Abstract
The present study contrasted physiological arousal in infants and toddlers with fragile X syndrome to typically developing control participants and examined physiological predictors early in development to autism severity later in development in fragile X syndrome. Thirty-one males with fragile X syndrome (ages 8–40 months) and 25 age-matched control participants were included. The group with fragile X syndrome showed shorter interbeat intervals (IBIs), lower vagal tone (VT), and less modulation of IBI. Data suggested a nonlinear effect with IBI and autistic behavior; however, a linear effect with VT and autistic behavior emerged. These findings suggest that atypical physiological arousal emerges within the first year and predicts severity of autistic behavior in fragile X syndrome. These relationships are complex and dynamic, likely reflecting endogenous factors assumed to reflect atypical brain function secondary to reduced fragile X mental retardation protein. This research has important implications for the early identification and treatment of autistic behaviors in young children with fragile X syndrome.
Keywords: fragile X syndrome, autism spectrum disorder, physiological arousal, heart activity
Fragile X syndrome is the most common heritable form of intellectual disability and the leading known biological cause of autism (Hagerman, 2002; Hagerman et al., 2008). Fragile X syndrome affects approximately 1 in 3,600 males and is caused by a CGG triplet expansion at Xq27.3, resulting in reduced production of fragile X mental retardation protein (FMRP; Crawford et al., 2002). Core phenotypic features of fragile X syndrome include intellectual disabilities (Hagerman et al., 2009), lowered adaptive behavior skills (Sarimski, 2010), and social atypicalities (Hall, Lightbody, Huffman, Lazzeroni, & Reiss, 2009). Symptom severity varies across individuals, and females tend to exhibit milder symptoms than males (Bailey, Raspa, Holiday, Bishop, & Olmsted, 2009).
One of the most common comorbid conditions in fragile X syndrome is autism, with 30% to 60% of children with fragile X syndrome meeting Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association, 1994) autism diagnostic criteria (Bailey et al., 1998; Kaufmann et al., 2004; Rogers, Wehner, & Hagerman, 2001) and up to 90% displaying at least one autistic symptom (Hagerman, 2002). In very young children with fragile X syndrome (21–48 months), 33% met DSM IV diagnostic criteria for autism (Rogers et al., 2001). The co-occurrence of autism and fragile X syndrome is associated with debilitating effects; thus, studies that focus on the characterization and underlying mechanisms of autism in fragile X syndrome are critical to increasing the understanding of the heterogeneity of autism and the manifestation of this disorder in fragile X syndrome. To date, however, most research has focused on prevalence rates, with virtually no data on the emergence, precursors, or underlying mechanisms of autism in young children with fragile X syndrome.
Early identification of autism in fragile X syndrome is critical to refining the phenotype, facilitating diagnosis, and guiding targeted treatment aimed at reduction, or even prevention, of autism as a secondary condition in fragile X syndrome. Few studies have examined emergence of autism in infants and toddlers with fragile X syndrome, and extant findings are inconsistent. In a study using retrospective video analysis in 11 infants (ages 9–12 months) with fragile X syndrome, results indicated that sensory–motor features were not associated with later autism diagnoses (Baranek et al., 2005). However, a follow-up prospective longitudinal study found that sensory processing in young boys with fragile X syndrome (ages 9–54 months) followed an age-related profile of hyporesponsiveness during the infant and early toddler years, changing to hyperresponsivess by preschool age (Baranek et al., 2008). In a longitudinal developmental study of 55 male infants and toddlers with fragile X syndrome (ages 8–48 months at study entry), elevated autism features appeared to emerge in the second year of life and were associated with slower rates of development (Roberts, Mankowski, et al., 2009). Last, in a mixed-methods study, cross-sectional results indicated that 12-month-old infants with fragile X syndrome (n = 13) displayed longer look durations than typically developing age- and gender-matched controls, and longitudinal data suggested that atypicalities in visual attention worsened over time for the infants with fragile X syndrome (Roberts, Hatton, Long, Anello, & Colombo, 2011). Given multiple age-related profiles and relationships reported in these preliminary studies, use of a developmental framework with multiple methods is critical to understand complex relationships such as autism in fragile X syndrome.
Evidence from developmental neuroscience has contributed to our understanding of the underlying neural mechanisms associated with the onset of autism in fragile X syndrome. These data have suggested that individuals with fragile X syndrome display increased overall brain volume (Chiu et al., 2007), with enlarged caudate and smaller amygdala volume reported in toddlers with fragile X syndrome (Hazlett et al., 2009). However, the overall brain structure in young children with fragile X syndrome, both with and without comorbid autism, appears different than in idiopathic (non–fragile X syndrome) autism, and brain–behavior relationships are unclear at young ages (Hazlett et al., 2009; Hoeft et al., 2010). Other studies aimed at identifying biological underpinnings of autism in fragile X syndrome have indicated a relationship between the hypothalamic–pituitary–adrenal (HPA) axis, which regulates stress reactivity, and the behavioral expression of autistic features (Hall, Lightbody, & Reiss, 2008; Hessl, Glaser, Dyer-Friedman, & Reiss, 2006; Roberts, Clarke, et al., 2009). Specifically, elevated baseline and postassessment cortisol levels, as well as dampened regulation of cortisol, have been associated with increased autistic behavior in males with fragile X syndrome (Roberts, Clarke, et al., 2009). Elevated heart rate, reduced vagal tone (VT), and blunted modulation in response to cognitive and social demands have also been associated concurrently with increased severity of autistic behavior in males with fragile X syndrome (Hall et al., 2009; Roberts, Boccia, Bailey, Hatton, & Skinner, 2001). However, specific autistic features (i.e., gaze aversion) have not consistently been related to heart activity (Hall et al., 2009). Last, in the only study of heart activity with infants with fragile X syndrome, preliminary results indicated that 12-month-old infants with fragile X syndrome (n = 13) had higher heart rates than age- and gender-matched controls and displayed increasingly blunted physiological modulation across time (9, 12, and 18 months of age), which was associated with elevated autistic symptoms (Roberts et al., 2011). These findings are consistent with evidence that children with idiopathic autism (non–fragile X syndrome) display increased heart rate and suppressed VT, a measure of parasympathetic influence (Bal et al., 2010; Van Hecke et al., 2009). These findings regarding fragile X syndrome and idiopathic autism suggest that investigations of the relationship between autistic behavior and biomarkers holds promise for increasing researchers’ understanding of the underlying mechanisms of autism in fragile X syndrome.
In summary, multiple biomarkers have been associated with autism and disturbances in social interaction in fragile X syndrome. However, existing research is limited, with small samples, little focus on younger children, a lack of developmental consideration, and a focus on concurrent, not predictive, relationships. Accordingly, the in the current study, we examined the relationship between heart activity and autistic symptomology in infants and toddlers with fragile X syndrome to determine if heart activity at an early age is predictive of the severity of later emerging autistic symptoms. We addressed two primary research questions:
Does heart activity differ between infants and toddlers with fragile X syndrome and typically developing control participants?
What is the relationship among autistic behavior, age, and heart activity in infants and toddlers with fragile X syndrome?
In light of the previous literature, we hypothesized that children with fragile X syndrome would demonstrate higher heart rates, lower VT, and poorer physiological modulation compared with typically developing controls. Within the sample of infants with fragile X syndrome, we hypothesized that severity of physiological atypicalities would predict more severe autistic symptoms. We also hypothesized the relationship between physiology and autistic outcomes would become more pronounced at older ages, both across and within individuals.
Method
Participants
Infants and toddlers with fragile X syndrome
Data were drawn from an extant dataset for two independent, yet related, longitudinal studies focused on early development in fragile X syndrome. Fragile X syndrome was verified by genetic report. The first study included 13 male infants with fragile X syndrome assessed three times, at 9, 12, and 18 months of age. The follow-up study included 11 of these 13 infants with 34 additional male participants with fragile X syndrome assessed up to three times at 18-month intervals (age of entry between 12 and 40 months). Our method of combining data across studies allowed us to maximize our sample due to missing data, resulting in a sample of 31 males with fragile X syndrome between 8 and 40 months of age (M = 20.31, SD = 10.85) with complete data for one assessment. For participants with multiple complete assessments, we selected the earliest physiological assessment due to our focus on early emergence of symptoms. Participants were drawn from around the United States and recruited through ongoing and completed studies, fragile X syndrome family support groups, and a fragile X syndrome parent electronic mailing list. Parents provided written consent. Table 1 provides a summary of characteristics of participants with fragile X syndrome.
Table 1.
Descriptive Statistics of Sample With Fragile X Syndrome
| Variable | M | SD | Min. | Max. |
|---|---|---|---|---|
| Age of first assessment (mo.) | 20.31 | 10.85 | 8.4 | 40.0 |
| Household income | $97,288 | $75,092 | $30,000 | $383,333 |
| CARS* score | 28.63 | 5.26 | 18.0 | 40.0 |
| Age at CARS testing (in months) | 60.52 | 18.89 | 18.0 | 106.0 |
| Mullen score at first assessment | 11.35 | 5.54 | 5.25 | 28.75 |
Note. Min. = minimum; Max. = maximum; mo. = months; CARS = Childhood Autism Rating Scale (Schopler, Reichler, & Renner, 1988); Mullen = Mullen Scales of Early Learning (Mullen, 1995).
Typically developing control participants
Both fragile X syndrome studies included chronological age–matched, typically developing control participants. Participants were considered typical if they were born full term and had no history or current suspicion of developmental delay per parental report and research assessment results. This study represents complete data from 25 typically developing control participants, matched to the fragile X syndrome group by gender and age (M age = 21.9 months, SD = 10.8).
Measures
Developmental age
The Mullen Scales of Early Learning (MSEL; Mullen, 1995) consists of five scales: Visual Reception, Gross and Fine Motor skills, and Receptive and Expressive Language skills. The scales demonstrated satisfactory internal consistency (median reliability coefficients varying from .75 to .83 across the five scales). Test–retest reliabilities ranged from .76 to .96 (across scales and ages) and interrater reliabilities ranged from .91 to .96. As in previous studies (Roberts et al., in press), mental age composite was derived and used as a continuous variable to examine the effect of mental age on heart activity and autistic behavior in the group with fragile X syndrome.
Autism behavior ratings
For the fragile X syndrome group only, autistic behavior was measured using the Childhood Autism Rating Scale (CARS; Schopler, Reichler, & Renner, 1988), an examiner rating of autistic behavior that represents a continuum of autistic symptoms. The CARS has been used in numerous studies with individuals with fragile X syndrome (Bailey, Hatton, Skinner, & Mesibov, 2001; Hatton et al., 2002, 2006) and consists of 15 items that tap a range of behavioral responses, including imitation, adaptation to change, communication, and fear or anxiousness. Each item is rated on a scale from 1 (within normal limits) to 4 (severely abnormal), for a total score that ranges from 15 to 60. Scores above 30 indicate mild to severe autistic behaviors. The CARS has an internal consistency rating of .94 and is highly correlated with clinical ratings of autism (range = .80–.88). The CARS was completed through consensus of two examiners following each visit per standardized administration guidelines. The CARS total score at each participant’s final assessment (M age = 60.5 months) was the dependent variable in this study. The later CARS score was used due to a general lack of stability in measures during the first years of life, evidence that autistic behaviors increase in severity with age (Hatton et al., 2006), and our focus on predicting later autistic behavior from early physiological measures in very young children. The mean CARS score in our sample was 28.6 (SD = 5.3) and ranged from 18 to 40, with 12 participants scoring in the mild to severe autistic symptom range (39% of our sample), consistent with previous reports with larger samples (Bailey et al., 2001; Hatton et al., 2006).
Heart activity
We collected participants’ heart activity during the arm-restraint procedure of the laboratory-based assessment of temperament (Lab-TAB; Goldsmith & Rothbart, 1994). The Lab-TAB is highly correlated with parental ratings of temperament (Kochanska, Murray, Jacques, & Koenig, 1996; Rothbart, Ahadi, & Evans, 2000) and has been shown to elicit a set of negatively reactant behaviors (Lewis, Alessandari, & Sullivan, 1990; Sherman & Sherman, 1925) with consistency across contexts and restrainers (Fox, 1989; Stifter & Jain, 1996). The arm-restraint task has been shown to reflect physiological changes, including VT in infants (Fox, 1989; Stifter & Jain, 1996), and has been used with children with Williams syndrome (Jones et al., 2000), autism (Goldsmith, Lemery-Chalfant, Schmidt, Arneson, & Schmidt, 2007), fragile X syndrome (Shanahan, Roberts, Hatton, Reznick, & Goldsmith, 2008), as well as typically developing children (Porter, Jones, Evans, & Robinson, 2009). The arm-restraint epoch is designed for infants throughout the prelocomotor and locomotor age and has been used in studies of infants with fragile X syndrome and typically developing control participants up to 40 months of age (Shanahan et al., 2008). Procedures for the Lab-TAB require that the child be seated in a high chair and in a neutral state for at least 15 s (e.g., not crying, fussing, or yawning) prior to starting the arm-restraint procedure. Next, a toy is presented to the child (a shiny sphere spinning on a track), and the child is allowed approximately 1 min of unstructured toy play (toy-play phase). All children demonstrated interest in the toy, as reflected by physically reaching for or watching the toy. After the brief toy-play phase, the mother gently restrained the child’s hands down to their side, preventing access to the toy for 30 s (per standardized procedures) to induce frustration (reactivity phase).
Heart activity data were continuously recorded while the participants completed the toy-play and reactivity phases of the experiment. From the 60 s of toy-play data, only the last 30 s were retained for analysis to allow for initial adjustment to the toy and calculation of a consistent time frame across both toy-play and reactivity phases. The Mini-Logger 2000 (Mini Mitter Co., 1994), a telemetry system that uses a chest belt to transmit heart activity to a receiver, was used to collect heart activity. This system has been shown to be tolerable to a wide age range of children with fragile X syndrome and has shown adequate sensitivity and reliability across systems (Hall et al., 2009; Roberts et al., 2001; Roberts, Boccia, Hatton, Skinner, & Sideris, 2006). Heart activity data were edited for artifacts and then analyzed using the MxEdit program (MxEdit, 1989). We analyzed two heart activity indicators: (a) interbeat interval (IBI; milliseconds between successive R waves), reflecting both parasympathetic and sympathetic influences to heart activity and (b) VT, reflecting parasympathetic system influences on the heart. These indicators were derived for three variables: toy play, reactivity, and change (reactivity –toy play). Shorter IBIs reflect faster heart rates.
Procedure
Assessments were scheduled midmorning to control for circadian effects on the heart activity data. Each session began with a developmental assessment to allow for informal and flexible interaction with the child prior to onset of the Lab-TAB. Data were entered twice and verified prior to conducting a series of analyses to address our research questions. First, we compared group differences in heart activity between the typically developing and fragile X syndrome groups. We then compared group difference among the typically developing sample and two fragile X syndrome groups: children with fragile X syndrome and high levels of autistic symptoms as defined as a total CARS score of at 30 or more (fragile X syndrome + autism spectrum disorder; n = 12), and children with fragile X syndrome and low levels of autistic symptoms (CARS score < 30; fragile X syndrome; n = 19). Next, we conducted analyses to examine the relationship of autistic behavior and age to heart activity within the sample of children with fragile X syndrome. Chronological age at the time heart activity was collected and included in all models. Mental age was not related to heart activity (r = .10), so it was not included in the models. Chronological age at the time of the CARS score was not related to the CARS total score in our sample (r = .06) and was also not included in analyses.
Results
Group Differences in Heart Activity
In the first step of analyses, we used multivariate analysis of covariance (MANCOVA) to determine whether IBI and VT differed between the fragile X syndrome and typically developing groups for both the two-group (fragile X syndrome, typically developing) and three-group (fragile X syndrome, fragile X syndrome + autistic symptoms, typically developing) analyses. Phase (toy play, reactivity, change) was included in the both the 2 (group) × 3 (phase) and 3 (group) × 3 (phase) MANCOVA models, which were conducted using Wilks’ criterion. Table 2 includes means and age-adjusted estimated marginal means for both fragile X syndrome and typically developing groups.
Table 2.
Fragile X Syndrome and Typically Developing Groups’ Descriptive Heart Activity Data Across Phases
| Phase | Group | Vagal tone
|
Interbeat interval
|
||||
|---|---|---|---|---|---|---|---|
| Observed M |
Marginal M |
SE | Observed M |
Marginal M |
SE | ||
| Reactivity | TD | 4.53 | 4.43 | 0.20 | 473.71 | 469.24 | 8.72 |
| All FXS | 3.83 | 3.87 | 0.18 | 442.95 | 447.92 | 7.88 | |
| FXS + ASD | 4.23 | 3.92 | 0.29 | 463.94 | 449.43 | 12.72 | |
| FXS − ASD | 3.57 | 3.83 | 0.24 | 429.69 | 446.88 | 10.49 | |
|
| |||||||
| Toy Play | TD | 4.67 | 4.55 | 0.21 | 517.08 | 512.73 | 7.16 |
| All FXS | 3.82 | 3.92 | 0.20 | 459.35 | 462.96 | 6.47 | |
| FXS + ASD | 4.04 | 3.65 | 0.31 | 475.98 | 461.78 | 10.44 | |
| FXS − ASD | 3.67 | 4.10 | 0.26 | 448.26 | 463.76 | 8.61 | |
|
| |||||||
| Change | TD | −0.14 | −0.13 | 0.20 | −43.37 | −43.51 | 6.57 |
| All FXS | −0.03 | −0.05 | 0.19 | −15.16 | −15.04 | 5.99 | |
| FXS + ASD | .019 | 0.27 | 0.30 | −12.04 | −12.35 | 9.66 | |
| FXS − ASD | −0.18 | −0.27 | 0.24 | −17.24 | −16.89 | 7.97 | |
Note. TD = typically developing; FXS = fragile X syndrome; ASD = autism spectrum disorders. For the typically developing and all fragile X syndrome groups, marginal means are reported from the multivariate analysis of covariance of typically developing versus the whole fragile syndrome sample.
IBI
For the two-group comparison, the combined dependent variables were significantly affected by group membership for IBI, F(2, 51) = 15.50, p = .00. Compared with typically developing controls, the fragile X syndrome group showed shorter IBIs during toy play (p = .00) and less change in heart rate between phases (p = .00). Group differences during the reactivity phase approached significance (p = .08). For the three-group comparison, the combined dependent IBI variables also were significantly affected by group membership, F(4, 100) = 6.74, p = .00. Groups significantly differed in IBI during toy play (p = .00) and in change of IBI across phases (p = .01). Groups did not differ in IBI during the reactivity phase (p = .21). Toy-play IBI was longest (indicating slowest heart rate) in the typically developing group (marginal M = 512.73), followed by the fragile X syndrome (M = 463.76) and fragile X syndrome + autistic symptoms (M = 461.78) groups. Change scores indicated all groups showed decreases in IBI (faster heart rate) during the arm-restraint episode compared with toy play, although groups differed in magnitude of change across phases. The typically developing group showed the largest change in IBI across phases (−43.47), followed by the fragile X syndrome (−16.89) and fragile X syndrome + autistic symptoms (−12.35) groups. For both toy-play IBI and change scores, post hoc, least significant difference (LSD), pairwise comparisons confirmed significant group differences between typically developing and both fragile X syndrome and fragile X syndrome + autistic symptoms groups (ps < .02). Fragile X syndrome and fragile X syndrome + autistic symptoms groups were not significantly different. Figure 1 compares marginal means across phases and groups.
Figure 1.
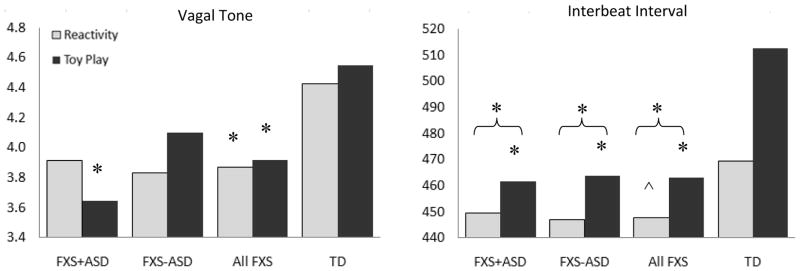
Estimated marginal means of FXS + ASD, FXS, and TD groups across reactivity and toy-play phases, controlling for age at assessment. FXS = fragile X syndrome; ASD = autism spectrum disorders; TD = typically developing. *Indicates significant post hoc group differences from TD controls. ^ Indicates post hoc group differences that approached significance (.05 < p <.08). Curved brackets indicate change scores. The fragile X syndrome + autism spectrum disorders, and fragile X syndrome − autism spectrum disorders groups did not significantly differ from each
VT
For the two-group comparison, the combined dependent variables approached significance for VT, F(2, 51) = 2.94, p = .06. The fragile X syndrome group showed lower VT during toy-play (p = .03) and reactivity (p = .04) phases, although groups did not significantly differ in change across phases (p = .79). For the three-group comparison, the overall F test for VT was not significant across all variables, F(4, 100) = 1.93, p = .11. Group differences in VT during toy play, however, approached significance (p = .06), although neither reactivity (p = .13) nor change (p = .38) of VT differed significantly across groups. During toy play, the fragile X syndrome + autistic symptoms group showed the lowest VT (estimated marginal M = 3.65), followed by the fragile X syndrome (M = 4.10) and typically developing (M = 4.55) groups. Post hoc, LSD, pairwise comparisons confirmed significant differences between the typically developing and fragile X syndrome + autistic symptoms groups (p = .02). Additional group comparisons were not significant (ps > .05).
Effects of Autism and Age on Heart Activity Within Fragile X Syndrome
Our first step of analyses demonstrated group differences between fragile X syndrome and typically developing groups, as well as suggested trends in group differences between children with fragile X syndrome and high versus low levels of autistic symptoms. In light of research suggesting autism is characterized as a spectrum (Short & Schopler, 1988; Yoder, Stone, Walden, & Malesa, 2009), we next used linear regression to examine the relationship between autistic outcomes and physiology across ages, using the CARS score as a continuous variable. Each model included chronological age at the time of heart activity collection, as well as the interaction of Age × Heart Activity. Within each model, significant interactions were probed to estimate ages at which heart activity predicted CARS in our sample. Regions of significance were estimated using techniques by Johnson and Fay (1950; Hayes & Matthes, 2009). Main effects and interactions from the regression models are listed in Table 3. Figures 2 and 3 visually depict Age × Physiology interactions.
Table 3.
Effects of Regression Model Parameters Predicting CARS Outcomes
| Phase | Effect | Estimate | SE | t | p | |
|---|---|---|---|---|---|---|
|
|
||||||
| Vagal tone (VT) | Toy play | Age | 1.02 | 0.30 | 3.41 | .002a |
| VT | 2.39 | 1.45 | 1.65 | .11 | ||
| Age × VT | −0.17 | 0.07 | −2.65 | .01a | ||
|
| ||||||
| Reactivity | Age | 0.80 | 0.40 | 2.0 | .06a | |
| VT | 3.42 | 2.02 | 1.7 | .10 | ||
| Age × VT | −0.15 | 0.09 | −1.7 | .10 | ||
|
| ||||||
| Change | Age | 0.19 | 0.08 | 2.34 | .03a | |
| VT | −0.60 | 2.40 | −0.25 | .80 | ||
| Age × VT | 0.09 | 0.10 | 0.90 | .38 | ||
|
| ||||||
| Interbeat interval (IBI) | Toy play | Age | 3.00 | 0.94 | 3.19 | .004a |
| IBI | 0.13 | 0.04 | 2.89 | .008a | ||
| Age × IBI | −0.006 | 0.002 | −3.08 | .005a | ||
|
| ||||||
| Reactivity | Age | 0.84 | 0.85 | 0.99 | .33 | |
| IBI | 0.07 | 0.05 | 1.55 | .13 | ||
| Age × IBI | 0.002 | 0.002 | −0.90 | .38 | ||
|
| ||||||
| Change | Age | 0.29 | 0.10 | 3.04 | .005a | |
| IBI | −0.08 | 0.06 | −1.26 | .22 | ||
| Age × IBI | 0.005 | 0.003 | 1.87 | .07 | ||
Note. CARS = Childhood Autism Rating Scale (Schopler, Reichler, & Renner, 1988).
Significant at p < .05.
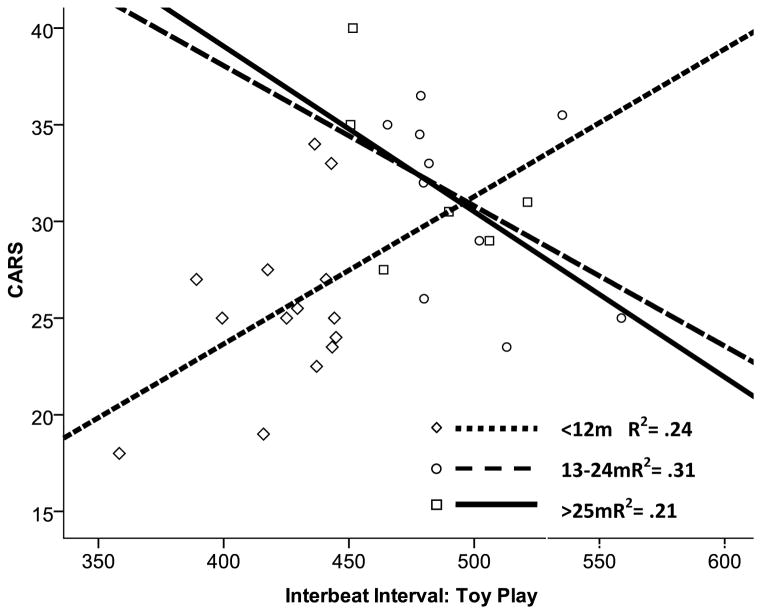
Figure 2.
Cross-sectional interaction between age and interbeat interval (IBI) during toy play in group with fragile X syndrome. CARS = Childhood Autism Rating Scale (Schopler, Reichler, & Renner, 1988). At younger ages, IBI was positively related with CARS outcomes. At older ages, IBI was negatively related with CARS outcomes. m = months.
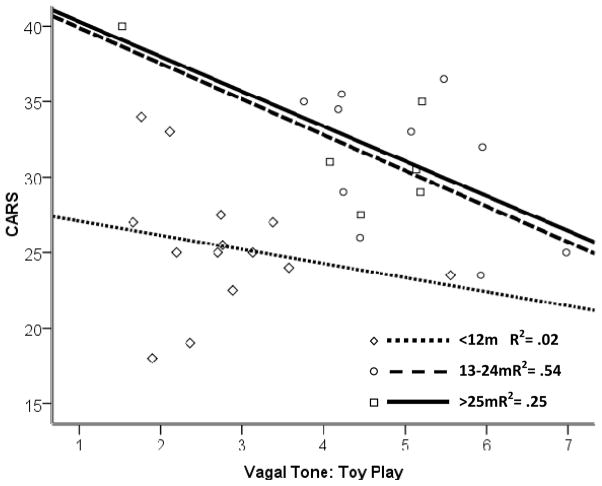
Figure 3.
Cross-sectional interaction between age and vagal tone (VT) during toy play in group with fragile X syndrome. CARS = Childhood Autism Rating Scale (Schopler, Reichler, & Renner, 1988). At older ages, higher CARS outcome was associated with lower VT. m = months.
IBI
Mean IBI was shorter in the toy-play phase than reactivity phase. Results indicated a main effect of IBI during toy play (p = .008) as well as main effects of age for toy play (p = .002) and change (p = .005). No main effects reached significance for the reactivity model. However, an interaction between IBI and age during toy play emerged (p = .005). Estimates indicated a curvilinear pattern, with a positive relationship between toy-play IBI and CARS below 10 months and a negative relationship after 37 months. CARS scores were also marginally predicted by the interaction of age and change in IBI (p = .07).
VT
Vagal tone was similar across toy-play and reactivity phases. Results indicated main effects of age for toy play (p = .002) and change (p = .03). There was no main effect of VT for the reactivity model. Consistent with the IBI data, CARS scores were predicted by the interaction of age with VT during toy play (p = .01). Estimates indicated a significant negative relationship between VT and CARS scores beginning at 22 months.
Discussion
Increasing our understanding of the developmental pathway and underlying mechanisms of autistic behavior in fragile X syndrome is critical to addressing the latent heterogeneity in autism and guiding diagnostic and treatment efforts for young children with neurodevelopmental disorders and their families. Our results indicated that heart activity measured at an early age during nondemanding baseline-simulating epochs predicts severity of autistic behavior at later age in a sample of male infants and toddlers with fragile X syndrome. This relationship appears evident by the first year of life, implicating endogenous factors assumed to reflect atypical brain function secondary to FMRP reduction. In addition to our finding that these relationships emerge very early in development, we found a striking and unanticipated developmental shift in these relationships. Thus, the relationship of heart activity in infants and toddlers with fragile X syndrome to autistic behavior is complex and likely reflects the effect of multiple dynamic processes.
As hypothesized, male infants and toddlers with fragile X syndrome display elevated physiological arousal compared with typically developing age- and gender-matched control participants, suggesting atypicalities in general autonomic function. To our knowledge, this study is the first to document these arousal differences in a sample younger than 12 months of age. These group differences were observed across multiple conditions, including nondemanding toy play and a frustration-eliciting task, and were reflected in both VT and IBI estimates of heart activity. Of interest, the data suggested that shortened IBI (a generalized index of heart activity) did not distinguish between typical control participants and groups with fragile X syndrome with and without elevated autistic behavior. In contrast, lower VT (a reflection of parasympathetic activity) differentiated between participants with fragile X syndrome and high levels of autistic behavior and typically developing control participants, whereas VT was not different in participants with fragile X syndrome and low levels of autistic behavior and typical controls.
In support of our hypothesis, we found that shortened IBIs and lower VT in infants and toddlers with fragile X syndrome predicted the later emergence of autistic symptoms. Our findings are consistent with previous work associating shorter IBIs and lower VT with concurrent autistic symptoms, both in idiopathic autism (Bal et al., 2010; Ming, Julu, Brimacombe, Connor, & Daniels, 2005; Van Hecke et al., 2009) and fragile X syndrome (Hall et al., 2008; Roberts et al., 2001) samples. Because higher baseline VT has been linked to environmental awareness and adaptability (Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996) and lower levels of problem behaviors in typically developing children (Huffman et al., 1998), the relationship of lower VT with more severe autistic symptoms is not surprising, considering the difficulties with adaptability and problem behaviors inherent in the autistic phenotype.
Of interest, we found developmental trends indicating a shift from underarousal to overarousal related to severity of autistic behavior over time. Within IBI data, we identified a complex age-related pattern, with higher autistic outcomes associated with lower arousal at approximately 10 months of age and then shifting to a relationship between higher autistic behavior and higher arousal by 37 months of age. For VT, a linear, age-related profile was evident, suggesting no relationship until 22 months of age, at which time a relationship with higher VT and elevated autistic outcomes emerged. These findings of developmental shifts in the relationship of heart activity to severity of autistic behavior outcomes are consistent with preliminary behavioral findings that later autistic behavior is associated with increased look duration at 12 and 18 months of age, as well as with a developmental lag in disengagement of attention between 9 and 12 months (Roberts, McDonald, Kelleher, & Hatton, 2010) and a shift from hypo- to hyperreactivity to sensory processing (Baranek et al., 2005). The marginally significant (p = .07) interaction of age and IBI modulation (change score) in our sample is also consistent with evidence of a relationship between autistic behavior and reduced cortisol reactivity (Hessl et al., 2006; Roberts, Clarke, et al., 2009), supporting blunted physiological reactivity as a potential biomarker for autism in boys with fragile X syndrome. However, direct associations between behavioral indicators of autism and physiological factors in fragile X syndrome have not yet been examined, in part, due to very small samples. Thus, these conclusions are speculative at this time.
Findings from the current study and other work (Roberts et al., 2010) parallel findings from work with infants with idiopathic (non–fragile X syndrome) autism that have showed visual attention abnormalities within the first year of life and a shift from underreactivity to overreactivity predicting autism diagnoses (Zwaigenbaum et al., 2005). Likewise, our findings of age-related shifts in arousal in infants and toddlers with fragile X syndrome support neuroimaging and head circumference data reflecting age-dependent volumetric differences (Hazlett et al., 2009; Hoeft et al., 2008). In contrast to these neuroimaging data that identify differences by 2 years of age, we found a relationship between heart activity and autistic symptoms that began to emerge earlier, in the first year of life. These differences in the age of emergence likely reflect different brain–behavior functional relationships, which may be more easily detected and emerge earlier than structural brain differences. Collectively, our data and extant literature suggest that autistic behavior in fragile X syndrome emerges within the first year and is associated with an atypical level and trajectory of arousal, most likely reflecting neural abnormalities associated with reduced FMRP.
Although this study is important because of the strong association of autism in fragile X syndrome and lack of data investigating underlying physiological markers of autism in young children with fragile X syndrome and idiopathic autism, there are a number of limitations. First, we used the CARS as a measure of autistic symptom severity instead of gold-standard measures such as the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000) or the Autism Diagnostic Interview-Revised (ADI-R; Le Couteur et al., 1989). An additional weakness of our study is that our analyses focused on a short epoch of heart activity during a single session of the Lab-TAB rather than over multiple measures of temperament or other stimulus conditions. In addition, our study did not include any clinical comparison group and excluded girls due to the well-established phenotypic differences, which resulted in interpretations restricted to males with fragile X syndrome. Despite these limitations, our current study exhibited multiple strengths, including a relatively large sample of infants with fragile X syndrome compared with existing research, an explicit examination of age effects within a developmental framework, and a focus on infants and toddlers with fragile X syndrome, which has rarely occurred in extant literature. This research also represents one of few studies to physiologically examine the underlying mechanisms of problem behavior in fragile X syndrome or idiopathic (non–fragile X syndrome) autism in such a young sample. Last, although our heart activity data were collected during a short epoch, our findings were consistent with data showing a relationship between heart activity and autistic symptoms in studies with longer epochs of 5 min (Roberts et al., 2001) and 25 min (Hall et al., 2009).
Our results suggest several promising areas of future research. First, although our data indicated that a relationship between physiological arousal and later autistic symptoms may emerge in the first year of life, additional research is needed to determine more specifically when these relationships emerge and what other behaviors may be associated with physiological indicators. Second, longitudinal studies that adequately examine the within-subject growth over time are essential to more fully understand these relationships. Future studies may tease apart these relationships by comparing patterns and relationships across other high-risk samples, such as infant siblings of children with idiopathic autism. Last, future research should explore whether physiological arousal during social stressors predicts autistic symptoms in young children with fragile X syndrome, because our data represented a primarily nonsocial task. In addition to the novel findings reported in the present study, these future directions may further essential efforts to characterize the developmental relationship between biomarkers and autistic outcomes in high-risk children with fragile X syndrome.
Acknowledgments
This study was supported by the National Institute of Child Health and Human Development (Grant P30-HD003110-35S1) and the Office of Special Education Programs, U.S. Department of Education (Grant H324C990042) awarded to Don Bailey.
References
- Bailey DB, Jr, Hatton DD, Skinner M, Mesibov G. Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. Journal of Autism and Developmental Disorders. 2001;31:165–174. doi: 10.1023/a:1010747131386. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Mesibov GB, Hatton DD, Clark RD, Roberts JE, Mayhew L. Autistic behavior in young boys with fragile X syndrome. Journal of Autism and Developmental Disorders. 1998;28:499–508. doi: 10.1023/a:1026048027397. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Jr, Raspa M, Holiday D, Bishop E, Olmsted M. Functional skills of individuals with fragile X syndrome: A lifespan cross-sectional analysis. American Journal on Intellectual and Developmental Disabilities. 2009;114:289–303. doi: 10.1352/1944-7558-114.4.289-303. [DOI] [PubMed] [Google Scholar]
- Bal E, Harden E, Lamb D, Van Hecke AV, Denver JW, Porges SW. Emotion recognition in children with autism spectrum disorders: Relations to eye gaze and autonomic state. Journal of Autism and Developmental Disorders. 2010;40:358–370. doi: 10.1007/s10803-009-0884-3. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Danko CD, Skinner ML, Bailey DR, Hatton DD, Roberts JE, Mirrett PL. Video analysis of sensory-motor features in infants with fragile X syndrome at 9–12 months of age. Journal of Autism and Developmental Disorders. 2005;35:645–656. doi: 10.1007/s10803-005-0008-7. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Wakeford L, David FJ. Understanding, assessing, and treating sensory-motor issues. In: Chawarska K, Klin A, Volkmar FR, editors. Autism spectrum disorders in infants and toddlers: Diagnosis, assessment, and treatment. New York: Guilford Press; 2008. pp. 104–140. [Google Scholar]
- Chiu S, Wegelin JA, Blank J, Jenkins M, Day J, Hessl D, Hagerman R. Early acceleration of head circumference in children with fragile X syndrome and autism. Journal of Developmental and Behavioral Pediatrics. 2007;28:31–35. doi: 10.1097/01.DBP.0000257518.60083.2d. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Meadows KL, Newman JL, Taft LF, Scott E, Leslie M, Sherman SL. Prevalence of the fragile X syndrome in African-Americans. American Journal of Medical Genetics. 2002;110:226–233. doi: 10.1002/ajmg.10427. [DOI] [PubMed] [Google Scholar]
- Fox NA. Psychophysiological correlates of emotional reactivity during the first year of life. Developmental Psychology. 1989;25:364–372. [Google Scholar]
- Goldsmith H, Lemery-Chalfant K, Schmidt NL, Arneson CL, Schmidt CK. Longitudinal analyses of affect, temperament, and childhood psychopathology. Twin Research and Human Genetics. 2007;10:118–126. doi: 10.1375/twin.10.1.118. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Rothbart MK. Manual for the Laboratory Temperament Assessment Battery (Version 2.03) 1994. Unpublished manuscript. [Google Scholar]
- Hagerman RJ. The physical and behavioral phenotype. In: Hagerman RJ, Hagerman PJ, editors. Fragile X syndrome: Diagnosis, treatment, and research. 3. Baltimore: Johns Hopkins University Press; 2002. pp. 3–109. [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Tranfaglia M. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123:378–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Hall DA, Coffey S, Leehey M, Bourgeois J, Gould J, Hagerman PJ. Treatment of fragile X-associated tremor ataxia syndrome (FXTAS) and related neurological problems. Journal of Clinical Interventions in Aging. 2008;3:251–262. doi: 10.2147/cia.s1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Huffman LC, Lazzeroni LC, Reiss AL. Physiological correlates of social avoidance behavior in children and adolescents with fragile X syndrome. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48:320–329. doi: 10.1097/CHI.0b013e318195bd15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Reiss AL. Compulsive, self-injurious, and autistic behavior in children and adolescents with fragile X syndrome. American Journal on Mental Retardation. 2008;113:44–53. doi: 10.1352/0895-8017(2008)113[44:CSAABI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hatton DD, Hooper SR, Bailey DB, Skinner ML, Sullivan KM, Wheeler A. Problem behavior in boys with fragile X syndrome. American Journal of Medical Genetics. 2002;108:105–116. doi: 10.1002/ajmg.10216. [DOI] [PubMed] [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Jr, Roberts J, Mirrett P. Autistic behavior in children with fragile X syndrome: Prevalence, stability, and the impact of FMRP. American Journal of Medical Genetics. 2006;140A:1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behavior Research Methods. 2009;41:924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Lightbody AA, Gerig G, Macfall JR, Ross AK, Piven J. Teasing apart the heterogeneity of autism: Same behavior, different brains in toddlers with fragile X syndrome and autism. Journal of Neurodevelopmental Disorders. 2009;1:81–90. doi: 10.1007/s11689-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, Reiss AL. Social behavior and cortisol reactivity in children with fragile X syndrome. Journal of Child Psychology and Psychiatry. 2006;47:602–610. doi: 10.1111/j.1469-7610.2005.01556.x. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Carter JC, Lightbody AA, Hazlett H, Piven J, Reissa AL. Region-specific alterations in brain development in one- to three-year-old boys with Fragile X syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9335–9339. doi: 10.1073/pnas.1002762107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Lightbody AA, Hazlett HC, Patnaik S, Piven J, Reiss AL. Morphometric spatial patterns differentiating boys with fragile X syndrome, typically developing boys, and developmentally delayed boys aged 1 to 3 years. Archives of General Psychiatry. 2008;65:1087–1097. doi: 10.1001/archpsyc.65.9.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman LC, Bryan YE, del Carmen R, Pedersen FA, Doussard-Roosevelt JA, Porges SW. Infant temperament and cardiac vagal tone: Assessments at twelve weeks of age. Child Development. 1998;69:624–635. [PubMed] [Google Scholar]
- Johnson PO, Fay LC. The Johnson-Neyman technique, its theory and application. Psychometrika. 1950;15:349–367. doi: 10.1007/BF02288864. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, Stanard P. Autism spectrum disorder in fragile X syndrome: Communication, social interaction, and specific behaviors. American Journal of Medical Genetics. 2004;129A:225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Murray K, Jacques TY, Koenig AL. Inhibitory control in young children and its role in emerging internalization. Child Development. 1996;67:490–507. [PubMed] [Google Scholar]
- Le Couteur A, Rutter M, Lord C, Rios P, Robertson S, Holdgrafer M, McLennan J. Autism diagnostic interview: a standardized investigator-based instrument. Journal of Autism and Developmental Disorders. 1989;19:363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- Lewis M, Alessandri SM, Sullivan MW. Violation of expectancy, loss of control, and anger expressions in young infants. Developmental Psychology. 1990;26:745–751. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Ming X, Julu PO, Brimacombe M, Connor S, Daniels ML. Reduced cardiac parasympathetic activity in children with autism. Brain Development. 2005;27:509–516. doi: 10.1016/j.braindev.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Mullen EM. The Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Porter CL, Jones BL, Evans CA, Robinson CC. A comparative study of arm-restraint methodology: Differential effects of mother and stranger restrainers on infants’ distress reactivity at 6 and 9 months of age. Infancy. 2009;14:306–324. doi: 10.1080/15250000902840011. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Boccia ML, Bailey DB, Jr, Hatton DD, Skinner M. Cardiovascular indices of physiological arousal in boys with fragile X syndrome. Developmental Psychobiology. 2001;39:107–123. doi: 10.1002/dev.1035. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Boccia ML, Hatton DD, Skinner ML, Sideris J. Temperament and vagal tone in boys with fragile X syndrome. Journal of Developmental and Behavioral Pediatrics. 2006;27:193–201. doi: 10.1097/00004703-200606000-00003. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Clarke MA, Alcorn K, Carter JC, Long AJ, Kaufmann WE. Autistic behavior in boys with fragile X syndrome: Social approach and HPA-axis dysfunction. Journal of Neurodevelopmental Disorders. 2009a;1:283–291. doi: 10.1007/s11689-009-9028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Hatton DD, Long AC, Anello V, Columbo J. Visual attention and autistic behavior in infants with fragile X syndrome. Journal of Autism and Developmental Disorders. 2011 doi: 10.1007/s10803-011-1316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Mankowski JB, Sideris J, Goldman BD, Hatton DD, Mirrett PL, Bailey DB. Trajectories and predictors of the development of very young boys with fragile X syndrome. Journal of Pediatric Psychology. 2009b;34:827–836. doi: 10.1093/jpepsy/jsn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, McDonald LM, Kelleher BL, Hatton DD. Social responsivity in infants with fragile X syndrome over time. Paper presented at the 12th Annual Meeting of the National Fragile X Foundation; Detroit. 2010. Jul, [Google Scholar]
- Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: Symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. Journal of Developmental and Behavioral Pediatrics. 2001;22:409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: Origins and outcomes. Journal of Personality and Social Psychology. 2000;78:122–135. doi: 10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Sarimski K. Perceived quality of life in mothers of young boys with fragile X syndrome. Prax Kinderpsychol Kinderpsychiatr. 2010;59:389–403. doi: 10.13109/prkk.2010.59.5.389. [DOI] [PubMed] [Google Scholar]
- Schopler E, Reichler RJ, Renner BR. The Childhood Autism Rating Scale (CARS) Los Angeles: Western Psychological Services; 1988. [Google Scholar]
- Shanahan MM, Roberts JJ, Hatton DD, Reznick JJ, Goldsmith HH. Early temperament and negative reactivity in boys with fragile X syndrome. Journal of Intellectual Disability Research. 2008;52:842–854. doi: 10.1111/j.1365-2788.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- Sherman M, Sherman IC. Sensori-motor responses in infants. Journal of Comparative Psychology. 1925;5:53–68. [Google Scholar]
- Short AB, Schopler E. Factors relating to age of onset in autism. Journal of Autism and Developmental Disorders. 1988;18:207–216. doi: 10.1007/BF02211947. [DOI] [PubMed] [Google Scholar]
- Stifter CA, Jain A. Psychophysiological correlates of infant temperament: Stability of behavior and autonomic patterning from 5 to 18 months. Developmental Psychobiology. 1996;29:379–391. doi: 10.1002/(SICI)1098-2302(199605)29:4<379::AID-DEV5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Van Hecke A, Lebow J, Bal E, Lamb D, Harden E, Kramer A, Porges SW. Electroencephalogram and heart rate regulation to familiar and unfamiliar people in children with autism spectrum disorders. Child Development. 2009;80:1118–1133. doi: 10.1111/j.1467-8624.2009.01320.x. [DOI] [PubMed] [Google Scholar]
- Yoder P, Stone WL, Walden T, Malesa E. Predicting social impairment and ASD diagnosis in younger siblings of children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2009;39:1381–1391. doi: 10.1007/s10803-009-0753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Roberts W, Rogers T, Brian J, Szatmari P. Behavioral markers of autism in the first year of life. International Journal of Developmental Neurosciences. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]