Abstract
Patient biological material for isolation of β2-glycoprotein I (β2GPI) and high avidity IgG anti-β2-glycoprotein I antibodies (HAv anti-β2GPI) dictates its full utilization. The aim of our study was to evaluate/improve procedures for isolation of unnicked β2GPI and HAv aβ2GPI to gain unmodified proteins in higher yields/purity. Isolation of β2GPI from plasma was a stepwise procedure combining nonspecific and specific methods. For isolation of polyclonal HAv aβ2GPI affinity chromatographies with immobilized protein G and human β2GPI were used. The unknown protein found during isolation was identified by liquid chromatography electrospray ionization mass spectrometry and the nonredundant National Center for Biotechnology Information database. The average mass of the isolated unnicked purified β2GPI increased from 6.56 mg to 9.94 mg. In the optimized isolation procedure the high molecular weight protein (proteoglycan 4) was successfully separated from β2GPI in the 1st peaks with size exclusion chromatography. The average efficiency of the isolation procedure for polyclonal HAv anti-β2GPI from different matrixes was 13.8%, as determined by our in-house anti-β2GPI ELISA. We modified the in-house isolation and purification procedures of unnicked β2GPI and HAv anti-β2GPI, improving the purity of antigen and antibodies as well as increasing the number of tests routinely performed with the in-house ELISA by ~50%.
1. Introduction
Recent findings of antiphospholipid syndrome (APS) pathogenesis support the important role of β2-glycoprotein I (β2GPI), as one of the most studied antigens [1–3]. β2GPI is a ~50 kDa protein with a mean plasma concentration in the healthy population of ~180 mg/L. The protein consists of 326 amino acids folded into 5 domains [4, 5]. The first 4 domains contain approximately 60 amino acids, whereas the last, 5th domain consists of 82 amino acids containing specific segments of positively charged amino acids 281CKNKEKKC288 and a hydrophobic loop 313LAFW316, which along with 19 amino acids of the C-terminal extension form the binding site to negatively charged phospholipids [6, 7]. Plasmin can clip/nick β2GPI at amino acids L317T318 and consequently terminate its ability to bind phospholipids [8]. Furthermore, recently it was observed that β2GPI can exist in different conformations, that is, in a circular form that can change to an open (fishhook) conformation after exposure to anionic structures or negatively charged phospholipids, which can be stabilized by anti-β2GPI antibodies (anti-β2GPI) [9, 10].
The presence of anti-β2GPI in human sera or plasma is one of the defining laboratory criteria for classification of APS [3, 11]. High avidity IgG anti-β2GPI (HAv anti-β2GPI) represent a subgroup of anti-β2GPI associated with thrombotic [12, 13] and obstetric [14] manifestations in APS patients. On the other hand, several studies in past decades implied that anti-β2GPI were associated with the development of atherosclerosis in autoimmune patients (as reviewed in [15]) and represent a non-traditional risk factor for atherosclerosis-based cardiovascular diseases in patients without overt autoimmunity (reviewed in [16]).
In the context of fully utilizing the preparatory fractions for the isolation of human unnicked β2GPI and HAv anti-β2GPI, as well as optimizing their yield, we evaluated and improved the protocols and procedures/methods involved [17, 18]. The primary aim was to gain unmodified, endogenous proteins in higher yields and purity.
2. Materials and Methods
2.1. Isolation of β2-Glycoprotein I
For isolation of human unnicked β2GPI, AB plasma pooled from apparently healthy donors was used. Isolation included a stepwise procedure combining perchloric acid (PA) precipitation, heparin affinity, and cationic exchange chromatography as determined by Cucnik et al. [17], with modifications performed in the precipitation step. In contrast to the previously used isolation procedure (standard protocol) [17], precipitation with PA was carried out in 3 aliquots of 80–90 mL of plasma starting volume (~250 mL) diluted with an equal volume of physiological solution of sodium chloride. Precipitation was carried out in an ice bath (0°C) using 60% PA, which was added dropwise to a final concentration of 0.285 M. The addition of PA in the optimized protocol lasted ~20 min per aliquot, as compared to the standard protocol which involved a precipitation step lasting ~60 min or more, due to the higher starting volume of plasma (~250 mL). Immediately after precipitation the suspension was centrifuged at 4°C and the pH of supernatants was adjusted to 8.0 with 1 M NaOH. Following precipitation, centrifugation and adjustment of pH, all aliquots were combined and dialyzed against 0.02 M Tris-HCl/0.03 M NaCl, pH 8.0 overnight. The following steps were without modifications as previously described [17]. Briefly, the dialysed supernatants were concentrated using 350 mL Amicon Stirred Ultrafiltration cell unit (Millipore, Bedford, MA, USA) and Ultrafiltration Membranes from regenerated cellulose with a molecular weight cut-off (MWCO) lower than 10 kDa (Millipore, Carrigtwohill Co., Cork, Ireland). In the next step concentrated dialysed supernatants were applied to a 10 mm × 20 cm Heparin Sepharose CL-6B column (GE Healthcare Bio-Sciences AB, Uppsala, Sweden), which was equilibrated with the same buffer. For elution of bound proteins 0.02 M Tris-HCl/0.35 M NaCl, pH 8.0 was used. Eluted fractions containing proteins were then pooled and dialysed against 0.05 M acetate buffer/0.05 M NaCl, pH 4.8 overnight and finally applied to a 5 × 50 mm cation exchange column with polystyrene/divinylbenzene matrix and R-CH2-SO3− charged groups (Mono S 5/50 GL, GE Healthcare Bio-Sciences AB, Uppsala, Sweden). β2GPI was eluted over a linear gradient starting from 0.05 M NaCl at pH 4.8 to 0.65 M NaCl at pH 5.2 (Na+ in acetate buffer). After the last step (cationic exchange chromatography), three quantitatively different peaks were collected and dialyzed against phosphate buffered saline, pH 7.4 (PBS) with 0.02% NaN3 for 2 h (repeated twice). Protein concentrations were determined by NanoDrop 2000c Spectrophotometer (Thermo Fischer Scientific, Wilmington, Delaware, USA) at a wavelength of 280 nm using the excitation coefficient for β2GPI E 1 cm 1% = 10.0. The final preparations were aliquoted and stored at −80°C for further analysis and/or use.
2.2. Polyacrylamide Gel Electrophoresis
The purity of isolated β2GPI was checked by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) according to Laemmli protocol [19] in 4% stacking and 10% resolving gels on the Mini-Protean II apparatus (Bio-Rad Laboratories, Hercules, CA, USA). Briefly, isolated protein samples were diluted in SDS sample buffer with 2-mercaptoethanol as reduction agent. Samples were then heated at 98°C for 5 min and afterwards applied to the gels. Electrophoresis was run at 125 V (stacking gel) and 250 V (resolving gel) at 4°C. Staining was carried out with Coomassie Brilliant Blue R250 (CBB) and destaining with a 10% acetic acid solution in 25% ethanol. Gels were scanned using G-Box (Syngene, Cambridge, United Kingdom).
2.3. Mass Spectrometry Analysis
Liquid chromatography electrospray ionization mass spectrometry (LC-ESI-MS/MS) was used to determine an unknown protein band ~150–250 kDa coming from the 1st peaks of column chromatography and detected on 10% SDS-PAGE. Due to the higher purity demands for ESI-MS/MS analysis (to avoid keratin and any other contamination), samples were run on a premade 7.5% polyacrylamide gel (Bio-Rad Laboratories, Hercules, CA, USA) at 250 V in a laminar air flow chamber. All samples and buffers were filter sterilized. After staining with CBB and destaining with 30% methanol, the stained band was cut out and stored at −20°C in sterile autoclaved microtubes for further MS analysis. Further destaining was done with 25 mM ammonium bicarbonate/50% (V/V) acetonitrile and in-gel digestion was performed using mass spectrometry grade modified trypsin (Promega, Madison, WI, USA) in 25 mM ammonium bicarbonate overnight at 37°C. The resulting peptides were extracted with 50% acetonitrile/5% formic acid (V/V), concentrated and analyzed on an ion trap mass spectrometer 1200 series HPLC-Chip-LC/MSD Trap XCT Ultra (Agilent Technologies, Waldbronn, Germany). MS and MS/MS spectra were searched against the non-redundant National Center for Biotechnology Information (NCBI-nr) database using the Mascot software (Matrix Science Ltd., UK).
2.4. Size Exclusion Chromatography
Size exclusion chromatography was carried out using Sephacryl S-300 HR, as matrix on a 16 mm × 100 cm column (16/100 XK, Pharmacia Biotech, Uppsala, Sweden) in order to separate β2GPI from proteoglycan 4 (PRG4) in the 1st peaks with a fractionation range for globular proteins from 10 to 1500 kDa [20]. The 1st peaks from several β2GPI isolations were collected and concentrated with Amicon Ultra centrifugation filters from regenerated cellulose with MWCO lower than 10 kDa (Millipore, Carrigtwohill Co., Cork, Ireland) at 2000 ×g. The final volume of ~2.5 mL was applied to the column with PBS as the mobile phase. The flow used was 10 mL/h and the volume of collected fractions was ~2.3 mL. Fractions from corresponding peaks were concentrated using Amicon Ultra centrifugation filters from regenerated cellulose with MWCO 30 kDa (Millipore Corporation, Billerica, MA, USA) and stored at −20°C.
2.5. In-House Anti-β2-Glycoprotein I ELISA
In-house anti-β2GPI ELISA was used to check the functionality of purified unnicked β2GPI [21, 22]. The test was conducted with seven sera samples from different autoimmune patients, positive and negative controls with respect to IgG, IgM, and IgA subtypes. Results were presented in arbitrary IgG, IgM, and IgA titer units (negative < 2, positive ≥ 2 and high positive ≥ 16).
Avidity of patient samples used for isolation of anti-β2GPI was determined using a chaotropic variant of the in-house anti-β2GPI ELISA. Anti-β2GPI were arbitrarily defined as HAv when the binding in PBS-0.05% Tween-20 (PBS-Tw) with 0.5 M NaCl remained 65% or higher than the initial binding in PBS-Tw (0.15 M NaCl) [18, 23].
2.6. Isolation of High Avidity Anti-β2-Glycoprotein I Antibodies
For isolation of polyclonal HAv anti-β2GPI from human sera, plasma, or immunoadsorption fractions, a stepwise procedure combining affinity chromatographies with immobilized protein G and human unnicked β2GPI were used [18]. Improvements of the elution step from the β2GPI affinity column were done as previously described [19, 24].
IgG fractions were concentrated to volumes of <50 mL using Ultrafiltration Membranes from regenerated cellulose with MWCO 50 kDa (Millipore Corporation, Billerica, MA, USA) in a 350 mL Amicon Stirred Ultrafiltration Cell unit (Millipore, Bedford, MA, USA) at 4°C and applied onto a column with immobilized human unnicked β2GPI (30 mg of pure unnicked β2GPI was coupled with 10 mL of CNBr-activated Sepharose 4B (Sigma-Aldrich, St Louis, MO, USA)) [18]. HAv anti-β2GPI were eluted from the column with 0.1 M glycine/4 M NaCl, pH 2.5 followed by PBS. These two steps were repeated 3 times. Fractions were immediately neutralized with neutralization buffer to pH ~7 and then dialyzed against PBS at 4°C overnight. Afterwards, HAv anti-β2GPI were concentrated as described above, using Amicon Ultra centrifugation filters from regenerated cellulose with MWCO 30 kDa, followed by sterile filtration (0.2 μm; Minisart, Sartorius Stedim Biotech GmbH, Goettingen, Germany). The concentrations of HAv anti-β2GPI in each step of isolation were derived from the standard curve according to the defined dilutions of IgG Sapporo Standard (HCAL; INOVA Diagnostics, San Diego, CA, USA) by in-house anti-β2GPI ELISA [21, 22].
2.7. Statistical Analysis and Data Presentation
Data are presented in mean values ± standard deviation (SD). Where statistical analysis was applied, each set of data was normally distributed according to Shapiro-Wilkes test. For statistical analysis the two-tail Student's t-test with significance level P < 0.05 was used.
3. Results
Modification of the β2GPI isolation procedure with special emphasis on duration of precipitation with PA improved the efficiency of the procedure by 51.5% (Table 1, Scheme 1). The precipitation was carried out in three smaller aliquots (~80–90 mL) from similar starting volumes (~250 mL), regardless of the isolation procedure. Lowering the precipitation volume (i.e., dividing the starting volume to smaller aliquots which were precipitated separately) consequently shortened the duration of PA precipitation from an average of 63 ± 6 min to 20 ± 1 min per aliquot. Elution from cationic exchange chromatography resulted in three protein peaks (Figure 1). After purity and functionality check with SDS-PAGE and anti-β2GPI ELISA, the isolated mass of unnicked human β2GPI rose significantly from 6.56 ± 1.38 mg (n = 12; range 3.63 to 8.85 mg) using the standard protocol of isolation [17] to 9.94 ± 1.57 mg (n = 5; range 8.69 to 12.56 mg) (P = 0.004), following optimization of the standard protocol.
Table 1.
β2-Glycoprotein I antigen isolation procedure: comparison of standard and optimized protocols.
| Standard protocol | Optimized protocol | β2GPI isolation from the same starting material after both protocols | ||
|---|---|---|---|---|
| AB plasma donors (n) | 14 | 6 | 2 | |
| Number of isolations | 12 | 5 | 1 (isolation I) | 1 (isolation II) |
| Starting volume, mean ± SD (mL) | 254 ± 24 | 246 ± 8 | 250 | 255 |
| Number of aliquots | 1 | 3 | 1 | 3 |
| Precipitation duration, mean ± SD (min) | 63 ± 6 | 20 ± 1 | 61.25 | 21.25 |
| Mass of isolated β2GPI, mean ± SD (mg) | 6.56 ± 1.38* | 9.94 ± 1.57* | 7.22 | 10.21 |
| Efficiency improvement | 51.5% | 41.4% | ||
β2GPI, β2-glycoprotein I. *Significantly higher mass (P = 0.004) as compared to standard protocol (Student's t-test, two-tail).
Scheme 1.
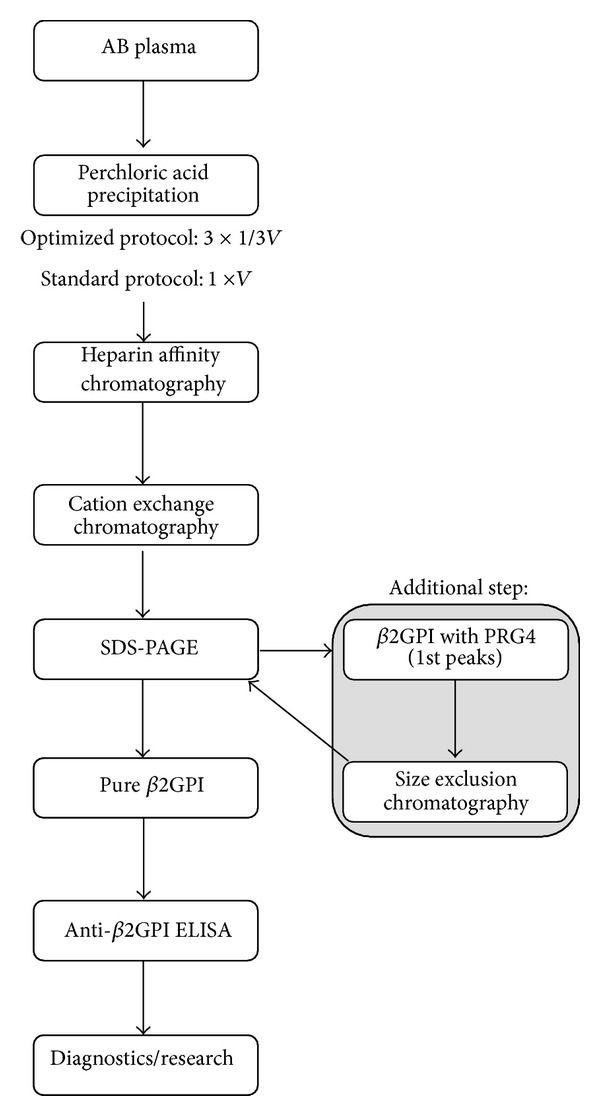
β2-Glycoprotein I isolation procedure after optimization and using size exclusion chromatography as an additional step. β2GPI: β2-glycoprotein I; PRG4: proteoglycan 4.
Figure 1.
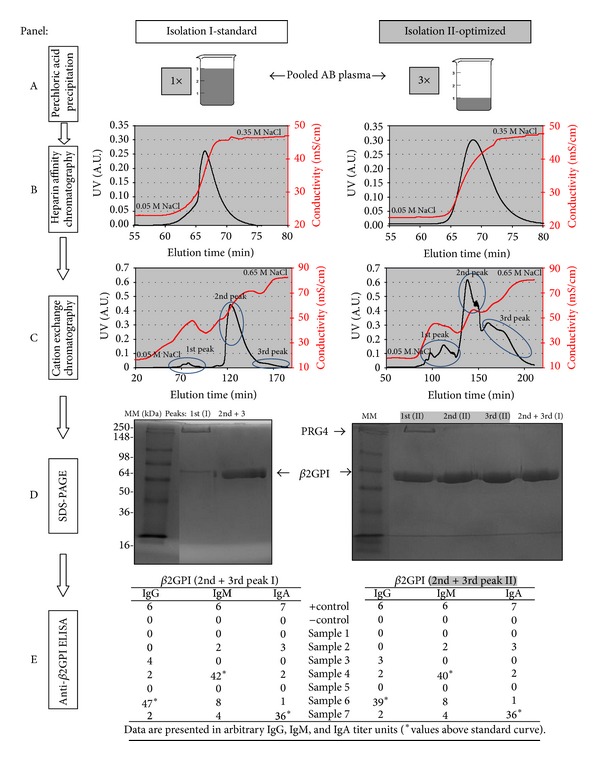
Flow chart and graphical data of β2-glycoprotein I isolation procedure: comparison between isolation data after standard (isolation I) and optimized (isolation II) protocols. Panel A: the perchloric acid precipitation was carried out with the whole volume (V) (isolation I) and in 3 aliquots (isolation II) from the same starting material (pooled plasma from 2 AB donors, see Table 1). Panel B: elution chromatograms with NaCl gradient after heparin affinity chromatography. Panel C: elution chromatograms with NaCl gradient after cation exchange chromatography. Panel D: purity check of protein fractions collected after cationic exchange chromatography as detected by Coomassie Brilliant Blue stained 10% SDS-PAGE (~5 μg of proteins/lane). Indicated are β2GPI in 2nd and 3rd peaks of isolations I and II, respectively, and PRG4 in 1st peak after optimized protocol (isolation II). Panel E: functionality check of isolated β2GPI. Pure β2GPI was used as antigen in anti-β2GPI ELISA. Data is presented in arbitrary IgG, IgM, and IgA units—negative < 2, positive ≥ 2, and high positive ≥ 16. Legend: A.U.: absorbance units; β2GPI: β2-glycoprotein I; MM: molecular weight marker; mS/cm: millisiemens per centimeter; PRG4: proteoglycan 4; SDS-PAGE: sodium dodecyl sulphate-polyacrylamide gel electrophoresis.
In one example, isolation data were compared after β2GPI isolation according to standard (isolation I) and optimized (isolation II) protocols from the same starting material, pooled from two AB plasma donors (Table 1, Figure 1). The starting volumes were 250 mL and 255 mL, with the precipitation time ~61 min according to standard and ~21 min per aliquot according to the optimized protocol, respectively. After elution from heparin affinity column the peak was higher in isolation II as compared to isolation I (Figure 1, Panel B). The next step yielded 3 peaks in isolations I and II (Figure 1, Panel C). Since in isolation I the total protein mass of the 3rd peak was very low and the amount of protein C inhibitor was negligible [17], this peak was not further separately examined. The purity check with 10% SDS-PAGE revealed 3 peaks with different quantity of β2GPI in isolation II, which were not different as compared to the band of unnicked β2GPI from the 2nd and 3rd peaks after the standard procedure (Figure 1, Panel D, bands >50 kDa). In the 1st peaks of both isolations a protein band with molecular weight over 250 kDa was present (described below). The functionality check with anti-β2GPI ELISA showed no differences between pure β2GPI isolated after the new optimized protocol (combined 2nd and 3rd peaks—isolation II) as compared to the unnicked β2GPI isolated according to the standard protocol (2nd peak—isolation I) (Figure 1, Panel E). In this specific case, the mass of isolated unnicked β2GPI rose by more than 41% (from 7.22 mg to 10.21 mg) (Table 1).
In the optimized isolation procedure we observed an impurity of high molecular weight (150–250 kDa) in the 1st peak (Figure 1, Panel D, isolation II). The unknown protein was determined to be proteoglycan 4 (PRG4; megakaryocyte stimulating factor or lubricin) using LC-ESI-MS/MS and NCBI-nr database searching of tryptic peptides. PRG4 was an impurity present in the highest amounts in the 1st peak. It was successfully separated from unnicked β2GPI by size exclusion chromatography.
From a functional prospective, 50 μL of 1 g/L β2GPI is routinely used for 96-well plates in our in-house ELISA for anti-β2GPI determination, which is sufficient for carrying out 48 tests (technical duplicates). Through the improvement of the isolation procedure, the calculated estimate for diagnostic use increased from ~6300 to ~9500 tests, gaining an additional ~3200 tests.
The average efficiency of the isolation procedure for polyclonal IgG anti-β2GPI from plasma on the protein G and β2GPI column was 8.9%, as determined by our in-house anti-β2GPI ELISA (Table 2). The average efficiency from different starting materials, such as immunoadsorption, sera, and plasma was 13.8% (ranging from 6% to 21.4%; data not shown). All isolated samples were established as HAv anti-β2GPI, due to their binding to antigen in PBS-Tw in a higher ionic strength environment (0.5 M NaCl) being ≥80% of the initial binding in PBS-Tw (0.15 M NaCl).
Table 2.
Isolation of human polyclonal high avidity IgG antibodies against β2-glycoprotein I: a representation of isolation efficiency calculation.
| Starting material (plasma) | V o (mL) | 205 |
| *C o HAv anti-β2GPI (μg/mL) | 33.9 | |
| *m o anti-β2GPI (μg) | 6950 | |
| 1. step | *m start HAv anti-β2GPI (μg) | 6950 |
| IgG isolation | *m isolated HAv anti-β2GPI (μg) | 6049 |
| (protein G column) | η (1. step) | 0.870 |
| 2. step | *m start HAv anti-β2GPI (μg) | 6049 |
| HAv aβ2GPI isolation | *m isolated HAv anti-β2GPI (μg) | 620 |
| (β2GPI column) | η (2. step) | 0.102 |
| η total (%) | 8.9% |
Concentrations and calculated masses were determined by in-house anti-β2GPI ELISA (*). β2GPI: β2-glycoprotein I; HAv anti-β2GPI: high avidity anti-β2GPI IgG antibodies; V o: volume of plasma used for isolation; C o: starting concentrations of HAv anti-β2GPI; m o, m start, and m isolated: calculated masses of HAv anti-β2GPI before isolation and before and after each step; and η: estimated efficiency of isolation.
4. Discussion
β2GPI and anti-β2GPI are important proteins in the pathology of APS, especially since anti-β2GPI represent one of the diagnostic markers/laboratory criteria for the disease classification. The main pathological functions of anti-β2GPI in APS rise from complexes formed by anti-β2GPI binding to β2GPI in the fishhook-open conformation and impacting the vascular system [25].
In order to improve the existing procedure for isolation of unnicked β2GPI, we found that the time of protein exposure to low pH was important. According to recent findings by Ağar et al. [9], we assume that the isolated and solubilized unnicked form of β2GPI is present in circular conformation, whereas in the anti-β2GPI ELISA β2GPI is expected to be unfolded, due to the physical and chemical characteristics of the microtiter plates. This would allow for hydrophobic and ionic binding (with respect to negatively charged carboxyl groups) to the fishhook conformation of β2GPI, making detection of anti-β2GPI possible and reproducible [21, 22]. Brighton et al. reported that purification of β2GPI using PA precipitation yields from 49.7–90.8% of unnicked β2GPI, whereas 9.2–50.3% of purified β2GPI was reported to be in the cleft form, depending on the elution peaks collected in the last step. Observed cleavage occurred at Lys317Thr318 and to a lesser extent at Ala314Phe315 as determined by N-terminal sequencing. Furthermore, in ELISA binding assays they observed no detectable binding to solid-phase anionic phospholipids in samples where 50% of β2GPI was cleft (~50% of β2GPI was unnicked), emphasizing the necessity of preserved structural integrity of β2GPI. The group speculated that β2GPI can be damaged in the above-mentioned segments in this isolation procedure, due to strong oxidative properties of PA [26]. Contrary to their observations, Cucnik et al. reported that the isolation with PA precipitation under controlled conditions, however different to Brighton et al. [26], yields unnicked β2GPI (wild-type) in each elution peak, as determined by SDS-PAGE, N-terminal sequencing, and anti-β2GPI ELISA [17]. If present, nicked β2GPI, could be found in quantities of <5%. One of the explanations for the different yields of unnicked/nicked β2GPI between the two reports could be the temperature at which precipitation with PA is carried out. Specifically, according to the standard protocol [17] Cucnik et al. added PA dropwise either for 3, 18, or 50 min on an ice bath (0–4°C), as compared to Brighton et al., where precipitation was conducted at room temperature (25°C) for ~15 min [26]. It could well be postulated that the lower temperature significantly slows the damaging and cleavage of β2GPI by PA. In the current report the time of precipitation with PA was significantly reduced to ~20 min, thus shortening the time of β2GPI contact with PA, as well as time of β2GPI in low pH. Compared to unnicked β2GPI gained after the standard protocol [17], the purified β2GPI after optimization exhibited similar characteristics on SDS-PAGE (Figure 1, Panel D) and anti-β2GPI ELISA (Figure 1, Panel E), indicating that after optimization we gain also structurally unmodified (e.g., unnicked) β2GPI.
Previously, the standard isolation procedure yielded three qualitatively different protein peaks with different quantity and purity of β2GPI [17]. In the 1st peaks also immune complexes of immunoglobulins and β2GPI were detected, the 2nd peaks yielded only β2GPI, whereas concomitant presence of β2GPI and protein C inhibitor was reported in the 3rd quantitatively smallest peaks [17]. Currently, we describe that using a modification of the β2GPI isolation procedure, the 1st elution peaks after cationic exchange chromatography, yielded a newly detected protein identified by LC-ESI-MS/MS as PRG4. This is a highly glycosylated human protein consisting of 1404 amino acids with a core molecular weight of ~150 kDA, ranging up to 400 kDa, depending on the O-glycosylation and glycosaminoglycan substitutions [27]. The main function of PRG4 is lubrication of articular cartilage in joints [28]. In synovial fluid, the observed average concentrations can be ~290 mg/L (287.1 ± 31.8 mg/L) [29], whereas in serum/plasma the concentrations of PRG4 have, to our knowledge, not been reported to date. For separation of β2GPI from PRG4 in the 1st peaks, a new step was added to the optimized protocol, specifically size exclusion chromatography (Scheme 1).
For diagnostic purposes of APS, the presence and quantity of anti-β2GPI are crucial. A subpopulation of anti-β2GPI, specifically HAv anti-β2GPI, was successfully isolated in the current report. The procedure involved multiple steps [24] including affinity binding onto a column with immobilized unnicked β2GPI. The average and the highest isolation efficiency from human sera, plasma, or immunoadsorption were determined by in-house anti-β2GPI ELISA as 13.8% and 21.4%, respectively. HAv anti-β2GPI present a clinically relevant subpopulation of anti-β2GPI, which can correlate with thrombosis in patients with APS [12, 13]. This correlation, as well as association of HAv anti-β2GPI with obstetric complications, was recently reported by Cucnik et al. [14, 30]. These data were confirmed by the European multicenter study analysing HAv anti-β2GPI that enrolled 226 out of 479 patients with primary APS and APS associated with other autoimmune diseases as well as patients with other non-APS autoimmune diseases [14]. Recently, it was also observed that HAv anti-β2GPI influenced human coronary artery endothelial cells to release chemotactic and inflammatory cytokines which consequently resulted in a higher migration of peripheral blood mononuclear cells to preconditioned supernatants [24].
5. Conclusions
Modification of the in-house isolation and purification procedures for unnicked β2GPI and polyclonal IgG anti-β2GPI of high avidity led to increased purity of both as well as a substantial elevation in the number of diagnostic tests performed.
Acknowledgments
This study was approved by the National Ethical Committee (no. 163/02/09) and is in accordance with the Helsinki Declaration of 1975, as revised in 1983, and was supported by the National Research Programme no. P3-0314 from the Ministry of Higher Education, Science and Technology, Slovenia.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Mehdi AA, Uthman I, Khamashta M. Antiphospholipid syndrome: pathogenesis and a window of treatment opportunities in the future. European Journal of Clinical Investigation. 2010;40(5):451–464. doi: 10.1111/j.1365-2362.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- 2.de Laat B, Mertens K, de Groot PG. Mechanisms of disease: antiphospholipid antibodies—from clinical association to pathologic mechanism. Nature Clinical Practice Rheumatology. 2008;4(4):192–199. doi: 10.1038/ncprheum0740. [DOI] [PubMed] [Google Scholar]
- 3.Favaloro EJ, Wong RCW. Laboratory testing for the antiphospholipid syndrome: making sense of antiphospholipid antibody assays. Clinical Chemistry and Laboratory Medicine. 2011;49(3):447–461. doi: 10.1515/CCLM.2011.064. [DOI] [PubMed] [Google Scholar]
- 4.Lozier J, Takahashi N, Putnam FW. Complete amino acid sequence of human plasma β2-glycoprotein I. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(12):3640–3644. doi: 10.1073/pnas.81.12.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin F, Murphy R, White B, et al. Circulating levels of β2-glycoprotein I in thrombotic disorders and in inflammation. Lupus. 2006;15(2):87–93. doi: 10.1191/0961203306lu2270oa. [DOI] [PubMed] [Google Scholar]
- 6.Hunt JE, Simpson RJ, Krilis SA. Identification of a region of β2-glycoprotein I critical for lipid binding and anti-cardiolipin antibody cofactor activity. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(6):2141–2145. doi: 10.1073/pnas.90.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarzenbacher R, Zeth K, Diederichs K, et al. Crystal structure of human β2-glycoprotein I: implications for phospholipid binding and the antiphospholipid syndrome. EMBO Journal. 1999;18(22):6228–6239. doi: 10.1093/emboj/18.22.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horbach DA, Van Oort E, Lisman T, Meijers JCM, Derksen RHWM, De Groot PG. β2-glycoprotein I is proteolytically cleaved in vivo upon activation of fibrinolysis. Thrombosis and Haemostasis. 1999;81(1):87–95. [PubMed] [Google Scholar]
- 9.Ağar Ç, Van Os GMA, Mörgelin M, et al. β2-Glycoprotein I can exist in 2 conformations: implications for our understanding of the antiphospholipid syndrome. Blood. 2010;116(8):1336–1343. doi: 10.1182/blood-2009-12-260976. [DOI] [PubMed] [Google Scholar]
- 10.De Laat B, Derksen RHWM, Van Lummel M, Pennings MTT, De Groot PG. Pathogenic anti-β2-glycoprotein I antibodies recognize domain I of β2-glycoprotein I only after a conformational change. Blood. 2006;107(5):1916–1924. doi: 10.1182/blood-2005-05-1943. [DOI] [PubMed] [Google Scholar]
- 11.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) Journal of Thrombosis and Haemostasis. 2006;4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 12.Čučnik S, Božič B, Kveder T, Tomšič M, Rozman B. Avidity of anti-β2-glycoprotein I and thrombosis or pregnancy loss in patients with antiphospholipid syndrome. Annals of the New York Academy of Sciences. 2005;1051:141–147. doi: 10.1196/annals.1361.055. [DOI] [PubMed] [Google Scholar]
- 13.de Laat B, Derksen RHWM, de Groot PG. High-avidity anti-β2 glycoprotein I antibodies highly correlate with thrombosis in contrast to low-avidity anti-β2 glycoprotein I antibodies. Journal of Thrombosis and Haemostasis. 2006;4(7):1619–1621. doi: 10.1111/j.1538-7836.2006.02002.x. [DOI] [PubMed] [Google Scholar]
- 14.Cucnik S, Kveder T, Artenjak A, et al. Avidity of anti-beta2-glycoprotein I antibodies in patients with antiphospholipid syndrome. Lupus. 2012;21(7):764–765. doi: 10.1177/0961203312440057. [DOI] [PubMed] [Google Scholar]
- 15.Shoenfeld Y, Gerli R, Doria A, et al. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation. 2005;112(21):3337–3347. doi: 10.1161/CIRCULATIONAHA.104.507996. [DOI] [PubMed] [Google Scholar]
- 16.Artenjak A, Lakota K, Frank M, et al. Antiphospholipid antibodies as non-traditional risk factors in atherosclerosis based cardiovascular diseases without overt autoimmunity. A critical updated review. Autoimmunity Reviews. 2012;11(12):873–882. doi: 10.1016/j.autrev.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Cucnik S, Krizaj I, Rozman B, et al. Concomitant isolation of protein C inhibitor and unnicked beta2-glycoprotein I. Clinical Chemistry and Laboratory Medicine. 2004;42(2):171–174. doi: 10.1515/CCLM.2004.031. [DOI] [PubMed] [Google Scholar]
- 18.Žager U, Irman Š, Lunder M, et al. Immunochemical properties and pathological relevance of anti-β2-glycoprotein I antibodies of different avidity. International Immunology. 2011;23(8):511–518. doi: 10.1093/intimm/dxr043. [DOI] [PubMed] [Google Scholar]
- 19.Omersel J, Žager U, Kveder T, Božič B. Alteration of antibody specificity during isolation and storage. Journal of Immunoassay and Immunochemistry. 2010;31(1):45–59. doi: 10.1080/15321810903405027. [DOI] [PubMed] [Google Scholar]
- 20.Omersel J, Avberšek-Lužnik I, Grabnar PA, Kveder T, Rozman B, Božič B. Autoimmune reactivity of IgM acquired after oxidation. Redox Report. 2011;16(6):248–256. doi: 10.1179/174329211X13190184351680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cucnik S, Ambrozic A, Bozic B, Skitek M, Kveder T. Anti-β2-glycoprotein I ELISA: methodology, determination of cut-off values in 434 healthy Caucasians and evaluation of monoclonal antibodies as possible international standards. Clinical Chemistry and Laboratory Medicine. 2000;38(8):777–783. doi: 10.1515/CCLM.2000.111. [DOI] [PubMed] [Google Scholar]
- 22.Avcin T, Ambrozic A, Bozic B, et al. Estimation of anticardiolipin antibodies, anti-beta2 glycoprotein I antibodies and lupus anticoagulant in a prospective longitudinal study of children with juvenile idiopathic arthritis. Clinical and Experimental Rheumatology. 2002;20(1):101–108. [PubMed] [Google Scholar]
- 23.Čučnik S, Kveder T, Križaj I, Rozman B, Božič B. High avidity anti-β2-glycoprotein I antibodies in patients with antiphospholipid syndrome. Annals of the Rheumatic Diseases. 2004;63(11):1478–1482. doi: 10.1136/ard.2003.017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Artenjak A, Kozelj M, Lakota K, et al. High avidity anti-b2-glycoprotein I antibodies activate human coronary artery endothelial cells and trigger peripheral blood mononuclear cell migration. European Journal of Inflammation. 2013;11(2):385–396. [Google Scholar]
- 25.Meroni PL, Borghi MO, Raschi E, Tedesco F. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nature Reviews Rheumatology. 2011;7(6):330–339. doi: 10.1038/nrrheum.2011.52. [DOI] [PubMed] [Google Scholar]
- 26.Brighton TA, Dai Y-P, Hogg PJ, Chesterman CN. Microheterogeneity of beta-2 glycoprotein I: implications for binding to anionic phospholipids. Biochemical Journal. 1999;340(1):59–67. [PMC free article] [PubMed] [Google Scholar]
- 27.Steele BL, Alvarez-Veronesi MC, Schmidt TA. Molecular weight characterization of PRG4 proteins using multi-angle laser light scattering (MALLS) Osteoarthritis Cartilage. 2013;21(3):498–504. doi: 10.1016/j.joca.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis and Rheumatism. 2007;56(3):882–891. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 29.Ludwig TE, McAllister JR, Lun V, et al. Diminished cartilage-lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4: restoration through proteoglycan 4 supplementation. Arthritis & Rheumatism. 2012;64(12):3963–3971. doi: 10.1002/art.34674. [DOI] [PubMed] [Google Scholar]
- 30.Čučnik S, Kveder T, Ulcova GZ, et al. The avidity of anti-β2-glycoprotein i antibodies in patients with or without antiphospholipid syndrome: a collaborative study in the frame of the European forum on antiphospholipid antibodies. Lupus. 2011;20(11):1166–1171. doi: 10.1177/0961203311406308. [DOI] [PubMed] [Google Scholar]


