of discussion, the 14-membered analogs are numbered according to Figure 2. The simple cyclic analogs were designed to embody the ‘western’ portion of 3 but with different ring sizes and substitutions on ring B (C17). It was hypothesized that the ‘western’ portion of 3 composed the minimum pharmacophore, where variation of the torsional angle (C1-O2-C3-C4) would modulate activity. The highly constrained 14-membered analogs would contain fewer degrees of freedom and simultaneously constrain both the biphenyl ether angle (C1-O2-C3) and the torsional angle (C1-O2-C3-C4) compared to the larger 18-membered analogs. Varying the substitution on ring B may reveal electronic effects upon the binding of bastadin analogs to the RyR1/FKBP12 complex.
Figure 2.

Representative nOe's of 29
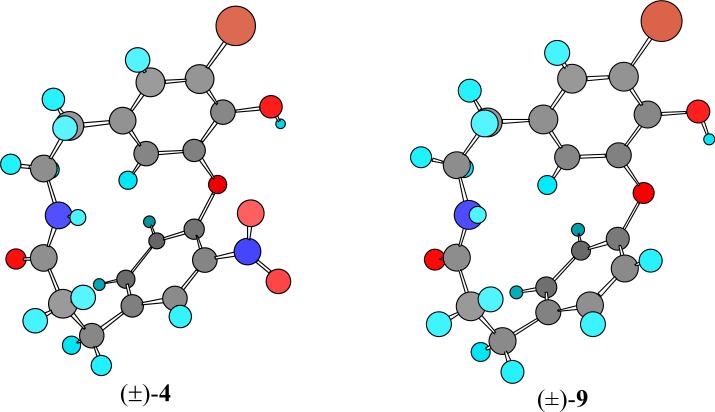
Solution and solid-state conformation studies of the analogs 4-15 were ascertained from NMR nOe and chemical shift analysis (Figure 2, Table 1), and X-Ray crystal structure analysis (Figure 3). With the exception of compound 9, the 14-membered cyclic analogs are chiral and exhibit atropisomerism due to restricted rotation. Consequently the latter compounds were synthesized as racemates. Evidence for atropisomerism is seen in the NMR data as illustrated by spectral interpretation of lactam 29 (the precursor to 7) as follows (Figure 2, Table 1). The 1H NMR spectrum of 29 showed a diagnostic two-proton signal with an ‘AB quartet’ J coupling pattern (δ 5.18, d, 1H, J=11 Hz; δ 5.34, d, 1H, J=11 Hz) corresponding to diastereotopic O-benzyloxy protons. Restricted rotation about the catechol ether linkage (C1-O2-C3) was also evident from diamagnetism due to ring current effects leading to an exceptionally high field H19 aryl proton signal (δ 5.06, 1H, J=2.0 Hz).
Table 1.
NMR data for 29 (400 MHz, CDCl3)
| # | 1H δ, m, J (Hz) | 13C (ppm) | COSY | HMBC | nOe |
|---|---|---|---|---|---|
| 1 | 152.9 | ||||
| 3 | 154.6 | ||||
| 4 | 136.4 | ||||
| 5 | 117.9 | ||||
| 6 | 6.88 dd (2.0, 1.2) | 125.8 | H19, H8 | C5, C7, C19, C8 | |
| 7 | 142.8 | ||||
| 8 | 2.64 m | 30.5 | H9 | H6, H9, H10; H19 | |
| 9 | 3.29 m | 39.7 | H8; H10 | H8, H10; H18 | |
| 10 | 4.90 m | H9 | H8, H9, H12, H19 | ||
| 11 | 171.1 | ||||
| 12 | 2.29 m | 41.0 | H13 | C11, C13, C14 | H10, H13, H15, H18 |
| 13 | 3.00 m | 31.7 | H12 | C11, C12, C14; C15, C18 | H12, H15; H18 |
| 14 | 140.6 | ||||
| 15 | 7.23 dd (8.4, 2.4) | 130.4 | H16; H18 | C1, C13, C15; C17 | H13, H16 |
| 16 | 7.05 d (8.4) | 126.0 | H15 | C1, C18 | H15, H19 |
| 17 | 118.7 | ||||
| 18 | 7.51 d (2.4) | 134.6 | H15 | C1, C13, C15; C17 | H12, H13 |
| 19 | 5.06 d (2.0) | 113.1 | H6 | C3, C7, C8, C6 | H9, H10; H16 |
| 20a | 5.34 d (10.8) | 75.2 | H20b | C4, C21; C (22- 26) | |
| 20b | 5.18 d (10.8) | 75.2 | H20a | C4, C21, C (22- 26) | |
| 21 | 137.0 | ||||
| 22-26 | 7.3- 7.4 m; 7.61- 7.64 m | 128.8, 128.4, 128.2 | H22-26 | C20, C22-C26) |
Figure 3.
X-Ray crystal structures of (±)-4 and 9
The constrained conformation of 29 places H19 in ring A within the shielding region that is close to a perpendicular extended from center of ring B. Complexity in 1H NMR signals of other CH2 signals in 29 was also consistent with diastereotopic methylene groups and, likewise, seen in the 1H NMR spectra of 7 and other products derived from 28, except 9. The debromo analog 9, where free rotation about the 1,4-axis of the disubstitued phenyl ring is allowed and the barrier to macrocyclic torsional inversion is relaxed, shows no atropisomerism.
A rigid, compact conformation of analog 29 was supported by nOe experiments (Figure 2, Table 1). Representative nOe's of 29 are consistent with a conformation where ring B lies close to parallel with a plane that bisects ring A (Figure 2). The solid-state conformations of 4 and 9 were determined by X-ray crystallography (Figure 3). The catechol ether torsional angles in 4 are 83.0° (C3-O2-C1-C17) and 152.4° (C1-O2-C3-C4). The bond angle (C1-O2-C3) of 110.7° deviated slightly from that expected from sp3 hybridization. Slight bending of the aromatic ring B from planarity (C15-C14-C18-C17, 9.5°) was also evident, compensating in part for the ring strain. Few differences between the solution state conformation and the solid conformation were ascertained; this is not unexpected due to the rigidity imposed by torsional strain within these compounds. Comparison of the X-ray crystal structures of analogs 4 and 9 revealed identical bond angles and distances of their respective carbon skeletons. As expected, the 18-membered counterparts 11-15 did not show atropisomerism due to the additional degrees of freedom conferred by the β-alanine linker.
The barrier to rotation in the 14-membered ring analog 29 was briefly examined by temperature dependent 1H NMR (d6-DMSO). At temperatures up to T=105 °C the 1H NMR lineshapes of the O-Bn signals in 29 remained sharp and revealed no tendency towards coalescence or broadening which suggests a barrier to rotation >17 kcal/mol.35
Biological Activity
Biological evaluation of the synthetic analogs 4-15, together with bastadin-5 (3) was carried out using the [3H]-ryanodine binding assay (Table 2). The assay provides a ‘readout’ of the open state of the Ca2+ channel by detection of [3H]-ryanodine bound to the protein which occurs only in the open state.13 In earlier studies, we showed that [3H]-ryanodine binding correlates with the functional properties of bastadin-5 (3), in particular, transport of Ca2+ across the channel and alteration of channel gating (open state probability).13
Table 2.
Structure-activity relationships of bastadin-5 (3) and analogues.a

| ||||||
|---|---|---|---|---|---|---|
| Entry | Compound | Skeleton | X | Y | EC50 (μM)b | IC50 (μM)c |
| 1 | 3 | A | – | – | 2.2 ± 0.1 | – |
| 2 | (±)-4 | B | NO2 | H | 20.9 ±10.8 | – |
| 4 | (±)-5 | B | NH2 | H | 33.0 ±12.2 | – |
| 5 | (±)-6 | B | N3 | H | – | 63 ±8.1 |
| 6 | (±)-7 | B | Br | H | 10.9 ±2.6 | – |
| 7 | (±)-8 | B | Br | Br | >100 | – |
| 8 | (±)-9 | B | H | H | >100 | – |
| 9 | (±)-10 | B | I | 28.6 ±14.2 | – | |
| 10 | 11 | C | NO2 | H | 24 ± 3.9 | – |
| 11 | 12 | C | NH2 | H | 21 ±5.7 | – |
| 12 | 13 | C | N3 | H | 20.0 ± 2.3 | – |
| 13 | 14 | C | Br | H | – | 6.2 ±1.2 |
| 14 | (±)-15 | C | Br | Br | – | 47 ± 15 |
| 15 | 49 | D | Br | H | >100 | |
| 16 | 52 | D | Br | Br | 6.5 ±0.7 | |
| 17 | 53 | >100 | ||||
Equilibrium binding of 1 nM [3H]-ryanodine to skeletal junctional sarcoplasmic reticulum (SR) was performed in assay buffer containing 250 mM KCl, 15 mM NaCl, 20 mM HEPES, 20 μM Ca2+, pH 7.4. Nonspecific binding was determined in the presence of 1 μM cold ryanodine.
agonist-like activity (enhanced 3[H]-ryanodine binding)
antagonist-like (reduced 3[H]- ryanodine binding)
Equilibrium binding of [3H]-ryanodine to skeletal JSR was measured in the presence of analog and solvent DMSO, or solvent alone (DMSO, 0.5% v/v final concentration), together with assay buffer containing 250 mM KCl, 15 mM NaCl, 20 mM HEPES, 20 μM Ca2+, pH 7.4. Nonspecific binding was determined in the presence of 1 μM cold ryanodine.
Most of the 14-membered ring analogs retained at least some of the potency of 3 on the ryanodine binding to the RyR1/FKBP12 complex. The most potent agonist was 7 (EC50= 11 μM) with a structure corresponding to the substitution pattern of bastadin-5. Substitution of the bromine at C17 in ring B by different functional groups (NO2, NH2, H, I, N3) diminished ryanodine binding. Replacement of the bromine with a nitro group in compound 4 resulted in a twofold loss of activity (EC50= 21 μM), whereas the amino analog 5 (EC50= 33 μM) and the iodo analog 10 (EC50= 28 μM) retained only a third of the activity of 7. The debromo analog 9 (EC50< 100 μM) was nearly inactive underscoring the importance of the dibromocatechol ether moiety at the western edge of the bastadin-5 structure. Despite almost identical conformations (X-ray), analogs 4 and 9 showed the largest difference in ryanodine binding among the 14-membered ring analogs (EC50= 21 and >100 μM, respectively). Since the nitro group is expected to occupy space equivalent to the van der Waals radius of a Br atom, it is tempting to speculate that stereoelectronic effects may be responsible for differences in biological activity. Unfortunately, we cannot be more definitive without additional data on the locus of binding of bastadin-5 at the receptor surface and knowledge of the putative amino acid residue contacts that mediate the binding contacts. The addition of a third Br atom on ring B in analog 8 resulted in total loss of agonist activity in this 14-membered ring analog (EC50< 100 μM) although, curiously, this was not the case in the corresponding 18-membered ring analog (see below).
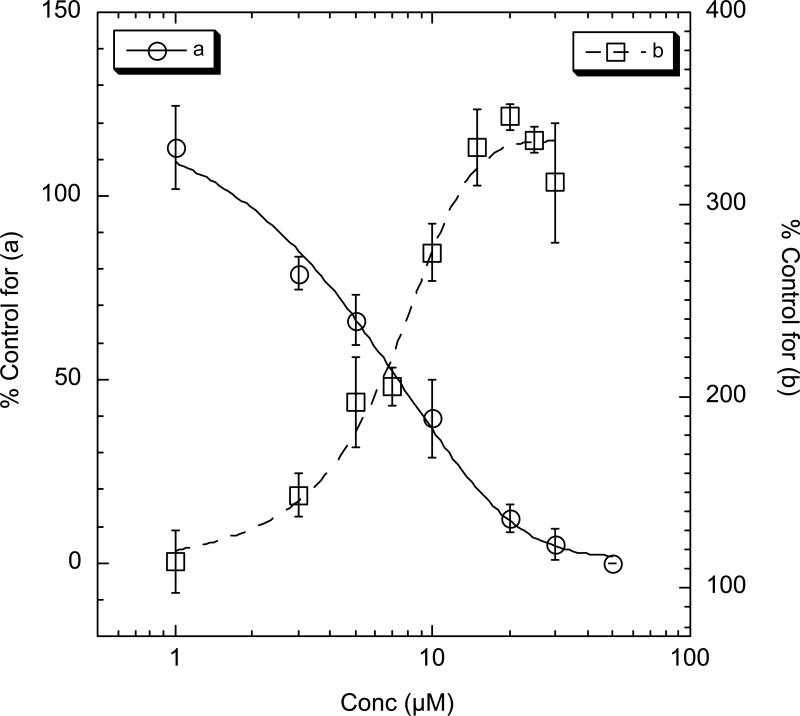
An unexpected trend was revealed by measurements of ryanodine binding in the presence of the 18-membered ring analogs – two of the five compounds were inhibitors of ryanodine binding, which suggests inhibition of Ca2+ channel opening. While the amino- and azido-substituted 18-membered ring compounds (12 and 13, respectively) showed weak activity in promoting channel activation (EC50 21 μM and 24 μM), compound 14 with the bastadin-5-like substitution pattern, was a more potent inhibitor (IC50= 6 μM, Figure 4), in diametric opposition to that of the 14-membered ring 7 with the same aryl substitution pattern which is agonist-like (EC50 11 μM). The remaining compounds in the 18-membered ring series showed weaker agonist-like activity (EC50's 24-58 μM).
Figure 4.
Concentration-dependent enhancement/inhibition of the binding of 1 nM [3H]-ryanodine to RyR1-FKBP12 in skeletal SR by bastadin analogs (a) 14 and (b) 52. Equilibrium binding of 1 nM [3H]-ryanodine to skeletal junctional sarcoplasmic reticulum (SR) was performed in assay buffer containing 250 mM KCl, 15 mM NaCl, 20 mM HEPES, 20 μM Ca2+, pH 7.4. Control (1% DMSO aq) = 0.175 pmol [3H]-ryanodine/mg SR. Nonspecific binding was determined in the presence of 1 μM cold ryanodine. Measurements were carried out in triplicate; error bars represent ±SD.
These results suggest the interaction of simple bastadin-5 analogs with the RyR1/FKBP12 is more complex and shows a broader range of action than expected. Each of the active 14-membered ring analogs were synthesized as racemic mixtures, but we predict that specific protein-drug contacts with the binding site should favor only one of the two enantiomers of each compound. Consequently, it would be of interest to investigate the ryanodine binding and Ca2+ transport activity of an enantiopure preparation of the most active analog, 7.
Photoaffinity Analogues
With the ryanodine-binding properties of both 14-membered and 18-membered ring analogs in hand, we asked the question, ‘can a simple photoaffinity analog of 3 be designed which retains high potency for the RyR1/FKBP12 complex?’ Using the key design principles from the 18-membered ring series, we replaced the β-alanine with a β-lysine residue to prepare an 18-membered macrolactam that contains a primary amino group that would could be acylated at the ε-NH2 group with a photoreactive azidobenzoic acid. Photolysis of the derived azidobenzamide analog of 3 would produce a nitrene that may covalently bond to the RyR1/FKPB12 complex. Tryptic digestion and MS analysis of the photolabelled RyR1/FKPB12-derived peptides from the exposed surface may reveal the consensus amino acid sequence of the bastadin binding site.
Differentially N-protected β-lysine (41) was coupled (EDCI, HOBt) to bromotyramine 34 (Scheme 7), followed by removal of the β-N Fmoc protecting group (81%) and coupling to the substituted dihydrocinnamic acid 17 to give the N,N’-diacyl β-lysine 44 (86% over 2 steps). Exposure of 44 to standard SNAr macroetherification conditions gave cyclic analog 45 in very good yield (75%). Transformation of the NO2 group in 44 to the corresponding analogs (replacement of NO2 by Br (47) and the overbrominated product 50) was achieved, as before, by reduction-diazonium salt displacements. Finally, simultaneous deprotection of the aryl ethers and ε-N-Boc protecting group in each of the latter compounds, followed by coupling to 2-azido-5-iodobenzoic acid37 (EDCI, HOBt) gave the corresponding photoaffinity analogs 49 and 52, respectively, in acceptable yields (two steps, 33% and 48%, respectively).
Scheme 7.
Synthesis of photoaffinity analogs by SNAr macroetherification
a. i. EDCI, HOBt, CH2Cl2, 4136; ii. H+, 81%; b. N,N,N,N-tetramethylethylenediamine, CH2Cl2; c. EDCI, HOBt, CH2Cl2, 17 86% two steps; d. K2CO3, 4Å mol. sieves, DMSO, 75%; e. CrCl2, DMF, 85%; f. t-BuONO, CuBr2, CH3CN, 38% of 47, 42% of 50; g. BBr3, CH2Cl2; h. 2-azido-5-iodobenzoic acid37, EDCI, HOBt, DMF, 48, 33% two steps; i. BBr3, CH2Cl2 j. 2-azido-5-iodobenzoic acid37, EDCI, HOBt, DMF, 51, 48% two steps.
When tested in the ryanodine binding assay, each of the photoaffinity analogs gave very different results. Compound 49 was essentially inactive (EC50 >100 μM), however, dibromo compound 52 showed very potent agonist-like activity (EC50 = 6 μM, Figure 4). It should be noted that the methyl ester 53 prepared from 2-azido-5-iodobenzoic acid (CH2N2, Et2O, 0°C), was inactive in this assay. Compound 52 shows binding affinity for the receptor similar to that of bastadin-5 (3), and its synthesis is amenable to introduction of radiolabel for preparation of [125I]-52. Consequently, 52 should provide a suitable probe for photoaffinity labeling of the RyR-FKBP12 complex.
Conclusion
The synthesis of simple, ring-constrained cyclic analogs of bastadin-5, with structures that embody the ‘western’ edge of 3, was accomplished by an efficient, macrocyclization based on intramolecular SNAr substitution. Assay of two classes of analogs that differ in ring size and aryl substitution patterns in the [3H]-ryanodine binding assay – which detect the open state of the RyR1/FKBP12 Ca2+ channel – revealed an interesting bi-modal range of activity represented by compounds with agonistic and antagonistic properties. In each of the two families, the compound structures that embodied the same ‘western edge’ substitution pattern found in native bastadin-5 (dibromocatechol ether) showed the highest activities. Members of the smaller 14-membered ring family, constrained by a conformationally rigid macrocycle, exhibited atropisomerism. The synthesis of an active photoaffinity probe 52, based on the structure of bastadin-5, has been achieved. The synthetic design of 52 should facilitate preparation of [125I]-52 for use in defining the bastadin-5 binding locus on the surface of the heterotetrameric RyR1/ FKBP12 complex that constitutes the membrane bound Ca2+ channel of the SR. The unexpected antagonistic activity of the 18-membered ring analogs suggests more complex interactions occur between the receptor site and this family of ligands. A bi-modal gating model that accommodates binding-activation-inactivation of the RyR1/ FKBP12 complex by bastadin-5 analogs suggests the possibility that Ca2+ release from the SR may be modulated by ‘fine tuning’ of stereoelectronic factors. Tuned release of Ca2+ from stores by custom-designed ligand-gate interactions between small molecule analogs of 3 and the RyR1/FKBP12 complex is an attractive idea that may have practical benefits in treatment of disease conditions that arise from complications of defective SR Ca2+ channel activity, including arrythmias, heart failure, and malignant hypothermia.38
Supplementary Material
Figure 1.
Natural products that interact with FKBP12 or the RyR1/FKBP12 complex
Scheme 3.
Synthesis of Common Intermediate 17
Key: (a) RhCl(PPh3)3, H2 (3 atm), toluene, 8 h, 95%; (b) i. LiOH, THF, H2O, MeOH (4:1:1); ii. HCl aq. (0.5M), 86%.
Acknowledgements
We thank S. Goth and T-A. Ta for assistance with some of the binding assays. Funding for this study was provided by the U.S. National Institutes of Health (GM57560 to TFM, and ES11269 to INP) and the U.S. Environmental Protection Agency (R829388 to INP).
Experimental
General
TLC was carried out on aluminum plates coated with silica (0.2 mm) containing a fluorescent indicator. Spots were visualized was under a UV lamp then sprayed with a solution of vanillin in ethanolic–H2SO4, or ninhydrin in ethanol followed by heating. Purity of each compound was established as >95% by 1H NMR and HPLC. Pyridine, triethylamine, dimethylsulfoxide (DMSO), and dimethylformamide (DMF) were distilled from glass over CaH2. Dichloromethane, acetonitrile, toluene, tetrahydrofuran, and 1,4-dioxane were dried through commercial alumina cartridge. Optical rotations were recorded on a Jasco DIP-370 instrument. IR spectra were recorded on a Mattson Galaxy FTIR. . 1H and 13C NMR were recorded at 400 MHz and 100 MHz, respectively, in the stated solvent (≥ 99.5% atom D) and referenced to residual protonated solvent signal (δΗ CDCl3 7.24 ppm; CD3OD, 3.30 ppm; δCCDCl3 77.00 ppm, CD3OD, 49.00 ppm).
[3H]-Ryanodine Binding Asssay
Specific binding of [3H]-ryanodine to high affinity sites on rabbit skeletal membrane vesicles13,39 was determined by incubating SR protein (25 μg), containing the RyR1-FKBP12 complex, with [3H]-ryanodine (1 nM) for 3.5 h at 37° C in binding assay buffer containing KCl (250 mM), NaCl (15mM), HEPES (20 mM), CaCl2 (20 μM) and at pH 7.4 (500 μL, final volume). The binding reaction was initiated by addition of a solution of the drug in DMSO (final DMSO conc. ~1%) to the complete assay medium and the incubation was terminated by filtration through Whatman GF/B glass fiber filters using a Brandel cell harvester (Gaithersburg, MD). Separation of bound and free [3H]-ryanodine was performed by washing the filters with ice-cold buffer (3 × 500 μL) containing Tris-HCl (20 mM), KCl (250 mM), NaCl (15 mM) at pH 7.4. Filters were placed in scintillation vials containing scintillant (5 mL). Treatments and controls were measured in triplicate and bound radioactivity (dpm) was measured by scintillation counting and corrected for background. Ryanodine affinity curves were plotted and fitted to sigmoidal functions (Origin, Microcal Software, Inc., Northampton, MA). Error bars represented in Figure 4 are ±1 standard deviation. Positive controls were bastadin-5 (EC50 2.0 μM) and PCB95 (2,2',3,5',6-pentachlorobiphenyl)40,41 and nonspecific binding was determined in the presence of 100-fold ‘cold’ ryanodine.
5-Bromo-4-hydroxy-17-nitro-2-oxa-10-aza-tricyclo[12.2.2.10,0]nonadeca-1(17),3(19),4,6,14(18),15-hexaen-11-one (4)
BBr3 (20 μL, 0.21 mmol) was added to a solution of lactam 27 (20 mg, 0.04 mmol) in CH2Cl2 (300 μL) at –78 C. The orange solution was stirred (1h, –78 °C), quenched with a NaHCO3 (aq., satd.) and extracted with EtOAc (3 × 10 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 4. Flash chromatography (SiO2, CH2Cl2/MeOH 20:1) gave 4 (15.0 mg, 91%) as an amorphous solid: mp 259-260 °C (CH2Cl2/MeOH); IR (ZnSe, neat) ν 3282, 2933, 1642, 1531, 1346 cm-1; 1H NMR (300 MHz, CD3COCD3) δ 2.40 (t, J= 6.3 Hz, 2H), 2.60-2.64 (m, 2H), 3.00-3.20 (m, 4H), 5.28 (d, J= 2.0 Hz, 1H), 6.89 (d, J= 2.0 Hz, 1H), 6.93 (brs, 1H) 7.29 (d, J= 8.4 Hz, 1H), 7.63 (dd, J= 8.4, 2.4 Hz, 1H), 8.00 (d, J= 2.4 Hz, 1H), 8.90 (brs, 1H); 13C NMR (75 MHz, CD3COCD3) δ 30.8 (CH2), 32.0 (CH2), 40.2 (CH2), 40.6 (CH2), 110.3 (C), 114.3 (CH), 126.7 (CH), 127.4 (CH), 127.9 (CH), 134.0 (C), 137.4 (C), 141.9 (C), 142.3 (C), 144.9 (C), 150.3 (C), 151.1 (CH), 170.9 (C); HRMS (DEI) found m/z 406.0179 [M]+, C17H15N2O5Br requires 406.0164.
17-Amino-5-bromo-4-hydroxy-2-oxa-10-aza-tricyclo[12.2.2.10,0]nonadeca-1(17),3(19),4,6,14(18),15-hexaen-11-one (5)
BBr3 (2 μL, 0.02 mmol) was added to a solution of lactam 28 (3 mg, 0.006 mmol) in CH2Cl2 (300 μL) at –78 C. The orange solution was stirred (1h, –78 °C), quenched with a NaHCO3 (aq., satd.) and extracted with EtOAc (3 × 5 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 5. Flash chromatography (SiO2, CH2Cl2/MeOH 20:1) gave 5 (1.4 mg, 58%) as an amorphous solid: IR (ZnSe, neat) ν 3371, 2927, 1635, 1502, 1434 cm-1; 1H NMR (400 MHz, CD3OD) δ 2.15-2.23 (m, 1H), 2.32-2.38 (m, 1H), 2.57-2.71 (m, 2H), 2.86-2.94 (m, 2H), 3.25-3.57 (m, 2H), 4.89 (brs, 1H), 5.36 (d, J= 1.6 Hz, 1H), 5.79 (brs, 1H), 6.62-6.67 (m, 2H), 6.82-6.84 (m, 2H); 13C NMR (100 MHz, CD3OD) δ 30.2 (CH2), 32.2 (CH2), 39.7 (CH2), 41.1 (CH2), 109.2, 112.7, 120.2, 124.7, 125.06, 131.3, 132.4, 140.3, 141.0, 142.2, 171.8; HRMS (DEI) found m/z 376.0434 [M]+, C17H17N2O3Br requires 376.0423.
17-Azido-5-bromo-4-hydroxy-2-oxa-10-aza-tricyclo[12.2.2.10,0]nonadeca-1(17),3(19),4,6,14(18),15-hexaen-11-one (6)
NaNO2 (0.2 mg, 3 μmol) was added in one portion into a chilled solution (0 °C, ice bath) of aryl amine 5 (1.0 mg, 0.003 mmol) in AcOH/H2O (20 μL, 9:1). The solution was allowed to stir 15 min followed by the addition of NaN3 (1.0 mg, 0.015 mmol) in one portion. After 0.5 h, the reaction was quenched with H2O and was extracted with CH2Cl2 (3 × 5 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 6. HPLC purification (C18 5μm Microsorb 10 × 250 mm, MeOH/H2O, 3:2, 4 mL/min, rt, 6.4 min) provided 6 (0.7 mg, 66%) as an oil: IR (neat) ν 3241, 2923, 2115, 1639, 1500 cm-1; 1H NMR (400 MHz, CD3OD) δ 2.33-2.38 (m, 2H), 2.63-2.67 (m, 2H), 2.94-2.99 (m, 2H), 3.13-3.17 (m, 2H), 5.18 (d, J= 2.0 Hz, 1H), 6.87 (d, J= 2.0 Hz, 1H), 7.01-7.11 (m, 3H), 7.80 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 30.7 (CH2), 32.6 (CH2), 40.5 (CH2), 41.4 (CH2), 114.0 (CH), 123.3 (CH), 126.7 (CH), 127.1 (CH), 128.6 (CH), 134.0 (C), 136.2 (C), 141.9 (C), 174.4 (C); HRMS (DEI) found m/z 402.0336 [M]+, C17H15N4O3Br requires 402.0327.
5,17-Dibromo-4-hydroxy-2-oxa-10-aza-tricyclo[12.2.2.10,0]nonadeca-1(17),3(19),4,6,14(18),15-hexaen-11-one (7)
BBr3 (2 μL, 0.02 mmol) was added to a solution of lactam 29 (4.0 mg, 7 μmol) in CH2Cl2 (200 μL) at –78 C. The orange solution was stirred (1h, –78 °C), quenched with a NaHCO3 (aq., satd.) and extracted with EtOAc (3 × 5 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 7. Flash chromatography (SiO2, CH2Cl2/MeOH 20:1) gave 7 (3.0 mg, 91%) as an amorphous solid: IR (ZnSe, neat) ν 3291, 2933, 1637, 1504, 1224 cm-1; 1H NMR (400 MHz, CD3OD) δ 2.18-2.25 (m, 1H), 2.32-2.38 (m, 1H), 2.61-2.64 (m, 2H), 2.98-3.02 (m, 2H), 3.20-3.36 (m, 2H), 4.85 (brs, 1H), 5.07 (d, J= 2.0 Hz, 1H), 5.85 (brs, 1H), 6.85 (d, J= 2.0 Hz, 1H), 7.11 (d, J= 8.4 Hz, 1H), 7.24 (dd, J= 8.4, 2.0 Hz, 1H), 7.49 (d, J= 2.0 Hz, 1H); 13C NMR (100 MHz, CD3OD) δ 30.3 (CH2), 31.6 (CH2), 39.5 (CH2), 41.0 (CH), 112.7 (CH), 118.5 (C), 125.6 (CH), 126.0 (CH), 130.3 (CH), 132.0 (C), 134.3 (CH), 140.9 (C), 148.5 (C); HRMS (DCI/NH3) found m/z 439.9493 [M]+, C17H16O3NBr2 requires 439.9497.
5,15,17-Tribromo-4-hydroxy-2-oxa-10-aza-tricyclo[12.2.2.10,0]nonadeca-1(17),3(19),4,6,14(18),15-hexaen-11-one (8)
Trifluoroacetic acid (0.5 mL) was added to lactam 30 (5 mg, 8 μmol) and was allowed to stir at room temperature for 24 h. The trifluoroacetic acid was removed under reduced pressure and trace trifluoroacetic acid were removed by reevaporation from toluene to give crude 8. Reversed phase HPLC purification (C18, 5 μm Microsorb, 10 × 250 mm, MeOH/H2O, 60:40, 3 mL/min) provided 8 (3.2 mg, 75%) as a colorless amorphous solid: IR (ZnSe, neat) ν 3284, 2937, 1644, 1503, 1463, 1232, 1058 cm-1; 1H NMR (400 MHz, CD3OD) δ 2.28-2.36 (m, 1H), 2.47-2.70 (m, 3H), 3.00-3.09 (m, 1H), 3.20-3.26 (m, 2H), 3.38-3.47 (m, 1H), 4.90 (d, J= 6.8 Hz, 1H), 5.14 (d, J= 1.6 Hz, 1H), 5.78 (brs, 1H), 6.87 (d, J= 1.6 Hz, 1H), 7.35 (s, 1H), 7.53 (s, 1H); 13C NMR (100 MHz, CD3OD) δ 30.5 (CH2), 32.0 (CH2), 37.1 (CH2), 39.9 (CH2), 109.6 (C), 112.5 (CH), 118.1 (C), 122.5 (C), 125.9 (CH), 129.4 (CH), 132.6 (C), 136.8 (CH), 139.5 (C), 140.6 (C), 148.1 (C), 153.2 (C), 170.8 (C); HRMS (DCI/NH3) found m/z 517.8594 [M]+, C17H15O3NBr3 requires 517.8602.
5-Bromo-4-hydroxy-2-oxa-10-aza-tricyclo[12.2.2.10,0]nonadeca-1(17),3(19),4,6,14(18),15-hexaen-11-one (9)
Trifluoroacetic acid (0.5 mL) was added to lactam 31 (4 mg, 9 μmol) and was allowed to stir at room temperature for 24 h. The trifluoroacetic acid was removed under reduced pressure and trace trifluoroacetic acid were removed by reevaporation from toluene. Reversed phase HPLC purification (C18, 5μm Microsorb, 10 × 250 mm, MeOH/H2O, 60:40, 3 mL/min) provided 9 (2.2 mg, 70%) as a colorless solid: mp 250-251 °C (CH2Cl2/MeOH); IR (ZnSe, neat) ν 2929, 1627, 1501, 1429 cm-1; 1H NMR (400 MHz, CD3OD) δ 2.34-2.37 (m, 2H), 2.58-2.63 (m, 2H), 2.96-3.00 (m, 2H), 3.10-3.13 (m, 2H), 4.60 (brs, 1H), 5.03 (d, J= 2.0 Hz, 1H), 6.79 (dd, J= 2.0, 0.8 Hz, 1H), 7.02, (d, J= 8.4 Hz, 2H), 7.30 (d, J= 8.4 Hz, 2H); 13C NMR (100 MHz, CD3OD) δ 30.7 (CH2), 32.7 (CH2), 40.7 (CH2), 41.4 (CH2), 110.8 (C), 115.4 (CH), 125.4 (CH), 125.8 (CH), 132.2 (CH), 133.8 (C), 140.0 (C), 153.3 (C), 158.3 (C), 174.7 (C); HRMS (DCI/NH3) found m/z 362.0399 [M+H]+, C17H17O3NBr requires 362.0392.
5-Bromo-4-hydroxy-17-iodo-2-oxa-10-aza-tricyclo[12.2.2.10,0]nonadeca-1(17),3(19),4,6,14(18),15-hexaen-11-one (10)
Sodium nitrite (1.6 mg, 0.023 mmol) was added in one portion to concentrated sulfuric acid (50 μL) chilled with an ice bath. AcOH (60 μL) was added dropwise to this solution at 0 °C. The solution was stirred for 30 min and then treated with lactam 28 (9.4 mg, 0.021 mmol) in portions over 1 h. This mixture was stirred for 1 h at 0 °C, then room temperature for 20 min. KI (5.6 mg, 0.034 mmol) in 2M HCl (0.2 mL) was added to this mixture and the mixture was stirred at room temperature for 15 min, then 70 °C for 10 min. The mixture was chilled by ice bath and treated with Na2SO3 (aq., satd.) followed by extraction with EtOAc (4 × 5 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 10. Flash chromatography (SiO2, CH2Cl2/MeOH, 9:1) followed by reversed phase HPLC (C18, 5μm Microsorb, 10 × 250 mm, MeOH/H2O, 65:35, 3 mL/min) gave 10 (1.6 mg, 16%) as a pale yellow amorphous solid: IR (ZnSe, neat) ν cm-1 3289, 2928, 1643, 1502, 1433, 1217; 1H NMR (400 MHz, CD3OD) δ 2.50-2.54 (m, 2H), 2.78- 2.84 (m, 2H), 3.10-3.16 (m, 2H), 3.30-3.36 (m, 2H), 5.20 (d, J= 2.0 Hz, 1H), 7.02, (d, J= 2.0 Hz, 1H), 7.27 (d, J= 8.4 Hz, 1H), 7.49 (dd, J= 2.0, 8.4 Hz, 1H), 7.92, (d, J= 2.4 Hz, 1H); 13C NMR (100 MHz, CD3OD) δ 24.7 (CH2), 26.1 (CH2), 34.6 (CH2), 35.4 (CH2), 108.4 (CH), 119.9 (CH), 120.5 (CH), 126 (C), 135.8 (CH), 135.9 (CH), 151.9 (C); HRMS (DEI) found m/z 486.9296 [M]+, C17H15NO3Br requires 486.9280.
5-Bromo-4-hydroxy-20-nitro-2-oxa-10,14-diaza-tricyclo[16.2.2.10,0]tricosa-1(21),3(23),4,6,18(22),19-hexaene-11,15-dione (11)
BBr3 (4 μL, 0.042 mmol) was added to a solution of lactam 37 (8 mg, 0.014 mmol) in CH2Cl2 (100 μL) at –78 ° C. The orange solution was stirred (1h, –78 °C), quenched with NaHCO3 (aq., satd.) and extracted with EtOAc (3 × 20 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 11. Flash chromatography (SiO2, CH2Cl2/MeOH, 20:1) gave 11 (6 mg, 82%) as an oil: IR (ZnSe, neat) ν 3405, 3303, 1648, 1533 cm-1; 1H NMR (400 MHz, CD3OD) δ 2.16-2.13 (m, 2H), 2.40-2.34 (m, 2H), 2.65-2.60 (m, 2H), 3.10-3.00 (m, 2H), 3.31-3.27 (m, 2H), 3.51-3.46 (m, 4H), 5.29-5.25 (m, 1H), 5.95 (d, J= 2.0 Hz, 1H), 6.20 (bs, 1H), 6.23 (brt, J= 2.0 Hz, 1H), 6.99 (d, J= 2.0 Hz, 1H), 7.17 (d, J= 8.4 Hz, 1 H), 7.47 (dd, J= 8.4, 2.4 Hz, 1H), 7.81 (d, J= 2.4 Hz, 1H); 13C NMR (100 MHz, CD3OD) δ 31.3 (CH2), 33.4 (CH2), 34.0 (CH2), 35.4 (CH2), 38.9 (CH2), 41.0 (CH2), 110.3 (C), 114.2 (CH), 124.7 (CH), 126.1 (CH), 126.9 (CH), 131.8 (C), 135.1 (CH), 139.8 (C), 141.8 (C), 142.1(C), 145.7 (C), 145.9 (C), 170.6 (C), 171.9 (C); HRMS (DCI) found m/z 478.0629 [M+H]+, C20H21O6N3Br requires 478.0614.
21-Amino-4-hydroxy-5-bromo-2-oxa-10,14-diaza-tricyclo[16.2.2.10,0]tricosa-1(21),3(23),4,6,18(22),19-hexaene-11,15-dione (12)
BBr3 (4 μL, 0.042 mmol) was added to a solution of lactam 38 (7 mg, 0.013 mmol) in CH2Cl2 (100 μL) at –78 ° C. The orange solution stirred (1h, –78 °C), quenched with NaHCO3 (aq., satd.) and extracted with EtOAc (3 × 10 mL). The organic solution was combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 12. Flash chromatography (SiO2, CH2Cl2/MeOH, 20:1) gave 12 (3 mg, 51%) as an oil: IR (ZnSe, neat) ν 3328, 2929, 1648, 1509, 1423, 1278 cm-1; 1H NMR (400 MHz, CD3OD) δ 2.07-2.10 (m, 2H), 2.35-2.38 (m, 2H), 2.52-2.55 (m, 2H), 2.81-2.84 (m, 2H), 3.19-3.21 (m, 2H), 3.28-3.20 (m, 2H), 3.37-3.40 (m, 2H), 6.10 (d, J= 2.0 Hz, 1H), 6.54 (dd, J= 2.0, 8.4 Hz, 1H), 6.70 (d, J= 2.0 Hz, 1H), 6.81 (d, J= 8.4, 1H), 6.96 (d, J= 2.0 Hz, 1H); 13C NMR (100 MHz, CD3OD) δ 32.8, (CH2), 34.5 (CH2), 35.0 (CH2), 36.3 (CH2), 39.5 (CH2), 42.4 (CH2), 111.4 (C), 115.7 (CH), 118.9 (CH), 120.3 (CH), 123.3 (CH), 126.8 (CH), 133.4 (C), 139.9 (C), 140.8 (C), 141.8 (C), 143.8 (C), 148.2 (C), 173.8 (C), 174.9 (C); LRMS (ESI) found m/z 448.0 [M+H]+, C20H23N3BrO3 requires 448.0.
21-Azido-4-hydroxy-5-bromo-2-oxa-10,14-diaza-tricyclo[16.2.2.10,0]tricosa-1(21),3(23),4,6,18(22),19-hexaene-11,15-dione (13)
NaNO2 (0.8 mg, 12 μmol) was added in one portion to a chilled solution (0 °C, ice bath) of aryl amine 12 (4.8 mg, 11 μmol) in AcOH/ H2O (60 μL, 9:1). The solution was stirred for 15 min and treated with NaN3 (4.0 mg, 62 μmol) in one portion. After 0.5 h, the reaction was quenched with H2O and was extracted with CH2Cl2 (3 × 5 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 13. Purification by HPLC (C18 5 μm Microsorb 10 × 250 mm, MeOH/H2O, 3:2, 4 mL/min, rt. 6.0 min) provided 13 (3.5 mg, 68%) as an oil: IR (neat) ν 3303, 2925, 2117, 1648, 1500 cm-1; 1H NMR (400 MHz, CD3OD) δ 2.05-2.09 (m, 2H), 2.40-2.45 (m, 2H), 2.58-2.62 (m, 2H), 2.91-2.96 (m, 2H), 3.21-3.27 (m, 2H), 3.37-3.42 (m, 2H), 6.05 (d, J= 2.0 Hz, 1H), 6.95-7.10 (m, 4H), 7.67-7.74 (m, 2H); 13C NMR (100 MHz, CDCl3/CD3OD, 1:1) δ 31.9 (CH2), 33.8 (CH2), 34.1 (CH2), 35.4 (CH2), 39.1 (CH2), 41.3 (CH2), 111.0 (CH), 115.3 (CH), 121.6 (CH), 123.7 (CH), 127.0 (CH), 127.1 (CH), 132.3 (C), 133.0 (C), 139.7 (C), 143.0 (C), 145.7 (C), 147.5 (C), 173.1 (C), 173.4 (C); HRMS (DEI) found m/z 473.0694 [M]+, C20H20N5O4Br requires 473.0699.
5,21-Dibromo-4-hydroxy-2-oxa-10,14-diaza-tricyclo[16.2.2.10,0]tricosa- 1(21),3(23),4,6,18(22),19-hexaene-11,15-dione (14)
BBr3 (1 μL, 5 μmol) was added to a solution of lactam 39 (1 mg, 2 μmol) in CH2Cl2 (20 μL) at –78 °C. The orange solution was stirred (1h, –78 °C), quenched with a NaHCO3 (aq., satd.) and extracted with EtOAc (3 × 10 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 14. Flash chromatography (SiO2, CH2Cl2/MeOH 20:1) gave 14 (0.8 mg, 94%) as an amorphous solid: 1H NMR (400 MHz, CD3OD) δ 2.08-2.11 (m, 2H), 2.41-2.44 (m, 2H), 2.58-2.61 (m, 2H), 2.93-2.96 (m, 2H), 3.23-3.24 (m, 2H), 3.38-3.41 (m, 2H), 5.98 (d, J= 1.6 Hz, 1H), 7.05 (d, J= 2.0 Hz, 1H), 7.07 (d, J= 8.4 Hz, 1H), 7.21 (dd, J= 1.6, 8.4 Hz, 1H), 7.53 (d, J= 2.0 Hz, 1H); HRMS (DCI) found m/z 510.9853 [M+Na]+, C20H21O4N2Br2 requires 510.9868.
5,20,22-Tribromo-4-hydroxy-2-oxa-10,14-diaza-tricyclo[16.2.2.10,0]tricosa-1(21),3(23),4,6,18(22),19-hexaene-11,15-dione (15)
BBr3 (1M 50 μL) was added to a solution of lactam 40 (4 mg, 6 μmol) in CH2Cl2 (50 μL) at –78 °C. The orange solution was stirred (1h, –78 °C), was quenched with NaHCO3 (aq., satd.) and extracted with EtOAc (3 × 10 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 15. Flash chromatography (SiO2, CH2Cl2/MeOH 20:1) gave 15 (2.5 mg, 72%) as an amorphous solid: 1H NMR (400 MHz, CD3OD) δ 1.92-2.00 (m, 1H), 2.26-2.32, (m, 1H), 2.40-2.50 (m, 2H), 2.54-2.71 (m, 2H), 2.86-2.92 (m, 1H), 3.03-3.23 (m, 3H), 3.55-3.72 (m, 2H), 5.98 (d, J= 2.0 Hz, 1H), 7.07 (d, J= 2.0 Hz, 1H), 7.50 (s, 1H), 7.56 (s, 1H); LRMS (DCI) found m/z 610.9 [M+Na]+, C20H19O4N2Br3Na requires 610.9.
5-(2-Aminoethyl)-2-benzyloxy-3-bromophenol (16)
KOH (70 mg, 1.3 mmoles) and hydrazine (cat.) were added to a solution of carbamate 24 (22 mg, 0.05 mmol) dissolved in dry 1,4-dioxane (1 mL). This heterogeneous mixture was rapidly stirred and heated to 80 °C. After 0.5 h, the mixture was treated with HCl (1N) until the mixture was neutral by pH paper and was extracted with EtOAc (3 × 25 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to yield 16 as an oil (14 mg 92% crude). The product was carried forward without purification. 1H NMR (400 MHz, CDCl3) δ 2.58 (bs, 2H), 2.85 (bs, 2H), 4.20 (bs, 2H), 4.97 (s, 2H), 6.65 (bs, 1H), 6.89 (bs, 1H), 7.2-7.6 (m, 5H); HRMS (DCI/NH3) found m/z 322.0445 [M+H]+, C15H17O2NBr requires 322.0443.
3-(4-Benzyloxy-3-bromo-5-hydroxyphenyl)-N-[2-(4-fluoro-3-nitro-phenyl)-ethyl]propionamide (18)
Acid 17 (83 mg, 0.39 mmol), HOBt (55 mg, 0.41 mmol), and EDCI (78 mg, 0.41 mmol) were added sequentially to a solution of amine 16 (120 mg, 0.37 mmol) in CH2Cl2 (5 mL) at room temperature. After 1 h, the reaction was quenched with HCl (1N, 20 mL) and extracted with CH2Cl2 (4 × 20 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to provide crude 18 which was purified by flash chromatography (SiO2, CH2Cl2/MeOH, 9:1) yielding 18 (140 mg, 72%) as a amorphous solid: IR (KBr, pellet) ν 3392, 3075, 1639, 1529, 1425, 1349 cm-1; 1H NMR (300 MHz, CD3COCD3) δ 2.52 (t, J= 7.2 Hz, 2H), 2.65 (t, J= 7.2 Hz, 2H), 3.01 (t, J= 7.2 Hz), 3.38 (dt, J= 7.2, 6.0 Hz, 2H), 5.01 (s, 2H), 6.78 (d, J= 1.8 Hz, 1H), 6.88 (d, J= 1.8 Hz, 1H), 7.25 (brs, 1H), 7.30-7.45 (m, 4H), 7.50-7.60 (m, 2H), 7.64-7.69 (m, 1H), 7.99 (dd, J= 6.9, 2.1 Hz, 1H), 8.68 (bs, 1H); 13C NMR (75 MHz, CD3COCD3) δ 31.0 (CH2), 35.0 (CH2), 37.6 (CH2), 41.2 (CH2), 74.8 (CH2), 117.3 (CH), 117.6 (C), 118.7 (CH, J= 21.0 Hz), 124.3 (CH), 126.2 (CH, J= 3.0 Hz), 128.5 (CH), 128.8 (2 CH), 136.7 (CH, J= 8.0 Hz), 137.7 (C), 138.1 (C, J= 7.0 Hz), 139.6 (C, J= 4.3 Hz), 142.8 (C), 151.9 (C), 154.2 (C, J= 258.0 Hz), 171.9 (C); HRMS (DEI) found m/z 516.0686 [M]+, C24H22N2O5BrF requires 516.0696.
N-(2-{2-[4-Benzyloxy-3-bromo-5-triisopropyl-silanyloxy)-phenyl]-ethylcarbamoyl}-ethyl-3-(4-fluoro-3-nitro-phenyl)-propionamide (19)
EDCI (76 mg, 0.40 mmol) was added to a solution of amine 36 (110 mg, 0.20 mmol), HOBt (54 mg, 0.40 mmol), and acid 17 (47 mg, 0.22 mmol) in CH2Cl2 (5.0 mL). The solution stirred overnight at room temperature, quenched with HCl (1N) and extracted with CH2Cl2 (3 × 20 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 19. Flash chromatography (SiO2, CH2Cl2/MeOH, 20:1) gave 19 (139 mg, 93%) as an oil: IR (NaCl, neat) ν 3293, 2944, 2867, 1644, 1538 cm-1; 1H NMR (400 MHz, CDCl3) δ 1.07 (d, 7.2 Hz, 18H), 1.26 (sept, J= 7.2 Hz, 3H), 2.28 (t, J= 6.0 Hz, 2H), 2.45 (t, J= 7.2 Hz, 2H), 2.67 (t, J= 7.2 Hz, 2H), 2.99 (t, J= 7.2 Hz, 2H), 3.50-3.38 (m, 4H), 4.98 (s, 2H), 5.54 (brs, 1H), 6.39 (brs, 1H), 6.63 (d, J= 2.0 Hz, 1H), 6.94 (d, J= 2.0 Hz, 1H), 7.15 (dd, J= 10.8, 8.4 Hz, 1H), 7.38-7.29 (m, 3H), 7.48-7.42 (m, 1H), 7.52-7.48 (m, 2H), 7.86 (dd, J= 7.2, 2.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 12.9 (CH), 17.9 (CH3), 30.2 (CH2), 34.8 (CH2), 35.2 (CH2), 35.4 (CH2), 37.2 (CH2), 40.5 (CH2), 74.4 (CH2), 118.0 (C), 118.3 (d, J= 6.2 Hz, CH), 119.7 (CH), 125.1 (CH), 125.3 (d, J= 2.9 Hz, CH), 127.8 (CH), 128.0 (CH), 128.1 (CH), 135.5 (C), 135.6 (d, J= 5.1 Hz, CH), 136.0 (d, C), 136.9 (C), 137.8 (d, J= 4.3 Hz, C), 145.4 (C), 150.3 (C), 153.8 (d, J= 260.9 Hz, C), 171.0 (C), 171.4 (C); HRMS (FAB) found m/z 744.2478 [M+H]+, C36H48O6N3FSiBr requires 744.2478.
4-(Benzyloxy)-3-bromo-5-hydroxybenzaldehyde (21)
Li2CO3 (85 mg, 1.2 mmol) was added to a solution of 3-bromo-4,5-dihydroxybenzaldehyde 20 (100 mg, 0.46 mmol) in DMF (2 mL). This solution was vigorously stirred and heated (45 °C, 1h) followed by dropwise addition of benzylbromide (0.14 mL, 1.2 mmol). After 45 min, the reaction was quenched with HCl (aq., 1N) resulting in precipitation of the crude product 21. The precipitate was filtered, washed with water, dried under high vacuum and was purified by flash chromatography (SiO2, CH2Cl2/hexane, 9:1) to yield 21 (125 mg, 88%) as a pale yellow solid: mp 92-93 °C; IR (NaCl, neat) ν 3235, 1683 cm-1; 1H NMR (400 MHz, CDCl3) δ 5.14 (s, 2H), 5.85 (s, 1H), 7.34 (d, J= 2.0 Hz, 1H), 7.38-7.46 (m, 5H), 7.63 (d, J= 2.0 Hz, 1H), 9.79 (s, 1H); 13C NMR (75 MHz, CDCl3) δ 76.1 (CH2), 115.5 (CH), 117.0 (C), 126.7 (CH), 128.6 (CH), 129.0 (CH), 129.2 (CH), 133.9 (C), 148.3 (C), 150.9 (C), 190.0 (CH); HRMS (DCI/NH3) found m/z 306.9963 [M+H]+, C14H12O3Br requires 306.9969.
4-Benzyloxy-3-bromo-5-hydroxycinnamic acid (22)
Pyridine (0.55 mL, 6.8 mmol) and piperidine (0.16 mL, 1.62 mmol) were added to a solution of 4-benzyloxy-3-bromo-5-hydroxybenzaldehyde 21 (2.0 g, 6.5 mmol) and malonic acid (0.7 g, 6.8 mmol) in toluene (100 mL) within a round-bottom flask equipped with a Dean Stark trap and heated under reflux for 5h. The reaction was quenched with HCl (1N, 300 mL) resulting in precipitation of 22. This compound was collected and washed with water and dried under high vacuum to provide 22 (2.0 g, 88%) as a colorless solid: mp 173-174 °C; IR (NaCl, neat) ν 3249, 1650, 1633, 1616 cm-1; 1H NMR (300 MHz, CDCl3) δ 5.09 (s, 2H), 6.3 (d, J= 15.9 Hz, 1H), 7.03 (d, J= 1.8 Hz, 1 H), 7.29 (d, J= 1.8 Hz, 1H), 7.38-7.44 (m, 5H), 7.57 (d, J= 15.9 Hz, 1 H); 13C NMR (75 MHz, CDCl3/ drop of DMSO) δ 74.9 (CH2), 114.9 (CH), 117.5 (C), 118.7 (CH), 123.9 (CH), 128.3 (CH), 128.4 (CH), 128.5 (CH), 131.9 (C), 136.5(C), 143.3 (CH), 145.2 (C), 151.1 (C), 169.0 (C); HRMS (DCI/NH3) found m/z 349.0087 [M]+, C16H14O4Br requires 349.0075.
Ethyl(4-benzyloxy)-3-bromo-5-(ethoxycarbonyloxy)styrylcarbamate (23)
Diisopropylethylamine (1.4 mL) was added dropwise to a chilled solution (–10 °C) of 4-benzyloxy-5-bromo-3-hydroxy-cinnamic acid 22 (1.00 g) dissolved in acetone (40 mL), followed by the dropwise addition of ethylchloroformate (0.6 mL). After stirring for 2hr (–10 °C), a chilled aqueous solution of sodium azide (560 mg, 10 mL H2O) was added dropwise to the reaction. After stirring for 5 h at 0 °C, the solution was extracted with CH2Cl2 (3 × 100 mL). Extracts were combined, dried over anhydrous MgSO4, filtered, and volatiles were removed to give a colorless solid. The resulting solid was azeotroped dried with toluene (3 × 20 mL). Ethanol (5 mL) and toluene (50 mL) were added and this solution was heated to 80 °C for 12 h. The volatiles were removed to give crude 23 that was purified by flash chromatography (SiO2, CH2Cl2/EtOAc, 98:2) to yield 23 (0.89 g, 66%) as an amber viscous oil: IR (NaCl, neat) ν 3324, 2981, 1766, 1729, 1660 1525, 1475, 1257, 1224 cm-1; 1H NMR (400 MHz, CDCl3) δ 1.29 (t, J= 7.2 Hz, 6H), 4.21 (q, J= 7.2 Hz, 4H), 4.98 (s, 2H), 5.80 (d, J= 14.4 Hz, 1H,), 6.53 (d, J= 10.4 Hz, 1H,), 7.02 (d, J= 2.0 Hz, 1H), 7.14 (dd, J= 14.4, 10.4 Hz, 1H,), 7.5-7.3 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 14.5 (CH3), 61.8 (CH2), 65.2 (CH2), 75.6 (CH2), 107.7 (CH), 118.3 (C), 118.7 (CH), 125.6 (CH), 127.5 (CH), 128.2 (CH), 128.3 (CH), 128.4 (CH), 134.5 (C), 136.4 (C), 145.3 (C), 146.3 (C), 152.9 (C), 153.5 (C); HRMS (DCI/NH3) found m/z 463.0614 [M]+, C21H22O6NBr requires 463.0630.
Carbonic acid 2-benzyloxy-3-bromo-5-(2-methoxycarbonylamino-vinyl)-phenyl ester methyl ester
IR (KBr, neat) ν 3336, 2956, 1770, 1734, 1660, 1477, 1261, 943 cm-1; 1H NMR (300 MHz, CDCl3) δ 3.73 (s, 3H), 3.80 (s, 3H), 4.98 (s, 2H), 5.75 (d, J= 14.0 Hz, 1H), 6.87 (bd, J= 11.0 Hz, 1H), 7.01 (d, J= 2.0 Hz, 1H), 7.11 (bd, J= 11.0 Hz, 1H), 7.3-7.5 (m, 4H); 13C NMR (75 MHz, CDCl3) δ 52.7 (CH3), 55.7 (CH3), 75.6 (CH2), 107.8 (CH), 118.2 (C), 118.5 (CH), 125.5 (CH), 127.4 (CH), 128.1 (CH), 128.2 (2CH), 134.3 (C), 136.3 (C), 145.1 (C), 146.0 (C), 153.4 (C), 153.8 (C); HRMS (DCI/NH3) found m/z 349.0087 [M]+., C16H14O4Br requires 349.0075.
Carbonic acid 2-benzyloxy-3-bromo-5-(2-methoxycarbonylamino-ethyl)-phenyl ester ethyl ester (24)
Triethylsilane (265 uL, 1.67 mmol) was added to 23 (50 mg, 0.17 mmol) and the resulting heterogeneous mixture was rapidly stirred (–10 °C). Chilled (–10 °C) neat trifluoroacetic acid (1 mL) was rapidly transferred via canula to the reaction mixture. The heterogeneous mixture was allowed to rapidly stir for 20 min. The reaction was quenched with a NaHCO3 (aq., satd.) and was extracted with CH2Cl2 (4 × 25 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to provide 24 as a viscous oil (47 mg, 94%): IR (NaCl, neat) ν 3343, 2956, 1770, 1722, 1481, 1257 cm-1; 1H NMR (400 MHz, CDCl3) δ 2.75 (t, J= 7.0 Hz, 2H), 3.40 (dt, J= 7.0, 7.0 Hz, 2H), 3.65 (s, 3H), 3.80 (s, 3H), 4.74 (brs, 1H), 4.98 (s, 2H), 6.96 (d, J= 2.0 Hz, 1H), 7.29 (d, J= 2.0 Hz, 1H), 7.32-7.5 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 35.2 (CH2), 41.8 (CH2), 52.1 (CH3), 55.7 (CH3), 75.5 (CH2), 118.0 (C), 122.4 (CH), 128.1 (CH), 128.2 (2CH), 131.0 (CH), 136.2 (C), 136.4 (C), 144.9 (C), 146.6 (C), 153.3 (C), 156.7 (C); HRMS (DCI/NH3) found m/z 466.0864 [M+H]+, C21H25O6NBr requires 466.0865.
3-(4-Fluoro-3-nitro-phenyl)-propionic acid methyl ester (26)
In a glove box, olefin 25 (300 mg, 1.3 mmol) and Wilkinson's catalyst (44 mg, 0.05 mmol) were dissolved in toluene (25 mL) in a pressure vessel. The vessel was removed from the glove box and purged with H2 and pressurized with H2 (3 atm). The mixture was rapidly stirred and heated (60 °C). After 8 h, the toluene was removed resulting in a brown residue that was passed through a flash column (SiO2, EtOAc/CH2Cl2, 1:50) to provide 26 as an amorphous solid (290 mg, 95%): IR (NaCl, neat) ν 2954, 1735, 1537, 1349 cm-1; 1H NMR (400 MHz, CDCl3) δ 7.86 (dd, J= 7.2, 2.4 Hz, 1H), 7.45 (m, 1H), 7.17 (dd, J= 10.0, 8.4 Hz, 1H), 3.63 (s, 3H), 2.97 (t, J= 7.6 Hz, 2H), 2.63 (t, J= 7.6 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 172.2 (s, C), 153.9 (d, J= 348 Hz, C), 137.4 (d, J= 5.7 Hz, C) 136.8 (brs, C), 135.5 (d, J= 11 Hz, CH), 125.4 (d, J= 3.8 Hz, CH), 118.2 (d, J= 28 Hz, CH), 51.8 (s, CH3), 34.9 (s, CH2), 29.6 (s, CH2); HRMS (EI) found m/z 227.0601 [M]+, C10H10FNO4 requires 227.0593.
4-Benzyloxy-5-bromo-16-nitro-2-oxa-11-aza-tricyclo[12.2.2.10,0]nonadeca-1(17),3(19),4,6,14(18),15-hexaen-10-one (27)
K2CO3 (500 mg, 3.6 mmol) was added to a solution of phenol 18 (100 mg, 0.19 mmol) in DMSO (100 mL, 2 mM) containing 4 Å sieves at room temperature. After 3 h of vigorous stirring, the reaction mixture was diluted with water (100 mL) and extracted with EtOAc (5 × 20 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to provide crude 27 that was purified by flash chromatography (SiO2, CH2Cl2/MeOH, 9:1) yielding 27 (86 mg, 85%) as a viscous oil: IR (NaCl, neat) ν 3272, 2933, 1639, 1531, 1346 cm-1; 1H NMR (400 MHz, CDCl3) δ 2.2-2.5 (m, 3H), 3.0-3.4 (m, 5H), 5.05 (d, J= 1.6 Hz, 1H), 5.15 (d, J= 10.4 Hz, 1H), 5.30 (d, J= 10.4 Hz, 1H), 5.34 (brs, 1H), 6.86 (d, J= 1.6 Hz, 1H), 7.09 (d, J= 8.4 Hz, 1H), 7.3-7.4 (m, 3H), (dd, J= 8.4, 2.0 Hz, 1H), 7.6-7.62 (m, 2H), 7.92 (d, J = 2.0 Hz); 13C NMR (100 MHz, CDCl3) δ 30.3 (CH2), 31.5 (CH2), 39.6 (CH2), 39.9 (CH2), 75.2 (CH2), 113.2 (CH), 117.8 (C), 126.3 (CH), 126.4 (CH), 127.4 (CH), 128.2 (CH), 128.3 (CH), 128.6 (CH), 136.8 (C), 136.8 (CH), 137.3 (C), 140.5 (C), 142.6 (C), 143.9 (C), 149.4 (C), 155.2 (C), 171.0 (C); HRMS (DEI) found m/z 496.0624 [M]+, C24H21N2O5Br requires 496.0634.
17-Amino-4-benzyloxy-5-bromo-2-oxa-10-aza-tricyclo[12.2.2.10,0]nonadeca-1(17),3(19),4,6,14(18),15-hexaen-11-one (28)
CrCl2 (110 mg, 0.89 mmol) was added to a solution of lactam 27 (34 mg, 0.07 mmol) in DMF (1 mL) and the mixture stirred at room temperature. After 12 h, the volatiles were removed to give a residue that was dissolved in EtOAc (10 mL). The organic solution was washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 28 that was purified by flash chromatography (SiO2, CH2Cl2/MeOH, 9:1) yielding 28 (23 mg, 73%) as a viscous gum: IR (NaCl, neat) ν 3291, 2927, 1639, 1504, 1270, 1187 cm-1; 1H NMR (400 MHz, CDCl3) δ 2.2-2.3 (m, 2H), 2.6-2.7 (m, 2H), 2.8-2.9 (m, 2H), 3.2-3.3 (m, 2H), 4.85 (brs, 1H), 5.17 (d, J= 10.4 Hz, 1H), 5.27 (d, J= 10.4 Hz, 1H), 5.41 (s, 1H), 6.63 (brd, J= 7.2 Hz, 1H), 6.79 (d, J= 7.2 Hz, 1H), 6.88 (s, 1H), 7.3-7.4 (m, 3H), 7.5-7.6 (m, 2H), 7.99 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 30.4 (CH2), 32.2 (CH2), 39.4 (CH2), 41.1 (CH2), 75.3 (CH2), 113.8 (CH), 117.6 (CH), 120.0 (CH), 124.5 (CH), 125.7 (CH), 128.3 (CH), 128.4 (CH), 128.8 (CH), 136.4 (C), 136.9 (C), 140.0 (C), 141.0 (C), 142.4 (C), 142.7 (C), 154.6 (C), 171.9 (C); HRMS (DEI) found m/z 466.0908 [M]+, C24H23N2O3Br requires 466.0892.
5,17-Dibromo-4-benzyloxy-2-oxa-10-aza-tricyclo[12.2.2.10,0]nonadeca-1(17),3(19) ,4,6,14(18),15-hexaen-11-one (29)
tert-Butyl nitrite (20 μL, 17 μmol) was added to a solution of CuBr2 (3 mg, 14 μmol) in CH3CN (0.2 mL) at 0 °C. After stirring for 1h, a heterogeneous mixture of lactam 28 (8 mg, 17 μmol) in CH3CN (0.8 mL) was added dropwise over 20 min at 0 °C. After stirring for 2 h, the solution was allowed to warm to room temperature and quenched with HCl (1N, 4 mL) and extracted with CH2Cl2 (4 × 5 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 29. Flash chromatography (SiO2, CH2Cl2/MeOH, 9:1) followed by reversed phase HPLC (C18, 5μm Microsorb 10 × 250 mm, MeOH/H2O, 65: 35, 3 mL/min) gave 29 (6 mg, 66%) as a colorless amorphous solid: IR (NaCl, neat) ν 3286, 2928, 1640, 1480, 1214 cm-1; 1H NMR (400 MHz, CDCl3) δ 2.2-2.4 (m, 2H), 2.6-2.7 (m, 2H), 2.9-3.1 (m, 2H), 3.2-3.4 (m, 2H), 4.90 (brs, 1H), 5.06 (d, J= 2.0 Hz, 1H), 5.18 (d, J= 10.4 Hz, 1H), 5.34 (d, J= 10.4 Hz, 1H), 6.88 (dd, J= 2.0, 1.2 Hz, 1H), 7.05 (d, 8.4 Hz, 1H), 7.23 (dd, J= 8.4, 2.4 Hz, 1 H), 7.3-7.4 (m, 3H), 7.51 (d, J= 2.4 Hz, 1H), 7.6-7.5 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 30.5 (CH2), 31.7 (CH2), 39.7 (CH2), 41.0 (CH2), 75.2 (CH2), 113.1 (CH), 117.9 (C), 118.7 (CH), 125.8 (CH), 125.9 (CH), 128.2 (CH), 128.4 (CH), 128.8 (CH), 130.4 (CH), 134.6 (C), 136.4 (C), 137.0 (C), 140.6 (C), 142.8 (C), 152.9 (C), 154.6 (C), 171.1 (C); HRMS (DEI) found m/z 532.9852 [M]+, C24H21NO3Br2 requires 532.9847.
4-Benzyloxy-5,15,17-tribromo-2-oxa-10-azatricyclo[12.2.2.10,0]nonadeca-1 (17),3(19),4,6,14,(18),15-hexaene-11-one (30)
tert-Butyl nitrite (12 μL, 0.09 mmol) was added to a solution of CuBr2 (56 mg, 0.25 mmol) in CH3CN (0.5 mL) was added at 0 °C. After stirring for 1 h, a heterogeneous mixture of lactam 28 (12 mg, 0.03 mmol) in CH3CN (0.8 mL) was added drop-wise over 20 min at 0 °C. After stirring for 2 h, the mixture was allowed to warm to room temperature and quenched with HCl (1N, 4 mL) and extracted with EtOAc (4 × 5 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 30. Flash chromatography (SiO2, CH2Cl2/ MeOH, 9:1) followed by reversed phase HPLC (C18, 5μm Microsorb, 10 × 250 mm, MeOH/ H2O, 75:25, 3 mL/ min) gave 30 (5 mg, 32%) as a colorless amorphous solid: IR (NaCl, neat) ν 3273, 2928, 1638, 1463, 1218 cm-1; 1H NMR (400 MHz, CDCl3) δ 2.2- 2.8 (m, 4H), 3.0-3.5 (m, 4H), 4.93 (bd, J= 7.2 Hz, 1H), 5.16 (d, J= 10.4 Hz, 1H), 5.31 (d, J= 10.4 Hz, 1), 6.92 (d, J= 2.0 Hz, 1H), 7.25 (s, 1H), 7.3- 7.4 (m, 3H), 7.55 (s, 1H), 7.59-7.63 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 30.5 (CH2), 32.1 (CH2), 37.1 (CH2), 39.7 (CH2), 75.3 (CH2), 112.9 (CH), 118.1 (C), 118.7 (C), 122.7 (C), 126.2 (CH), 128.3 (CH), 128.4 (CH), 128.8 (CH), 129.4 (CH), 136.5 (CH), 136.7 (C), 136.8 (C), 139.3 (C), 142.7 (C), 153.2 (C), 154.1 (C), 170.9 (C); HRMS (DEI) found m/z 606.9021 [M]+, C24H20NO3Br3 requires 606.8993.
4-Benzyloxy-5-bromo-2-oxa-10-aza-tricyclo[12.2.2.10,0]nonadeca-1(17),3(19),4,6,14(18),15-hexaen-11-one (31)
tert-Butyl nitrite (40 μL, 0.34 mmol) was added to THF (0.3 mL) at 0 °C. After stirring for 1h, a heterogeneous mixture of lactam 28 (10 mg, 0.02 mmol) in THF (0.4 mL) was added dropwise over 20 min at 0 °C. After stirring for 2 h, the solution was allowed to warm to room temperature, quenched with HCl (1N, 4 mL) and extracted with EtOAc (4 × 5 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 31. Flash chromatography (SiO2, CH2Cl2/MeOH, 9:1) followed by reversed phase HPLC (C18, 5μm Microsorb, 10 × 250 mm, MeOH/H2O, 65:35, 3 mL/min) gave 31 (3.8 mg, 39%) as a colorless powder: IR (NaCl, neat) ν 3286, 2925, 1640, 1567, 1501, 1202 cm-1; 1H NMR (400 MHz, CDCl3) δ 2.26-2.29 (m, 2H), 2.58-2.62 (m, 2H), 3.00-3.04 (m, 2H), 3.24-3.28 (m, 2H), 4.77 (brs, 1H), 5.03 (d, J= 2.4 Hz, 1H), 5.22 (s, 2H), 6.83 (d, J= 2.4 Hz, 1H), 6.97 (d, J= 8.4 Hz, 2H), 7.27 (d, J= 8.4 Hz, 2H), 7.30-7.42 (m, 3H), 7.59-7.61 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 30.5 (CH2), 32.0 (CH2), 39.6 (CH2), 41.2 (CH2), 75.2 (CH2), 114.6 (CH), 117.6 (C), 124.4 (CH), 125.0 (CH), 128.1 (CH), 128.3 (CH), 128.7 (CH), 130.8 (CH), 135.9 (C), 136.8 (C), 138.7 (C), 142.2 (C), 156.3 (C), 156.6 (C), 171.4 (C); HRMS (DEI) found m/z 451.0795 [M]+, C24H22NO3Br requires 451.0783.
[2-(4-Benzyloxy-3-bromo-5-hydroxy-phenyl)-ethyl]-carbamic acid tert-butyl ester (32)
Phenethylamine 16, (28 mg, 86 μmol) was dissolved in CH3CN (0.75 mL) and was treated with a solution of di-tert-butyldicarbonate (46 mg, 210 μmol) in CH3CN (0.25 mL). After 3 h, the volatiles were removed and the resulting residue was redissolved in MeOH (0.5 mL), water (0.2 mL) and treated with sodium carbonate (50 mg). After stirring 12 h, the solution was extracted with CH2Cl2 (4 × 25 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to yield 32 as an oil (28 mg, 76%): IR (NaCl, neat) ν 3359, 2977, 1685, 1500, 1367, 1166 cm-1; 1H NMR (400 MHz, CDCl3) δ 1.42 (s, 9H), 2.65 (bt, J= 4.8 Hz, 2H), 3.31 (bd, 2H), 4.59 (bs, 1H), 5.01 (s, 2H), 5.94 (bs, 1H), 6.69 (bs, 1H), 6.90 (bs, 1H), 7.3-7.5 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 28.3 (CH3), 35.4 (CH2), 41.4 (CH2), 79.4 (C), 115.5 (CH), 116.3 (C), 124.6 (CH), 128.5 (CH), 128.7 (CH), 128.7 (CH), 136.4 (C), 137.3 (C), 141.8 (C), 150.2 (C), 155.9 (C); HRMS (DCI/NH3) found m/z 439.1221 [M+NH4]+, C20H28O4N2Br requires 439.1232.
{2-[4-Benzyloxy-3-bromo-5-(triisopropyl-silanyloxy)-phenyl]-ethyl}-carbamic acid tert- butyl ester (33)
TIPSCl (75 μL, 0.35 mmol) was added to a solution of amide 32 (122 mg, 0.299 mmol), and imidazole (50 mg, 0.72 mmol) in DMF (0.5 mL). The yellow solution was stirred overnight at room temperature, quenched with water and extracted with EtOAc (3 × 20 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 33. Flash chromatography (SiO2, CH2Cl2) gave 33 (164 mg, 95%) as an oil: IR (NaCl, neat) ν 3434, 3359, 2944, 2867, 1718, 1477, 1170 cm-1; 1H NMR (400 MHz, CDCl3) δ 1.08 (d, J= 7.6 Hz, 18H), 1.27 (sept, J=7.6, 3H), 1.42 (s, 9H), 2.65 (t, J= 6.4, 2H), 3.36-3.29 (m, 2H), 4.49 (brs, 1H), 4.98 (s, 2H), 6.64 (d, J= 2.0 Hz, 1H), 6.95 (d, J= 2.0 Hz, 1H), 7.40-7.30 (m, 3H), 7.53-7.50 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.0 (CH), 18.0 (CH3), 28.4 (CH3), 74.4 (CH2), 79.2 (C), 35.5 (CH2), 41.6 (CH2), 118.4 (C), 119.9 (CH), 125.3 (CH), 127.8 (CH), 128.0 (CH), 128.1 (CH), 136.0 (C), 137.0 (C), 145.4 (C), 150.2 (C), 155.6 (C); HRMS (FAB) found m/z 600.2131 [M+Na]+, C29H44O4NNaSiBr requires 600.2121.
2-[4-Benzyloxy-3-bromo-5-(triisopropyl-silanyloxy)-phenyl]-ethyl amine (34)
TFA (1.0 mL) was added to a solution of carbamate 33 (142 mg, 0.246 mmol) in CH2Cl2 (1.0 mL). The solution was stirred for 0.5 h at 0 °C, quenched with NaHCO3 (aq., satd.) and extracted with CH2Cl2 (3 × 20 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to yield 34 (112 mg, 95%) as an oil: IR (NaCl, neat) ν 2944, 2867, 1556, 1475 cm-1; 1H NMR (400 MHz, CDCl3) δ 1.08 (d, J= 7.2 Hz, 18H), 1.27 (sept, J= 7.2 Hz, 3H), 2.62 (t, J= 6.8 Hz, 2H), 2.92 (bt, J= 6.8 Hz, 2H), 4.98 (s, 2H), 6.66 (d, J= 2.0 Hz, 1H), 6.96 (d, J= 2.0 Hz, 1H), 7.40-7.25 (m, 3H), 7.55-7.49 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 13.0 (CH), 18.0 (CH3), 39.1 (CH2), 43.3 (CH2), 74.4 (CH2), 118.3 (C), 119.9 (CH), 125.3 (CH), 127.7 (CH), 128.0 (CH), 128.1 (CH), 136.6 (C), 137.1 (C), 145.2 (C), 150.2 (C); HRMS (DCI/NH3) found m/z 478.1759 [M+H]+, C24H37O2NSiBr requires 478.1777.
(2-{2-[4-Benzyloxy-3-bromo-5-(triisopropyl-silanyloxy)-phenyl]-ethylcarbamoyl}-ethyl)-carbamic acid tert- butyl ester (35)
EDCI (88 mg, 0.460 mmol) was added to a solution of amine 34 (110 mg, 0.23 mmol), HOBt (62 mg, 0.46 mmol), and N-Boc-β-alanine (65 mg, 0.35 mmol) in CH2Cl2 (1.0 mL). The solution was stirred overnight at room temperature, quenched with HCl (1N) and extracted with CH2Cl2 (3 × 20 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 34. Flash chromatography (SiO2, CH2Cl2/MeOH, 20:1) gave 34 (138 mg, 92%) as an oil: IR (NaCl, neat) ν 3315, 2945, 2867, 1691, 1558, 1477 cm-1; 1H NMR (400 MHz, CDCl3) δ 1.07 (d, J= 7.2 Hz, 18H), 1.26 (sept, J= 7.2 Hz, 3H), 1.41 (s, 9H), 2.34 (t, J= 6.0 Hz, 2H), 2.67 (t, J= 7.2 Hz, 2H), 3.37 (dt, J= 6.0, 6.0 Hz, 2H), 3.44 (dt, J= 7.2, 6.0 Hz, 2H), 4.99 (s, 2H), 5.16 (bs, 1H), 5.73 (bs, 1H), 6.64 (d, J= 2.0 Hz, 1H), 6.95 (d, J= 2.0 Hz, 1H), 7.40-7.29 (m, 3H), 7.52-7.48 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 12.8 (CH), 17.8 (CH3), 28.3 (CH3), 34.8 (CH2), 36.1 (CH2), 36.5 (CH2), 40.4 (CH2), 74.3 (CH2), 79.2 (C), 118.5 (C), 119.7 (CH), 125.2 (CH), 127.9 (CH), 128.1 (CH), 128.2 (CH), 135.8 (C), 137.0 (C), 145.6 (C), 150.4 (C), 156.1 (C), 171.3 (C); HRMS (FAB) found m/z 671.2512 [M+Na]+, C32H49O5N2NaSiBr requires 671.2492.
3-Amino-N-{2-[4-Benzyloxy-3-bromo-5-(triisopropyl-silanyloxy)-phenyl]-ethyl-propionamide (36)
TFA (1.0 mL) was added to a solution of carbamate 35 (136 mg, 0.209 mmol) in CH2Cl2 (1.0 mL) at room temperature. The solution was stirred for 0.5 h at 0 °C, quenched with NaHCO3 (aq., satd.) and extracted with CH2Cl2 (3 × 20 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to yield 36 (112 mg, 97%) as a oil: IR (NaCl, neat) ν 3315, 2945, 2867, 1691, 1646, 1558, 1477 cm-1; 1H NMR (400 MHz, CDCl3) δ 1.08 (d, J= 7.2 Hz, 18H), 1.27 (sept, J= 7.2 Hz, 3H), 2.29 (bs, 2H), 2.68 (t, J= 6.8 Hz, 2H), 2.97 (bs, 2H), 3.44 (dt, J= 6.8, 6.4 Hz, 2H), 4.99 (s, 2H), 6.65 (d, J= 2.0 Hz, 1H), 6.96 (d, J= 2.0 Hz, 1H), 7.08 (bs, 1H), 7.40-7.28 (m, 3H), 7.52-7.48 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 12.8 (CH), 17.9 (CH3), 28.3 (CH2), 34.9 (CH2), 37.9 (CH2), 40.2 (CH2), 74.4 (CH2), 118.3 (C), 119.8 (CH), 125.4 (CH), 127.9 (CH), 128.1 (CH), 128.2 (CH), 136.2 (C), 137.1 (C), 145.5 (C), 150.4 (C), 172.4 (C); HRMS (FAB) found m/z 549.2142 [M+H]+, C27H42O3N2NaSiBr requires 549.2148.
4-Benzyloxy-5-bromo-21-nitro-2oxa-10,14-diaza-tricyclo[16.2.2.10,0]tricosa-1(21),3(23),4,6,18(22),19-hexaene-11,15-dione (37)
CsF (43 mg, 0.29 mmol) was added to a solution of amide 19 (102 mg, 0.140 mmol) and 4 Å sieves in DMSO (75 mL, 2 mM). The solution was rapidly stirred overnight at room temperature, quenched with water and extracted with CH2Cl2 (5 × 20 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 37. Flash chromatography (SiO2, CH2Cl2/ MeOH, 20:1) gave 37 (65 mg, 84%) as an oil. IR (NaCl, neat) ν 3299, 2931, 1647, 1533 cm-1; 1H NMR (300 MHz, CDCl3) δ 2.12-2.16 (m, 2H), 2.33-2.40 (m, 2H), 2.63-2.69 (m, 2H), 3.05-3.08 (m, 2H), 3.28-3.37 (m, 2H), 3.46-3.52 (m, 2H), 5.14 (s, 2H), 5.36 (brs, 1H), 5.96 (d, J= 1.8 Hz, 1H), 6.22 (brs, 1H), 7.04 (d, J= 1.8 Hz, 1H), 7.07 (d, J= 8.4 Hz, 1H), 7.30-7.38 (m, 3H), 7.45 (dd, J= 8.4 Hz, 2.1 Hz), 7.54-7.58 (m, 2H), 7.82 (d, J= 2.1 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 31.2 (CH2), 33.6 (CH2), 33.8 (CH2), 35.3 (CH2), 38.9 (CH2), 40.8 (CH2), 75.3 (CH2), 115.0 (CH), 118.9 (C), 124.6 (CH), 125.8 (CH), 127.1 (CH), 128.2 (CH), 128.4 (CH), 128.6 (CH), 135.2 (CH), 136.6 (C), 136.7 (C), 139.6 (C), 142.6 (C), 143.8 (C), 146.0 (C), 152.0 (C), 170.8 (C), 172.0 (C); HRMS (FAB) found m/z 568.1061 [M+H]+, C27H27N3O6Br requires 568.1083.
21-Amino-4-benzyloxy-5-bromo-2-oxa-10,14-diaza-tricyclo[16.2.2.10,0]-tricosa-1(21),3(23),4,6,18(22),19-hexaene-11,15-dione (38)
CrCl2 (80 mg, 0.65 mmol) was added to a solution of lactam 37 (40 mg, 0.07 mmol) in DMF (1 mL) at room temperature. After 12 h of stirring, the DMF was removed under reduced pressure followed by dissolving the residue in EtOAc (10 mL). The organic solution was washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 38. Flash chromatography (SiO2, CH2Cl2/ MeOH, 9:1) gave 38 (21 mg, 55%) as a yellow viscous oil: IR (NaCl, neat) ν 3320, 2928, 1644, 1479, 1196 cm-1; 1H NMR (400 MHz, CD3OD) δ 2.10-2.14 (m, 2H), 2.38-2.63 (m, 2H), 2.60-2.63 (m, 2H), 2.84-2.87 (m, 2H), 3.25-3.27 (m, 2H), 3.40-3.43 (m, 2H), 5.16 (s, 2H), 6.21 (d, J= 2.0 Hz, 1H), 6.60 (dd, J= 8.0, 2.0 Hz, 1H), 6.75 (d, J= 8.0, 2.0 Hz, 1H), 6.75 (d, J= 2.0 Hz, 1H), 6.83 (d, J= 8.0 Hz, 1H), 7.11 (d, J= 2.0 Hz, 1H), 7.30-7.39 (m, 3H), 7.55-7.58 (m, 2H), 7.62 (bt, J= 6.0 Hz, 1H), 7.74 (bt, J= 6.0 Hz, 1H); 13C NMR (100 MHz, CD3OD) δ 32.8 (CH2), 34.5 (CH2), 35.2 (CH2), 36.3 (CH2), 39.5 (CH2), 42.1 (CH2), 76.4 (CH2), 117.2 (CH), 119.0 (CH), 119.1 (C), 120.8 (CH), 123.0 (CH), 127.2 (CH), 129.3 (CH), 129.4 (CH), 129.8 (CH), 138.4 (C), 139.1 (C), 140.1 (C), 140.2 (C), 141.8 (C), 144.7 (C), 153.4 (C), 173.8 (C), 174.9 (C); HRMS (DCI/NH3) found m/z 538.1330 [M+H]+, C27H29O4N3Br requires 466.0865.
4-Benzyloxy-5,21-dibromo-2-oxa-10,14-diaza-tricyclo[16.2.2.10,0]tricosa-1(21),3(23),4,6,18(22),19-hexaene-11,15-dione (39)
tert-Butyl nitrite (10 μL, 9 μmol) was added to a solution of CuBr2 (2.5 mg, 10 μmol) in CH3CN (0.5 mL) at 0 °C and allowed to stir for 1h. A mixture of lactam 38 (10 mg, 0.02 mmol) in CH3CN (0.8 mL) was then added drop-wise into the above solution over 20 min at 0 °C. After stirring for 2 h, the solution was allowed to warm to room temperature, quenched with HCl (1 N, 4 mL) and extracted with CH2Cl2 (4 × 5 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 39. Flash chromatography (SiO2, CH2Cl2/MeOH, 9:1) followed by reversed phase HPLC (C18, 5μm Microsorb, 10 × 250 mm, MeOH/H2O, 65:35, 3 mL/min) gave 39 (2.7 mg, 21%) as a colorless amorphous solid: 1H NMR (400 MHz, CD3OD) δ 2.15-2.13 (m, 2H), 2.45-2.41 (m, 2H), 2.66-2.63 (m, 2H), 2.98-2.95 (m, 2H), 3.27-3.23 (m, 2H), 3.44-3.36 (m, 2H), 5.18 (s, 2H), 6.05 (d, J= 1.6 Hz, 1H), 7.08 (d, J= 8.4 Hz, 1H), 7.15 (d, J= 1.6 Hz, 1H), 7.24 (dd, J= 8.4, 1.6 Hz, 1H), 7.32-7.40 (m, 3H), 7.54-7.57 (m, 3H); HRMS (FAB) found m/z 601.0312 [M+H]+, C27H27O4N2Br2 requires 601.0338.
4-Benzyloxy-5,20,22-tribromo-2-oxa-10,14-diaza-tricyclo[16.2.2.10,0]tricosa-1(21),3(23),4,6,18(22),19-hexaene-11,15-dione (40)
tert-Butyl nitrite (3.6 μL, 0.04 mmol) was added to a solution of CuBr2 (14 mg, 0.06 mmol) in CH3CN (0.5 mL) at 0 °C and allowed to stir for 1h. A mixture of lactam 38 (11 mg, 0.02 mmol) in CH3CN (0.5 mL) was added drop-wise into the above solution over 20 min at 0 °C. After stirring for 2 h, the solution was warmed to room temperature, quenched with HCl (1 N, 4 mL) and extracted with CH2Cl2 (4 × 5 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 40. Flash chromatography (SiO2, CH2Cl2/MeOH, 9:1) followed by reversed phase HPLC (C18, 5μm Microsorb, 10 × 250 mm, MeOH/H2O, 70:30, 3 mL/min) gave 40 (4 mg, 29%) as a colorless amorphous solid: IR (neat) ν 3307, 2925, 1648 cm-1; 1H NMR (400 MHz, CDCl3) δ 1.94-2.02 (m, 1H), 2.09-2.16, (m, 1H), 2.31-2.36 (m, 1H), 2.55-2.63 (m, 2H), 2.73-2.81 (m, 2H), 3.08-3.19 (m, 2H), 3.81-3.86 (m, 1H), 3.96-4.02 (m, 3H), 5.23 (d, J= 10.8 Hz, 1H), 5.24 (bs, 1H), 5.28 (d, J= 10.8 Hz, 1H), 5.85 (d, J= 2.0 Hz, 1H), 6.18 (bd, J= 6.8 hz, 1H), 7.03 (d, J= 2.0 Hz, 1H), 7.32-7.40 (m, 3H), 7.34 (s, 1H), 7.56-7.59 (m, 2H), 7.58 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 31.4 (CH2), 33.7 (CH2), 33.9 (CH2), 35.6 (CH2), 36.9 (CH2), 41.5 (CH2), 75.1 (CH2), 114.6 (CH), 115.1 (C), 119.0 (C), 123.2 (C), 126.4 (CH), 128.3 (CH), 128.4 (CH), 128.6 (2 × CH), 135.6 (CH), 136.5 (C), 136.8 (C), 139.6 (C), 143.4 (C), 149.9 (C), 151.7 (C), 171.1 (C), 171.9 (C); LRMS (ESI) found m/z 701.1 [M+Na]+, C27H25N2O4Br3 requires 700.9.
S-(–)-(6-{2-[4-Benzyloxy-3-bromo-5-(triisopropyl-silanyloxy)-phenyl]-ethylcarbamoyl}-5-tert butoxycarbonylamino-hexyl)-carbamic acid 9H-fluoren-9-ylmethyl ester (42)
A stirred solution of amine 34 (102 mg, 0.213 mmol) and acid 4136 (108 mg, 0.224 mmol) in CH2Cl2 (2 mL) was treated with HOBt (58 mg, 0.426 mmol) and EDCI (81 mg, 0.426 mmol) at room temperature. After 3 h, the reaction was quenched with HCl (0.5 N), extracted with EtOAc (3 × 10 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 42. Flash chromatography (SiO2, CH2Cl2/MeOH 5%) gave 42 (163 mg, 81%) as an oil: [α]25D –3.1° (c 0.75, CHCl3); IR (neat) ν 3313, 2944, 2867, 1687, 1641, 1538cm-1; 1H NMR (400 MHz, CDCl3) δ 1.08 (d, J= 7.2 Hz, 18H), 1.20-1.34 (m, 4H), 1.41(s, 9H), 2.31-2.43 (m, 2H), 2.66 (dd, J= 6.8 Hz, 2H), 2.85-3.15 (m, 2H), 3.29-3.49 (m, 2H), 3.80-3.90 (m, 1H), 4.19 (dd, J= 7.2 Hz, 1H), 4.35 (d, J=8.4 Hz, 2H), 4.65 (bs, 1H), 4.99 (s, 2H), 5.87 (bd, J= 8.0 Hz, 1H), 6.18 (bs, 1H), 6.65 (d, J=1.6 Hz, 1H), 6.94 (d, J= 1.6 Hz, 1H), 7.28-7.37 (m, 8H), 7.51 (d, J= 7.2 Hz, 4H), 7.58 (d, J= 7.2 Hz, 4H), 7.73 (d, J= 7.2 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ 12.8 (CH), 17.8 (CH3), 23.1 (CH2), 28.3 (CH2), 29.6 (CH2), 33.7(C), 34.8 (CH2), 39.9 (CH2), 40.4 (CH2), 40.5 (CH2), 47.1 (CH), 48.6 (CH), 66.5 (CH2), 74.3 (CH2), 79.0 (C) , 118.4 (C), 119.7 (CH), 119.9 (CH), 125.0 (CH), 125.3 (CH), 126.9 (CH), 127.6 (CH), 127.8 (CH), 128.1 (CH), 135.8 (C), 137.1 (C), 141.2 (C), 143.9 (C), 143.9 (C), 145.6 (C), 150.4 (C), 156.1 (C), 156.1 (C), 170.9 (C); HRMS (MALDI) found m/z 964.3905 [M+Na]+, C51H68N3O7BrSiNa requires 964.3902.
S-(–)-{6-{2-[4-benzyloxy-3-bromo-5-(triisopropyl-silanyloxy)-phenyl]-ethylcarbamoyl}-5-{3-(4-fluoro-3-nitro-phenyl)-propionylamino]-hexyl}-carbamic acid tert-butyl ester (44)
A stirred solution of amine 42 (72 mg, 0.076 mmol) in CH2Cl2 (1 mL) was treated with tris-(2-amino-ethyl)-amine (TAEA) (0.57 mL, 3.81 mmol) at room temperature. After 5 minutes, the reaction was quenched with NaCl (sat.) and extracted with CH2Cl2 (10 mL). The organic layers were combined, washed with brine (3 × 20 mL), phosphate buffer (pH= 5.5) (3 × 20mL), dried (Na2SO4), and the volatiles were removed to give crude amine 43. The crude amine 43 was carried forward without further purification. A stirred solution of amine 43 (55 mg, 0.076 mmol), and acid 17 (96 mg, 0.45 mmol) in CH2Cl2 (3 mL) was treated with HOBt (120 mg, 0.92 mmol) and EDCI (180 mg, 0.92 mmol) at room temperature. After 6 h, the reaction was quenched with HCl (0.5 N) and extracted with CH2Cl2 (3 × 10 mL). The organic layers were combined, washed with KOH (0.5 N) (3 × 30 mL) and brine, dried (Na2SO4), and the volatiles were removed to give crude amide 44. Flash chromatography (SiO2, CH2Cl2/EtOAc 20% then CH2Cl2/MeOH 5%) gave 44 (60 mg, 86%) as an amorphous solid: [α]25D –6.1° (c 0.58, CHCl3); IR (neat) ν 3295, 2942, 2867, 1697, 1646, 1538 cm-1; 1H NMR (400 MHz, CDCl3) δ 1.06 (d, J= 7.6 Hz, 18H), 1.21-1.30 (m, 3H), 1.40 (s, 9H), 2.21-2.35 (m, 2H), 2.48 (t, J= 7.2 Hz, 2H), 2.67 (t, J= 7.2 Hz, 2H), 2.98 (t, J= 7.2 Hz, 2H), 3.00-3.07 (m, 2H), 3.41 (q, J= 6.0 Hz, 2H), 4.00-4.10 (m, 1H), 4.58-4.62 (m, 1H), 4.97 (s, 2H), 6.25 (bs, 1H), 6.64 (d, J= 2.0 Hz, 1H), 6.95 (d, J= 2.0 Hz, 1H), 6.98 (bd, J= 7.6 Hz, 1H), 7.15 (dd, J= 8.4, 10.4 Hz, 1H), 7.37-7.28 (m, 3H), 7.44-7.51 (m, 3H), 7.87 (dd, J= 7.2, 2.0 Hz, 1Hz); 13C NMR (100 MHz, CDCl3) δ 12.9 (CH), 17.9 (CH3), 23.1 (CH2), 28.4 (CH3), 29.6 (CH2), 30.2 (CH2), 33.3 (CH2), 34.8 (CH2), 37.4 (CH2), 39.4 (CH2), 39.8 (CH2), 40.4 (CH2), 46.6 (CH), 74.4 (CH), 79.2 (C), 118.2 (d, J= 20.5, CH) ; 118.5 (C), 119.8 (CH), 125.3 (CH), 125.5 (d, J= 2.3 Hz, CH), 127.9 (CH), 128.2 (CH), 128.2 (CH), 135.6 (d, J= 8.4 Hz, CH), 135.7 (CH), 137.1 (d, J= 8.0 Hz, C), 137.1 (C), 138.0 (d, J= 4.5 Hz, C), 145.7 (C), 150.5 (C), 154.0 (d, J= 261 Hz, C), 156.3 (C), 170.6 (C), 171.3 (C); HRMS (MALDI) found m/z 937.3572 [M+Na]+, C45H64N4O8BrSiNa requires 937.3553.
S-(–)-[4-(4-Benzyloxy-5-bromo-21-nitro-11,15-dioxo-2-oxa-10,14-diaza-tricyclo[16.2.2.10,0tricosa-1(21), 3(23), 4, 6, 18(22), 19-hexaen-13-yl)-butyl]-carbamic acid tert-butyl ester (45)
A stirred solution of amide 44 (52 mg, 0.057 mmol) in DMSO (30 mL, 2 mM) containing 4 Å sieves was treated with CsF (86 mg, 0.57 mmol) at room temperature. After 3 h of rapid stirring, the reaction mixture was diluted with water (250 mL) and extracted with CH2Cl2 (5 × 20 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude amide 45. Flash chromatography (SiO2, 9.4:0.6 CH2Cl2/MeOH) gave 45 (31 mg, 75%) as an viscous oil: [α]25D –65.0° (c 0.600, CHCl3); IR (KBr, neat) ν 3301, 2929, 1683, 1644, 1531, 1284, 1236 cm-1; 1H NMR (400 MHz, CDCl3) δ 1.14-1.38 (m, 6H), 1.40 (s, 9H), 1.50-1.56 (m, 1H), 1.94-2.18 (m, 3H), 2.56-2.67 (m, 2H), 2.87-3.05 (m, 5H), 3.15-3.23 (m, 1H), 3.74-3.79 (m, 1H), 4.02-4.16 (m, 1H), 4.50-4.58 (m, 1H), 5.09-5.15 (m, 2H), 5.54-5.62 (m, 1H), 6.21 (s, 1H), 6.83 (bd, J= 7.2 Hz, 1H), 7.00 (d, J= 8.4 Hz, 1H), 7.05 (d, J= 2.0 Hz, 1H), 7.29-7.36 (m, 3H), 7.52-7.54 (m, 2H), 7.80 (d, J= 2.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 23.5 (CH2), 28.4 (CH2), 29.6 (CH2), 31.6 (CH2), 32.7 (CH2), 32.8 (CH2), 38.1 (CH2), 39.3 (CH2), 39.4 (CH2), 40.1 (CH2), 45.3 (CH), 75.4 (CH2), 79.1 (C), 115.5 (CH), 118.6 (C), 124.1 (C), 125.7 (CH), 127.6 (CH), 128.2 (CH), 128.3 (CH), 128.6 (CH), 135.1 (CH), 136.3 (C), 136.7 (C), 138.9 (C), 142.4 (C), 144.3 (C), 146.6 (C), 151.8 (C), 156.1 (C), 170.5 (C), 171.5 (C); HRMS (MALDI) found m/z 761.2150 [M+Na]+, C36H43N4O8BrNa requires 761.2157.
S-[4-(20-Amino-4-benzyloxy-5-bromo-11,15-dioxo-2-oxa-10,14-diaza-tricyclo[16.2.2.10,0]tricosa-1(21),3(23),4,6,18(22),19-hexaen-13-yl)-butyl]-carbamic acid tert-butyl ester (46)
CrCl2 (140 mg, 1.139 mmol) was added to a solution of lactam 45 (50 mg, 0.066 mmol) in DMF (1 mL) at room temperature. After 12 h of stirring, the DMF was removed under reduced pressure and the residue dissolved in EtOAc (10 mL). The organic solution was washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 46. Flash chromatography (SiO2, CH2Cl2/ MeOH, 9:1) gave 46 (40 mg, 85%) as a yellow viscous oil: IR (NaCl, neat) ν 3289, 2929, 1643, 1509, 1278 cm-1; 1H NMR (400 MHz, CD3OD/CDCl3, 1:1) δ 1.25-1.5 (m, 6H), 1.38 (s, 9H), 1.90-2.0 (m, 2H), 2.1-2.3 (m, 1H), 2.50-2.63 (m, 3H), 2.70-3.13 (m, 8H), 3.38-3.49 (m, 1H), 3.88-4.03 (m, 1H), 5.10 (s, 2H), 6.32 (s, 1H), 6.56 (d, J= 7.2 Hz, 1H), 6.65 (s, 1H), 6.81 (d, J= 7.2 Hz, 1H), 7.03 (s, 1H), 7.25-7.35 (m, 3H), 7.40-7.55 (m, 3H); 13C NMR (100 MHz, CD3OD/CDCl3, 1:1) δ 23.7 (CH2), 28.2 (CH3), 29.5 (CH2), 32.0 (CH2), 32.9 (CH2), 33.0 (CH2), 38.5 (CH2), 38.9 (CH2), 39.6 (CH2), 40.2 (CH2), 45.9 (CH), 75.7 (CH2), 79.1 (C), 116.5 (CH), 118,1 (CH), 120.4 (C), 122.1 (CH), 126.8 (CH), 128.5 (CH), 128.9 (CH), 136.8 (C), 137.3 (C), 137.8 (C), 138.3 (C), 141.6 (C), 144.0 (C), 152.0 (C), 157.2 (C), 172.3 (C), 172.9 (C); HRMS (MALDI) found m/z 731.2386 [M+Na]+, C36H45O6N4BrNa requires 731.2415.
S-(+)-[4-(4-Benzyloxy-5,20-dibromo-11,15-dioxo-2-oxa-10,14-diaza-tricyclo[16.2.2.10,0]tricosa-1(21),3(23),4,6,18(22),19-hexaen-13-yl)-butyl]-carbamic acid tert-butyl ester (47)
tBuONO (2.5 μL, 0.025 mmol) was added to a stirred solution of CuBr2 (5.6 mg, 0.01 mmol) in CH3CN (0.1 mL) at 0 °C. After 1 h, a mixture of lactam 46 (12 mg, 0.02 mmol) in CH3CN (0.8 mL) was added drop-wise into the above solution over 10 min at 0 °C. After stirring for 2 h, the mixture was allowed to warm to room temperature, quenched with HCl (1 N, 1 mL) and extracted with CH2Cl2 (4 × 5 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude lactam 47 and 50. Flash chromatography (SiO2, CH2Cl2/MeOH, 9:1) followed by silca HPLC (SiO2, 5μm Microsorb, 10 × 250 mm, CH2Cl2/MeOH, 99:1.5, 3 mL/min) gave 47 (4.8 mg, 38%) as a colorless amorphous solid and 50 (5.4 mg, 42%) as a colorless amorphous solid. 47: [α]D25 +69.4° (c 0.320, CHCl3); IR (KBr) ν 3303, 2927, 1681, 1644, 1527, 1488, 1280 cm-1; 1H NMR (400 MHz, CDCl3) δ 1.41 (s, 9H), 1.18-1.68 (m, 5H), 2.00-2.18 (m, 3H), 2.53-3.20 (m, 9H), 3.68-3.79 (m, 1H), 4.12-4.24 (m, 1H), 4.46-4.58 (m, 1H), 5.16 (d, J= 10.8 Hz, 1H), 5.25 (d, J= 10.8 Hz, 1H), 5.34 (bs, 1H), 5.99 (bs, 1H), 6.85 (bd, J=10 Hz, 1H), 7.01 (d, J= 8.4 Hz, 1H), 7.01 (d, J=2.0 Hz, 1H), 7.17 (dd, J= 8.4, 2.0 Hz, 1H), 7.29-7.38 (m, 3H); 7.48 (d, J= 2.0 Hz,1H), 7.58-7.59 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 23.6 (CH2), 28.4 (CH3), 29.6 (CH2), 31.7 (CH2), 32.7 (CH2), 32.9 (CH2), 38.2 (CH2), 39.7 (CH2), 40.2 (CH2), 40.5 (CH2), 45.2 (CH), 75.2 (CH2), 79.0 (C), 115.1 (CH), 116.2 (C), 118.6 (C), 123.7 (CH), 126.2 (CH), 128.2 (CH), 128.4 (CH), 128.7 (CH), 129.6 (CH), 134.2 (CH), 136.1 (C), 136.9 (C), 140.1 (C), 143.6 (C), 150.0 (C), 152.0 (C), 156.1 (C), 171.7 (C); HRMS (MALDI) found m/z 794.1425 [M+Na]+, C36H43N3O6Br2Na requires 794.1441.
S-(+)-[4-(4-Benzyloxy-5,20,22-tribromo-11,15-dioxo-2-oxa-10,14-diaza-tricyclo[16.2.2.10,0]tricosa-1(21),3(23),4,6,18(22),19-hexaen-13-yl)-butyl]-carbamic acid tert-butyl ester (50)
[α]D25 +38.3° (c 0.360, CHCl3); IR (KBr) ν 3334, 2925, 1697, 1652, 1513, 1247 cm-1; 1H NMR (400 MHz, CDCl3) δ 1.41 (s, 9H), 1.18-1.68 (m, 7H), 2.00-2.22 (m, 3H), 2.50-2.66 (m, 2H), 2.78-2.88 (m, 1H), 2.96-3.24 (m, 4H), 3.78-3.86 (m, 1H), 4.18-4.28 (m, 1H), 4.48-4.60 (m, 1H), 5.11 (d, J= 10.4 Hz, 1H), 5.28 (d, J= 10.4 Hz, 1H), 5.28 (bs, 1H), 5.95 (d, J= 2.0 Hz, 1H), 6.87 (bd, J= 9.6 Hz, 1H), 7.03 (d, J= 2.0 Hz, 1H), 7.32-7.39 (m, 3H); 7.36 (s,1H), 7.52 (s, 1H), 7.56-7.59 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 23.7 (CH2), 28.4 (CH3), 29.7 (CH2), 31.9 (CH2), 32.7 (CH2), 32.9 (CH2), 37.3 (CH2), 38.3 (CH2), 40.8 (CH2), 45.2 (CH), 75.2 (CH2), 79.0 (C), 114.5 (CH), 114.9 (C), 118.8 (C), 123.0 (C), 126.5 (CH), 128.2 (CH), 128.4 (CH), 128.7 (CH), 128.7 (CH), 135.4 (CH), 136.1 (C), 136.9 (C), 139.4 (C), 143.4 (C), 150.0 (C), 151.9 (C), 156.0 (C), 171.8 (C); HRMS (MALDI) found m/z 872.0502 [M+Na]+, C36H42N3O6Br3Na requires 872.0516.
S-2-Azido-N-[4-(5,20-dibromo-4-hydroxy-11,15-dioxo-2-oxa-10,14-diaza-tricyclo[16.2.2.10,0]tricosa-1(21),3(23),4,6,18(22),19-hexaen-13-yl)-butyl]-5-iodo-benzamide (49)
BBr3 (1 M, 10 μL, 10 μmol) was added to a solution of lactam 47 (3 mg, 3.8 μmol) in CH2Cl2 (50 μL) and the orange solution was allowed to stir overnight at room temperature. The solution was directly transferred onto a flash column (SiO2, CH2Cl2/MeOH/NH2OH, 10:1:0.1) to give the crude amine 48 (2.2 mg) as an amorphous solid that was immediately carried forward. EDCI (2 mg, 10 μmol) was added to a stirred solution of the crude amine (2.2 mg), HOBt (1.5 mg, 10 μmol), and 2-azido-5-iodo-benzoic acid37 (3 mg, 10 μmol) in DMF (5.0 mL) at room temperature. After 5 h, the reaction was quenched with HCl (1N) and extracted with CH2Cl2 (3 × 5 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 49. Flash chromatography (SiO2, CH2Cl2/MeOH, 20:1) followed by reversed phase HPLC (C18, MeOH/H2O, 3:1, 4 mL/min) gave pure 49 (0.56 mg, 33% over two steps) as a colorless amorphous solid. 1H NMR (400 MHz, CDCl3) δ 1.20-1.60 (m, 7H), 1.94-2.18 (m, 3H), 2.48-2.64 (m, 2H), 2.76-3.00 (m, 3H), 3.04-3.14 (m, 1H), 3.22-3.40 (m, 2H), 3.61-3.74 (m, 1H), 4.06-4.19 (m, 1H), 5.41 (bs, 1H), 5.98 (s, 1H), 6.06 (d, J= 2.0 Hz, 1H), 6.92 (d, J= 8.4 Hz, 1H), 6.96 (d, J= 2.0 Hz, 1H), 7.09 (d, J= 8.0 Hz, 1H), 7.19 (dd, J= 8.0, 2.0 Hz, 1H), 7.33 (bs, 1H), 7.75 (dd, J= 8.4, 2.0 Hz, 1H), 8.37 (d, J= 2.0 Hz, 1H); HRMS (MALDI) found m/z 874.9709 [M+Na]+, C31H31N6O5Br2NaI requires 874.9660.
S-2-Azido-5-iodo-N-[4-(5,20,22-tribromo-4-hydroxy-11,15-dioxo-2-oxa-10,14-diaza-tricyclo[16.2.2.10,0]tricosa-1(21),3(23),4,6,18(22),19-hexaen-13-yl)-butyl]-benzamide (52)
BBr3 (1 M, 10 μL, 10 μmol) was added to a stirred solution of lactam 50 (2 mg, 2.4 μmol) in CH2Cl2 (50 μL) at room temperature. The solution was directly transferred onto a flash column (SiO2, CH2Cl2/MeOH/NH2OH, 10:1:0.1) to give crude amine 51 (1.8 mg) as an amorphous solid that was immediately carried forward. EDCI (2 mg, 10 μmol) was added to a stirred solution of crude amine 51 (2.2 mg), HOBt (1.5 mg, 10 μmol), and 2-azido-5-iodo-benzoic acid37 (3 mg, 10 μmol) in DMF (5.0 mL) at room temperature. After 5 h, the reaction was quenched with HCl (1N) and extracted with CH2Cl2 (3 × 5 mL). The organic layers were combined, washed with brine, dried (Na2SO4), and the volatiles were removed to give crude 52. Flash chromatography (SiO2, CH2Cl2/MeOH, 20:1) followed by reversed phase HPLC (C18, MeOH/H2O, 3:1, 4 mL/min) gave 52 (0.80 mg, 48% over two steps) as a colorless amorphous solid. 1H NMR (400 MHz, CDCl3) δ 1.20-1.60 (m, 4H), 1.91-2.24 (m, 5H), 2.48-2.62 (m, 2H), 2.91-3.22 (m, 4H), 3.28-3.48 (m, 2H), 3.74-3.84 (m, 1H), 4.12-4.24 (m, 1H), 5.24 (bt, J= 5.6 Hz, 1H), 5.93 (s, 1H), 6.02 (d, J= 2.0 Hz, 1H), 6.92 (d, J= 8.4 Hz, 1H), 6.96 (dd, J= 8.4, 2.0 Hz, 1H), 6.99 (d, J= 2.0 Hz, 1H), 7.40 (bt, J= 5.6 Hz, 1H), 7.46 (s, 1H), 7.48 (s, 1H), 7.75 (dd, J= 8.4, 2.0 Hz, 1H), 8.37 (d, J= 2.0 Hz, 1H); HRMS (MALDI) found m/z 952.8767 [M+Na]+, C31H30N6O5Br3NaI requires 952.8765.
References
- 1.Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu. Rev. Immunol. 1996;14:483–510. doi: 10.1146/annurev.immunol.14.1.483. [DOI] [PubMed] [Google Scholar]
- 2.Marks AR. Cellular functions of immunophilins. Physiol. Rev. 1996;76:631–649. doi: 10.1152/physrev.1996.76.3.631. [DOI] [PubMed] [Google Scholar]
- 3.Snyder SH, Sabatini DM, Lai MM, Steiner JP, Hamilton GS, et al. Neural actions of immunophilin ligands. Trends Pharmacol. Sci. 1998;19:21–26. doi: 10.1016/s0165-6147(97)01146-2. [DOI] [PubMed] [Google Scholar]
- 4.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 5.Lorenz MC, Heitman J. TOR mutations confer rapamycin resistance by preventing interaction with FKBP12-rapamycin. J. Biol. Chem. 1995;270:27531–27537. doi: 10.1074/jbc.270.46.27531. [DOI] [PubMed] [Google Scholar]
- 6.Griffith JP, Kim JL, Kim EE, Sintchak MD, Thomson JA, et al. X-ray structure of calcineurin inhibited by the immunophilin-immunosuppressant FKBP12-FK506 complex. Cell. 1995;82:507–522. doi: 10.1016/0092-8674(95)90439-5. [DOI] [PubMed] [Google Scholar]
- 7.Choi J, Chen J, Schreiber SL, Clardy J. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science. 1996;273:239–242. doi: 10.1126/science.273.5272.239. [DOI] [PubMed] [Google Scholar]
- 8.Brunn GJ, Hudson CC, Sekulic A, Williams JM, Hosoi H, et al. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277:99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- 9.Pessah IN. Common immunophilin mechanism for noncoplanar PCBs and naturally occurring bromotyrosines from Ianthella basta. Organohalogen Compd. 1998;37:13–17. [Google Scholar]
- 10.Pessah IN, Molinski TF, Meloy TD, Wong P, Buck ED, et al. Bastadins relate Ryanodine-sensitive and -insensitive Ca2+ efflux pathways in Skeletal SR and BC(3)H1 cells. Amer. J. Physiol. (Cell Physiol.) 1997;41:C 601–C 614. doi: 10.1152/ajpcell.1997.272.2.C601. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Molinski TF, Pessah IN. Bastadin 10 stabilizes the open conformation of the Ryanodine-sensitive Ca2+ channel in an FKBP12-dependent manner. J. Biol. Chem. 1999;274:32603–32612. doi: 10.1074/jbc.274.46.32603. [DOI] [PubMed] [Google Scholar]
- 12.Franklin MA, Penn SG, Lebrilla CB, Lam TH, Pessah IN, et al. Bastadin 20 and Bastadin O-Sulfate Esters from Ianthella basta: Novel Modulators of the RyR1 FKBP12 Receptor Complex. J. Nat. Prod. 1996;59:1121–1127. doi: 10.1021/np960507g. [DOI] [PubMed] [Google Scholar]
- 13.Mack MM, Molinski TF, Buck ED, Pessah IN. Novel modulators of skeletal muscle FKBP12/calcium channel complex from Ianthella basta. Role of FKBP12 in channel gating. J. Biol. Chem. 1994;269:23236–23249. [PubMed] [Google Scholar]
- 14.Park SK, Jurek J, Carney JR, Scheuer PJ. Two more bastadins, 16 and 17, from an Indonesian sponge Ianthella basta. J. Nat. Prod. 1994;57:407–410. [Google Scholar]
- 15.Jaspars M, Rali T, Laney M, Schatzman RC, Diaz MC, et al. The search for inosine 5'-phosphate dehydrogenase (IMPDH) inhibitors from marine sponges. Evaluation of the bastadin alkaloids. Tetrahedron. 1994;50:7367–7374. [Google Scholar]
- 16.Gulavita NK, Wright AE, McCarthy PJ, Pomponi SA, Kelly-Borges M, et al. Isolation and structure elucidation of 34-sulfatobastadin 13, an inhibitor of the endothelin A receptor, from a marine sponge of the genus Ianthella. J. Nat. Prod. 1993;56:1613–1617. doi: 10.1021/np50099a026. [DOI] [PubMed] [Google Scholar]
- 17.Dexture AF, Garson MJ, Hemling ME. Isolation of a novel bastadin from the temperate marine Sponge Ianthella sp. J. Nat. Prod. 1993;56:782–786. [Google Scholar]
- 18.Butler MS, Lim TK, Capon RJ, Hammond LS. The bastadins revisited: new chemistry from the Australian marine sponge Ianthella basta. Aust. J. Chem. 1991;44:287–296. [Google Scholar]
- 19.Carney JR, Scheuer PJ, Kelly-Borges M. A new bastadin from the sponge Psammaplysilla purpurea. J. Nat. Prod. 1993;56:153–157. doi: 10.1021/np50091a025. [DOI] [PubMed] [Google Scholar]
- 20.Pordesimo EO, Schmitz FJ. New bastadins from the sponge Ianthella basta. J. Org. Chem. 1990;55:4704–4709. [Google Scholar]
- 21.Miao S, Andersen RJ, Allen TM. Cytotoxic metabolites from the sponge Ianthella basta collected in Papua New Guinea. J. Nat. Prod. 1990;53:1441–1446. doi: 10.1021/np50072a007. [DOI] [PubMed] [Google Scholar]
- 22.Kazlauskas R, Lidgard RO, Murphy PT, Wells RJ. Brominated tyrosine-derivated metabolites from the sponge Ianthella basta. Tetrahedron Lett. 1980;21:2277–2280. [Google Scholar]
- 23.Coll John C, Kearns Philip S, Rideout John A, Sankar V. Bastadin 21, a novel isobastarane metabolite from the Great Barrier Reef marine sponge Ianthella quadrangulata. J. Nat. Prod. 2002;65:753–756. doi: 10.1021/np010520n. [DOI] [PubMed] [Google Scholar]
- 24.Kazlauskas R, Lidgard RO, Murphy PT, Wells RJ, Blount JF. Brominated tyrosine-derived metabolites from the sponge Ianthella basta. Aust. J. Chem. 1981;34:765–786. [Google Scholar]
- 25.Bigot A, Zhu J. Synthesis of cycloisodityrosine revisited: A selective ring forming process. Tetrahedron Lett. 1998;39:551–554. [Google Scholar]
- 26.Bigot A, Dau METH, Zhu J. Total Synthesis of an Antitumor Agent RA-VII via an Efficient Preparation of Cycloisodityrosine. J. Org. Chem. 1999;64:6283–6296. [Google Scholar]
- 27.Masuno MN, Molinski TF. Regioselective cationic reduction of 2-aryl-1-N-(ethoxycarbonyl)enamines to 2-arylethylamine carbamates. Tetrahedron Lett. 2001;42:8263–8266. [Google Scholar]
- 28.Masuno MN, Molinski TF. Cationic Reduction of Bastadin-4 to Bastadin-5. Preparation of 5-[2H]-Bastadin-5 by Site-Specific Isotopic Labeling. J. Nat. Prod. 2003;66:112–114. doi: 10.1021/np020382h. [DOI] [PubMed] [Google Scholar]
- 29.Wymann WE, Davis R, Patterson JW, Jr., Pfister JR. Selective alkylations of certain phenolic and enolic functions with lithium carbonate-alkyl halide. Synth. Commun. 1988;18:1379–1384. [Google Scholar]
- 30.Jessup PJ, Petty CB, Roos J, Overman LE. 1-N-Acylamino-1,3-dienes from 2,4-pentadienoic acids by the Curtius rearrangement: benzyl trans-1,3-butadiene-1-carbamate. Org. Synth. 1980;59:1–9. [Google Scholar]
- 31.Park H, Cao B, Joullie MM. Regioselective asymmetric aminohydroxylation approach to a β-hydroxyphenylalanine derivative for the synthesis of ustiloxin D. J. Org. Chem. 2001;66:7223–7226. doi: 10.1021/jo010482c. [DOI] [PubMed] [Google Scholar]
- 32.Harmon RE, Parsons JL, Cooke DW, Gupta SK, Schoolenberg J. Homogeneous catalytic hydrogenation of unsaturated organic compounds. J. Org. Chem. 1969;34:3684–3685. [Google Scholar]
- 33.Hosoya T, Aoyama H, Ikemoto T, Hiramatsu T, Kihara Y, et al. [125I]-N-[(3-Azido-5-iodo)benzyl]dantrolene and [125I]-N-{[3-Iodo-5-(3-trifluoromethyl-3H-diazirin-3-yl)]benzyl}dantrolene: photoaffinity probes specific for the physiological Ca2+ release from sarcoplasmic reticulum of skeletal muscle. Bioorg. Med. Chem. Lett. 2002:3263–3265. doi: 10.1016/s0960-894x(02)00701-1. [DOI] [PubMed] [Google Scholar]
- 34.a Doyle MP, Siegfried B, Dellaria JF., Jr Alkyl nitrite-metal halide deamination reactions. 2. Substitutive deamination of arylamines by alkyl nitrites and copper(II) halides. A direct and remarkably efficient conversion of arylamines to aryl halides. J. Org. Chem. 1977;42:2426–2431. [Google Scholar]; b Doyle MP, Dellaria JF, Jr., Siegfried B, Bishop SW. Reductive deamination of arylamines by alkyl nitrites in N,N-dimethylformamide. A direct conversion of arylamines to aromatic hydrocarbons. J. Org. Chem. 1977;42:3494–3498. [Google Scholar]
- 35.Using the Eyring rate equation, a coalescence temperature of TC = 105 °C predicts an energy of activation of ΔG‡ = 16.8 kcal/mol for the 1H NMR frequencies of the OBn methylene rotor in 27. Gutsche CD, Bauer LJ. Calixarenes. 13. The Conformational Properties of Calix[4]arenes, Calix[6]arenes, Calix[8]arenes, and Oxacalixarenes. J. Am. Chem. Soc. 1985;107:6052–6059.
- 36.Guichard G, Abele S, Seebach D. Preparation of N-Fmoc-protected β2- and β3-amino acids and their use as building blocks for the solid-phase synthesis of β-peptides. Helv. Chim. Acta. 1998;81:187–206. [Google Scholar]
- 37.Latli B, Tomizawa M, Casida JE. Synthesis of a Novel [125I]Neonicotinoid Photoaffinity Probe for the Drosophila Nicotinic Acetylcholine Receptor. Bioconjugate Chem. 1997;8:7–14. doi: 10.1021/bc960066c. [DOI] [PubMed] [Google Scholar]
- 38.Berchtold MW, Brinkmeier H, Muntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol. Rev. 2000;80:1215–1265. doi: 10.1152/physrev.2000.80.3.1215. [DOI] [PubMed] [Google Scholar]
- 39.Saito A, Seiler S, Chu A, Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J. Cell Biol. 1984;99:875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong PW, Pessah IN. Ortho-substituted polychlorinated biphenyls alter calcium regulation by a ryanodine receptor-mediated mechanism: structural specificity toward skeletal- and cardiac-type microsomal calcium release channels. Mol. Pharmacol. 1996;49:740–751. [PubMed] [Google Scholar]
- 41.Wong PW, Pessah IN. Noncoplanar PCB 95 alters microsomal calcium transport by an immunophilin FKBP 12-dependent mechanism. Mol. Pharmacol. 1997;51:693–702. doi: 10.1124/mol.51.5.693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.