Abstract
Background
Bone fracture increases alarmins and pro-inflammatory cytokines in the blood, and provokes macrophage infiltration and pro-inflammatory cytokine expression in the hippocampus. We recently reported that stroke is an independent risk factor after bone surgery for adverse outcome, the impact of bone fracture on stroke outcome is unknown. We tested the hypothesis that bone fracture, shortly after ischemic stroke, enhances stroke-related injuries by augmenting the neuroinflammatory response.
Methods
Tibia fracture (bone fracture) was induced in mice one day after permanent occlusion of the distal middle cerebral artery (stroke). High-mobility-group box chromosomal protein-1 (HMGB1) was tested to mimic the bone fracture effects. HMGB1 neutralizing antibody and clodrolip (macrophage depletion) were tested to attenuate the bone fracture effects. Neurobehavioral function (n=10), infarct volume, neuronal death, and macrophages/microglia-infiltration (n=6–7) were analyzed three days after.
Results
We found that mice with both stroke and bone fracture had larger infarct volumes (mean percentage of ipsilateral hemisphere±SD: 30±7% vs. 12±3%, n=6, P<0.001) more severe neurobehavioral dysfunction, and more macrophages/microglia in the peri-infarct region than mice with stroke only. Intraperitoneal injection of HMGB1 mimicked, whereas neutralizing HMGB1 attenuated, the bone fracture effects and the macrophage/microglia infiltration. Depleting macrophages with clodrolip also attenuated the aggravating effects of bone fracture on stroke lesion and behavioral dysfunction.
Conclusions
These novel findings suggest that bone fracture shortly after stroke enhances stroke injury via augmented inflammation through HMGB1 and macrophage/microglia infiltration. Interventions to modulate early macrophage/microglia activation could be therapeutic goals to limit the adverse consequences of bone fracture after stroke.
Introduction
Stroke is the leading cause of disability in adults1 and an important risk factor for bone fracture.2 In the United States, approximately 70,000 stroke victims suffer from bone fracture within the first year after their stroke.3, 4 A small proportion of these stroke patients experience bone fracture within the first 24 hours after the ischemic stroke, but the impact of bone fracture on acute stroke lesion is unknown. In a database cohort study including more than 270,000 European patients hospitalized for stroke from 1987 to 1996, the authors found that overall, 9% of the stroke patients had a subsequent fracture.4 Interestingly, the increase in risk was most evident immediately after the stroke. Based on the data provided by Kanis et al,4 the estimated incidence of having a bone fracture within 24 hours of a stroke is 2.4–3.6/100,000, corresponding to 1–1.5% of stroke patients. Thus, about 7,000 to 11,000 patients in the U.S. and 167,000–250,000 worldwide will experience a fall-fracture within the first day of the stroke. More recently, an American study confirmed that the hazard ratio of suffering from a hip fracture during the first 24 hours after the stroke diagnosis was significantly increased by 3.9 (95% confidence interval of 2.1–7.3), when compared to a non-stroke population.3
Our retrospective review of more than 400,000 surgical patients revealed that stroke is an independent risk factor for poor outcome after orthopedic bone surgery but not abdominal aortic surgery,5 suggesting a specific interaction between stroke and bone surgery. Understanding the impact and underlying mechanism of this interaction will enable clinicians to intervene appropriately and to design target-selective neuroprotective strategies perioperatively.
In a model of aseptic bone fracture, circulating alarmins, including high-mobility-group box chromosomal protein-1 (HMGB1) and pro-inflammatory cytokines,6, 7 increase; in addition, aseptic bone fracture provokes macrophage infiltration and pro-inflammatory cytokine expression in the hippocampus.8 HMGB1, a non-histone DNA-binding protein stabilizes nucleosome formation and DNA repair;9 additionally, it activates pattern recognition receptors (e.g., Toll-like receptors 2 and 4, and the receptor for advanced glycation end-products [RAGE]) on bone marrow-derived monocytes and macrophages to initiate an innate immune response.10 Because systemic and local inflammation in the acute phase of ischemic stroke may have deleterious effects on stroke outcome,11–13 and because the risk of bone fracture in the stroke population is mainly just after the brain insult,3, 4 we tested the hypothesis that bone fracture, one day after ischemic stroke, aggravates brain damage and functional consequences of stroke.
Materials and Methods
Animals
All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco, and conformed to the National Institutes of Health Guidelines. All animals were fed standard rodent food and water ad libitum, and were housed (5 mice per cage) in sawdust-lined cages in an air-conditioned environment with 12-hour light/dark cycles.
Wild-type male mice (C57BL/6J, 10–12 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME). CCR2RFP/+CX3CR1GFP/+ mice (10–12 weeks old)14 were provided by Katerina Akassoglou, PhD, Israel F. Charo, MD, PhD, and Kim Baeten, PhD (Associate Investigator, Associate Director, and Postdoctoral Fellow, respectively, University of California, San Francisco Gladstone Institute, San Francisco, California). CCR2 and CXCR1 are acronyms for chemokine (C-C motif) receptor 2 (monocyte chemoattractant protein-1, MCP-1), highly expressed in bone marrow-derived macrophages, and CX3C chemokine receptor 1 (fractalkine receptor), highly expressed in resident microglia, respectively.
Animals were tagged and randomly allocated to each group before any treatment. Researchers blinded to the group assignment performed all neurobehavioral tests, infarct volume and cell counting. Based on preliminary data, in corner tests, there was a standard deviation of 15% in the percentage of left turns 3 days after pMCAO (permanent occlusion of the Middle Cerebral Artery). We estimated that a sample of 9 mice per group was necessary to find a significant difference between the pMCAO mice and the pMCAO+bone fracture mice with 80% of power if the difference was 20%. For this reason, we included n=10 mice per group for each behavior tests comparison.
Human Blood Samples
Under an approved protocol by the University of California, San Francisco Committee on Human Research (CHR, Study number: H5636-20263-09), four individuals presenting with osteoarthritis elective for total knee replacement under spinal anesthesia were enrolled. Blood was drawn immediately before and after the tourniquet was released using an uncoated tube. Blood samples were centrifuged at 1300 rpm for 10 min at room temperature and the serum samples were immediately frozen at −80°C.
Permanent Occlusion of the Middle Cerebral Artery (pMCAO) for Stroke Model
Following anesthesia (Isoflurane, 2%), under aseptic surgical condition, animals received a left craniotomy and a dissection of the dura. The left middle cerebral artery was permanently occluded (pMCAO) using electrical coagulation just proximal to the pyriform branch. Rectal temperature was maintained at 37±0.5°C using a thermal blanket throughout the surgical procedure. Surface cerebral blood flow was monitored during the procedure using a laser Doppler flowmeter (Vasamedics Inc, Little Canada, MN). Mice were excluded from further analysis when the surface cerebral blood flow in the ischemic core was more than 15% of the baseline after pMCAO, or if the artery injuries with the coagulator generate a massive bleeding. Animals were allowed to recover spontaneously from the anesthetic under warm conditions and received one intraperitoneal injection of buprenorphine (0.3 mg in 100 µl saline). Control mice were subjected to craniotomy without arterial occlusion but with the same amount and duration of anesthesia and the same amount of buprenorphine (0.3 mg in 100 µl saline) used for stroke mice. In this study, a total of 6 C57BL/6J mice were euthanized during the pMCAO procedures due to massive bleeding induced by vascular surgical injury, and were replaced by other mice from the same cage. No mouse was lost during the experiment’s 3-day duration.
Tibia Fracture Surgery for Bone Fracture Model
Twenty-four hours after the pMCAO procedure, animals were given general anesthesia with 2% isoflurane inhalation. Under aseptic surgical conditions, animals received an open tibia fracture of the right hind limb with an intramedullary fixation as previously described.6 Animals were allowed to recover spontaneously from the anesthetic under warm conditions and received one intraperitoneal injection of buprenorphine (0.3 mg in 100 µl saline). Rectal temperature was maintained at 37±0.5°C using a thermal blanket throughout the surgical procedure. Repeated measurements of arterial systolic blood pressure were performed using the tail cuff method (ML125M, AD Instruments, Colorado Spings, CO) as previously described.15 The mice subjected to craniotomy and pMCAO had similar tail arterial systolic blood pressure before the bone fracture procedure (data not shown). Control mice for bone fracture received hind limb hair shaving with the same amount and duration of anesthesia and analgesia (buprenorphine, 0.3 mg in 100 µl saline) as for the bone fracture mice. Body weight of the animals was measured before pMCAO and immediately after the neurobehavioral tests. Mice with pMCAO alone presented a significant loss of weight when compared to control mice but the groups of mice with pMCAO with and without bone fracture were not different. HMGB1ab and clodrolip did not influence the loss of body weight (data not shown). The tibia fracture surgery did not present any lethality.
Chemical Reagents
Based on serum levels of HMGB1 after mice tibia fracture,{Terrando, 2010 #24649} we injected 50 µg/Kg (100 µl) of recombinant HMGB1 (R&D System, Minneapolis, MN) intraperitoneally 24 hours after the stroke. Control animals received the same volume (100 µl) of the vehicle (saline). To neutralize HMGB1 in the blood, we injected anti-HMGB1 antibodies (chicken IgG, IBL International, Toronto, Canada), 200 µg in 100 µl (corresponding to 10 mg/kg) intraperitoneally, 60 minutes before the bone fracture. Control animals received the same volume (100 µl) of the control chicken IgG antibodies (IBL international, 10 mg/Kg). Clodronate liposomes (clodrolip) were obtained from clodronateliposomes.org (Vrije Universiteit, Amsterdam, Netherlands) at 7 mg/ml concentration and prepared as previously described.16, 17 Clodrolip (200 µl, about 100 mg/Kg) was injected intraperitoneally 60 minutes before the bone fracture. Control animals received 200 µl of control liposomal solution.
Behavioral Tests
All tests were conducted 3 days after the pMCAO.
Corner Test
As previously described,18 the corner test was used to detect sensorimotor and postural asymmetries. Mice were placed between two boards with identical dimensions (30 × 20 cm). When mice neared the corner, both sides of their vibrissae were stimulated. The mice would rear forward and upward, then turn back to face the open end. Normal mice would turn to the left or right side with equal frequency, whereas the stroke mice would turn more often to the ispilateral side of the lesion (left). The percentage of left turns was recorded in 3 different sets of 10 trials. Turning movements not incorporated in a rearing movement were not recorded.
Adhesive Removal Test
To assess the forepaw lateral sensitivity and point out a possible somatosensory neglect, we performed the adhesive removal test.19 Briefly, adhesive tape (0.3 × 0.3 cm) was applied on each paw. The time that it took for the mice to remove the tape from each paw was recorded with a maximum testing time of 120 seconds. Mice were trained three times daily for 4 days before the surgery to obtain an optimal level of performance.
Evaluation of Infarct Volume
Three days after the pMCAO, brain samples were collected after paraformaldehyde 4% perfusion. A series of 20-µm thick coronal sections was obtained. One in every 10 sections was stained with cresyl violet (one section per 200 µm thick tissue) and sections were digitized. After binary imaging, the infarct and the ipsilateral hemisphere areas were outlined using Image J software, then measured.20 The infarct and ipsilateral hemisphere volumes were estimated as the sum of each area multiplied by 200 µm. The ratio of infarct volume verse ipsilateral hemisphere volume was calculated.
Measurement of Mice HMGB1 in Serum
Six hours after the bone fracture procedure, blood was collected by cardiac puncture under general anesthesia (isoflurane, 3%). Blood samples were centrifuged at 1300 rpm for 10 min at room temperature and the serum was collected and frozen at −80°C. HMGB1 levels in the serum of the mice and the human samples were quantified using the HMGB1 ELISA kit (IBL International).
Measurement of Cytokines in the Brain Lesion
The left frontal-parietal cortical region of the mice was rapidly collected under a dissecting microscope 6 hours after the bone fracture, and placed in RNAlater™ solution (Qiagen, Valencia, CA). To avoid blood contamination, mice were perfused with saline for 5 minutes before sample collection. Total RNA was extracted using RNeasy Lipid tissue Kit (Qiagen) treated with recombinant DNase I using a RNase-Free Dnase set™ (Qiagen), and reverse-transcribed to complementary deoxyribonucleic acid with a High Capacity RNA to-cDNA Kit (Applied Biosystems, Carlsbad, CA). TaqMan Fast Advanced Master Mix (Applied Biosystems, CA) and gene specific primers and probes used for real-time polymerase chain reaction are: beta-actin (NM_007393.1), interleukin-6 (IL-6, Mm00446190_m1), tumor necrosis factor-α (Mm00443258_m1), and IL-1β (Mm01336189_m1). Real-time polymerase chain reaction was performed using StepOnePlus™ (Applied Biosystems). Each RNA sample was run in triplicate, and relative gene expression was calculated using the comparative threshold cycle ΔCT and normalized to ACTB. Results are expressed as fold-increases relative to controls.
Histological Analysis 3 Days After Ischemia Onset
Immunohistochemical staining was performed using a series of 20-µm thick coronal sections. All the quantifications were performed using the sections in the same anatomical region (bregma 1.2 to 1.4 mm). For immunostaining, sections were incubated with the following primary antibodies: CD68 (1:50, AbD Serotec, MCA1957, Raleigh, NC) and NeuN (Neuronal Nuclei, 1:500, MAB377, Millipore, Bedford, MA). Sections were then incubated with Alexa Fuor 647-conjugated, Alexa Fluor 594-conjugated, and Alexa Fluor 488-conjugated IgG (1:500, Invitrogen, Carlsbad, CA). Negative controls were performed by omitting the primary or the secondary antibodies in the staining procedures. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay was performed using the dedicated kit (ApopTag, Millipore) per instructions in the manual. The NeuN-TUNEL double staining was verified with confocal imaging and quantified using image J (NIH, USA) at the peri-infarct region inside the cortical infarct border, using three different pictures per mice taken under 40X objective (Figure 1). CD68+cells, CCR2+ cells, CX3CR1+ cells and CCR2+ with CX3CR1+ double-positive cells were counted separately, using three different pictures per mice taken under 40X objective (Figure 1) at the peri-infarct region outside the infarct border.21 CD68 staining and expression of CCR2-RFP and CX3CR1-GFP were verified using confocal images (data not shown) and quantified using Image J (National Institutes of Health, Bethesda, MD), with three different pictures/mice taken under 40X objective. The effectiveness of clodrolip in the depletion of macrophages was verified by CD68 staining of 20-µm thick spleen sections.
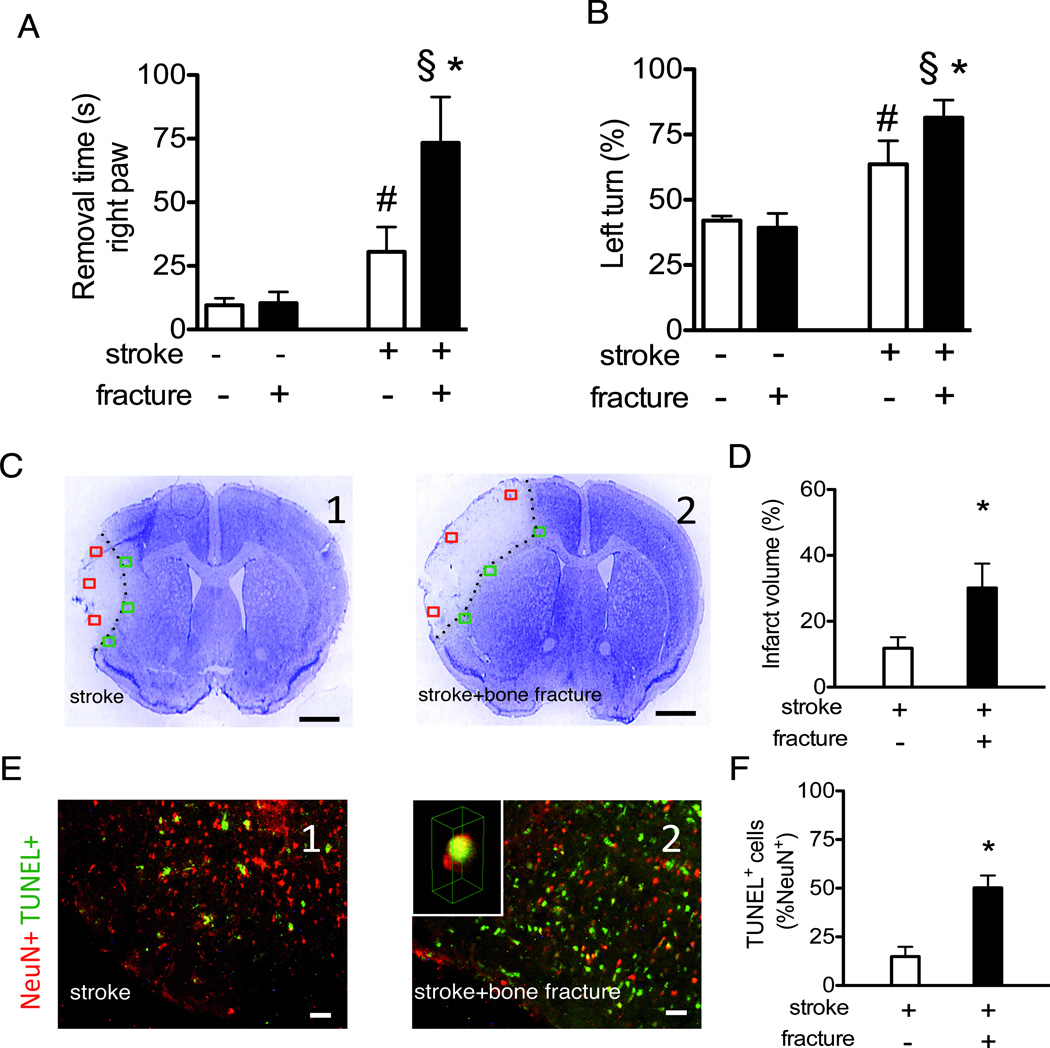
Figure 1. Bone fracture enhances neuronal injury.
A. Quantification of the time used to remove the tape from the right paw (contralateral) in adhesive removal test. (n=10, #: P<0.001 vs. mice subjected to sham procedures for stroke and bone fracture, §: P<0.001 vs. bone fracture group *: P<0.001 vs. stroke group). B. Quantification of corner test. (n=10, #: P<0.001 vs. mice subjected to sham procedures for stroke and bone fracture, §: P<0.001 vs. bone fracture group, *: P<0.001 vs. stroke group). C. Representative images of cresyl violet stained brain sections (bregma 1.3 mm, scale bar: 1 mm) of stroke (C1) or stroke and bone fracture mice (C2). The red squares correspond to the 3 regions used to quantify NeuN-TUNEL positive cells. The green squares correspond to the 3 regions used to quantify the CX3CR1, CCR2 and CD68 cells. D. The bar graph shows the quantification of infarct volumes (n=7, *: P<0.001). E. Representative NeuN (red) and TUNEL (green) co-stained pictures of stroke (E1) or stroke and bone fracture mice (E2). Insert in E2 is a 3-D reconstructed confocal image showing a nucleus stained positively for both NeuN and TUNEL (yellow). F. Bar graph shows the quantification of TUNEL+ neurons (n=7, *: P<0.001). NeuN: Neuronal Nuclei; TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling.
Statistical Analyses
Data are presented as mean ± standard deviation (SD). Gaussian distribution was tested with d’Agostino and Pearson omnibus normality test. Equalities of variances were tested with the F test. For dual comparisons, t-tests (Student, Mann-Wittney for non-Gaussian distribution, and Welch’s correction of unequal variances) were used when appropriate. For multiple comparisons, means were compared using one-way ANOVA analysis followed by Bonferroni post hoc correction.
Comparison of the human and mice HMGB1 expressions before and after bone fracture was performed with 2-way ANOVA. Correlations were analyzed using the Pearson r coefficient. A two-tailed P value < 0.05 was considered statistically significant. Prism 5 (GraphPad Software Inc, La Jolla, CA) was used to conduct the statistical analyses.
Results
Bone Fracture Aggravates Functional and Morphological Consequences of Stroke
Mice with stroke alone exhibited neurobehavioral deficits, e.g., increased time to remove adhesive on the contralateral right paw and more turns to the lesion side (left turn) in the corner test, than sham-operated mice subjected to craniotomy only (Fig 1A-B). These neurobehavioral abnormalities of stroke were significantly worsened by bone fracture, with longer latency times to remove the adhesive on the contralateral (P<0.001, Fig 1A) paw and higher percentage of left turns in the corner test (P<0.001, Fig 1B). Bone fracture alone did not affect neurobehavioral function and the time to remove adhesive on the ipsilateral paw was not increased in the groups of mice with stroke alone and bone fracture alone (data not shown).
Compared to mice with stroke alone, mice with both stroke and bone fracture had infarct volumes almost three times larger (P<0.001, Fig 1C-D) and more TUNEL positive neurons in the peri-infarct region right inside the border of the cortical infarct area (Fig 1E-F).
Bone Fracture Shortly After Stroke Exacerbates Brain Inflammation
Thirty hours after occlusion, mice with stroke alone exhibited higher transcript expression than controls in the left hemisphere (lesion side) for IL-1β and tumor necrosis factor-α but not for IL-6 (Fig 2A-C). Six hours after the bone fracture (corresponding to 30 hours after the stroke), mice with both injuries had higher transcript levels of IL-6 in the left hemisphere than mice with stroke (P=0.01, Fig 2A). The IL-1β level also trended higher in mice with both injuries than mice with stroke alone (Fig 2B, P=0.09). Furthermore, mice with both injuries presented higher transcript expression for IL-6, IL-1β and tumor necrosis factor-α than mice with bone fracture only (Fig 2A-C).
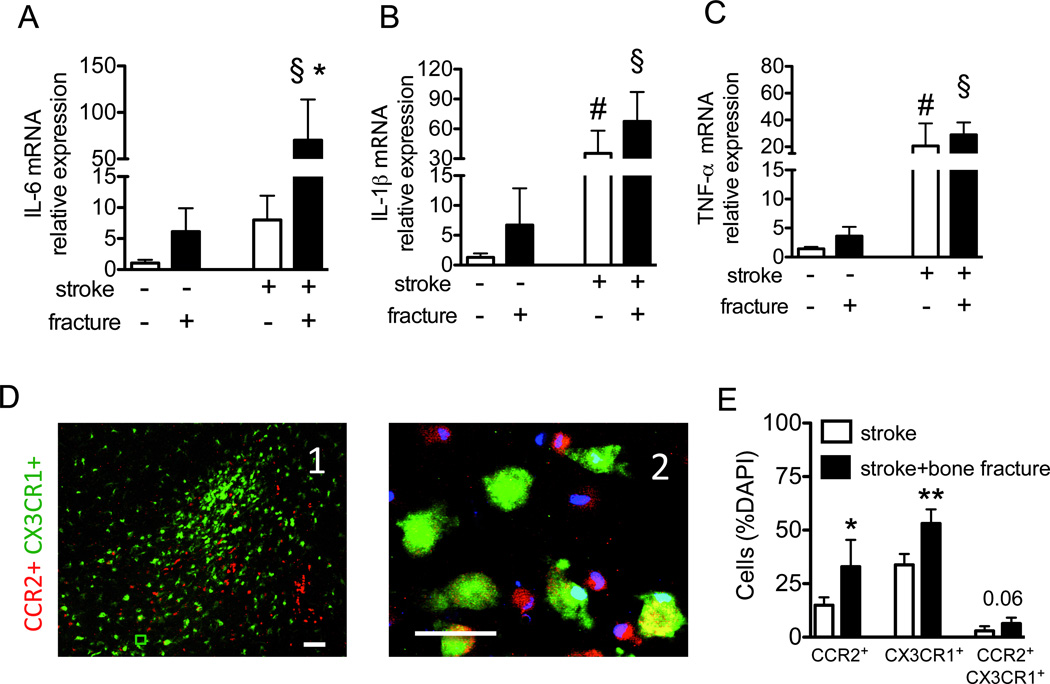
Figure 2. Bone fracture exacerbates brain inflammation.
Relative messenger RNA expression of IL-6 (A), IL-1β (B) and TNF-α (C) six hours after bone fracture and 30 hours after the stroke in the stroke lesion (n=5; (A) *: P=0.01 vs. stroke group without fracture, §: P=0.01 vs. bone fracture group; (B) #: P<0.001 vs. sham procedures for stroke §: P<0.001 vs. bone fracture group; (C) #: P=0.03 vs. the group subjected to sham procedures for stroke and §: P=0.002 vs. bone fracture group). The mice with stroke and bone fracture showed a trend towards higher IL-1β in the brain tissue then the mice with stroke only (P=0.09). D. Representative picture taken in the peri-infarct region of CCR2RFP/+CX3CR1GFP/+ mice with stroke and bone fracture. D1, A low magnified (scale bar: 100 µm) and D2, a high-magnified (scale bar: 50 µm) images show that there are many CCR2+ (red) and CX3CR1+ (green) cells.. Some cells are CCR2-CX3CR1 double positive (yellow). E. The bar graph shows quantification of the percentage of CCR2+, CX3CR1+ or CCR2&CX3CR1+ cells amount total (DAPI positive nuclei) cells in the peri-infarct region (n=5, *: P=0.01 and **: P<0.001). DAPI: 4',6-Diamidino-2-Phenylindole; IL: interleukin; mRNA: messenger ribonucleic acid; TNF: tumor necrosis factor.
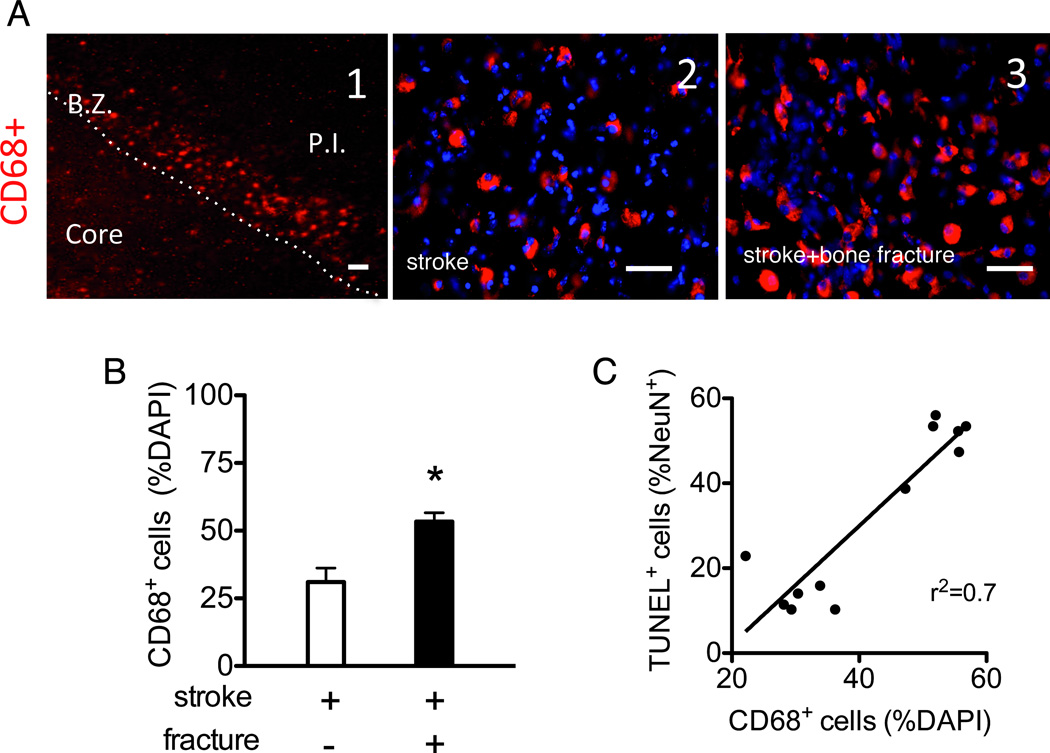
Using CCR2RFP/+CX3CR1GFP/+ mice,14 we demonstrated that bone fracture increased both bone marrow-derived CCR2+ macrophages (P=0.01) and resident CX3CR1+ microglia in the peri-infarct region (P<0.001), compared to mice with stroke only (Fig 2D-E). About 20% of the CCR2+ cells and ≈10% of the CX3CR1+ cells expressed both CX3CR1 and CCR2 (data not shown). Mice with both stroke and bone fracture showed a trend toward more double-positive cells than mice with stroke only. The number of CCR2+ cells positively correlated with CX3CR1+ cell number (r=0.40, P=0.03). CCR2+macrophages and active CX3CR1+ microglia in the peri-infarct region expressed CD68, whereas CX3CR1+ cells in the contralateral hemisphere (inactive microglia) did not (data not shown);22 mice with both stroke and bone fracture had more CD68+ cells in the peri-infarct region than mice with stroke alone (P<0.001, Fig 3A-B). The ratio of CD68 positive cells correlated with the ratio of NeuN-TUNEL positive cells (Fig 3C; r2=0.70, P<0.001).
Figure 3. Bone fracture exacerbates the recruitment of activated macrophages.
A. Representative images of CD68 antibody stained section. A1. A low magnified picture showing the border zone (B.Z.) and the peri-infarct region outside (P.I.) and inside the border (core). CD68+ cells formed a band just outside the infarct border. scale bar: 100 µm A2 and A3 are representative pictures taken in the peri-infarct region outside the infarct border of mice with stroke (A2) or stroke plus bone fracture mice (A3). Scale bar: 50 µm. B. Bar graph shows the quantification of CD68+ cells in the peri-infarct region (n=7, *: P<0.001). C. Correlation between the numbers of CD68+cells and TUNEL+ neurons (r2=0.70, P<0.001). TUNEL: Terminal deoxynucleotidyl transferase dUTP nick end labeling.
HMGB1 Plays a Causative Role in the Exacerbating Effects of Bone Fracture on Stroke Injury
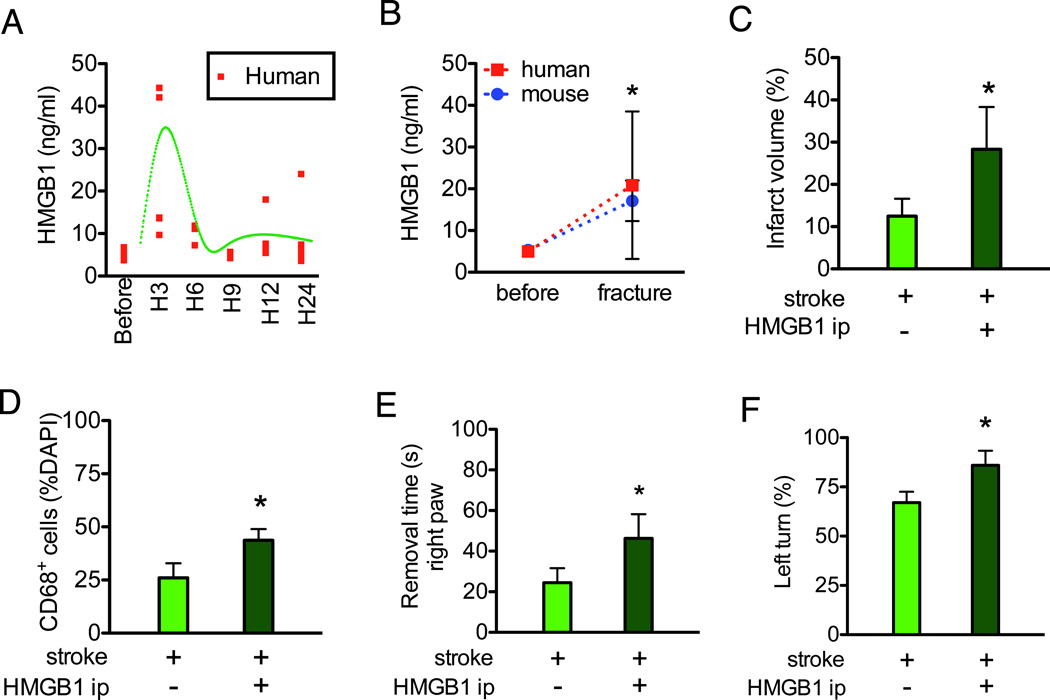
HMGB1 increased in the serum within six hours of bone fracture in both patients and mice (Fig4A-B). HMGB1 (50 µg/kg), injected intraperitoneally one day after stroke, produced significant larger infarct volumes (Fig 4C) and more TUNEL positive neurons in the infarct area than the saline-treated stroke mice (13±2% vs. 43±10%, P<0.001, n=6). HMGB1 treatment increased CD68+ cells in the peri-infarct region (Fig 4D). HMGB1-treated stroke mice demonstrated more severe neurobehavioral dysfunction with increased adhesive removal time (Fig 4E) and percentage of left turns in the corner test (Fig 4F).
Figure 4. HMGB1 injection mimics the negative impact of bone fracture on stroke injury.
A. HMGB1 levels in the human serum before, and three, six, nine, 12 and 24 hours after total knee replacement surgery. Red dots represent the levels in individual patients. The green curve shows the global shape of the time course (n=4) B. Comparison of HMGB1 in human and mice serum before and after bone fracture (n=4 for human and n=5 for mice *: P<0.001 vs. before bone fracture). C. Intraperitoneal injection of HMGB1 increased the infarct volume of stroke mice (n=6, *: P=0.003). D. Intraperitoneal injection of HMGB1 increased CD68+ cells in the peri-infarct region (n=6, *: P<0.001). E. Intraperitoneal injection of HMGB1 increased the time for stroke mice to remove the tape from the right paws (n=10, *: P<0.001). F. Intraperitoneal injection of HMGB1 increased the percentage of left turn in the corner test (n=10, *: P<0.001). DAPI: 4',6-Diamidino-2-Phenylindole; HMGB1:High-mobility-group box chromosomal protein-1; ip: intraperitoneal.
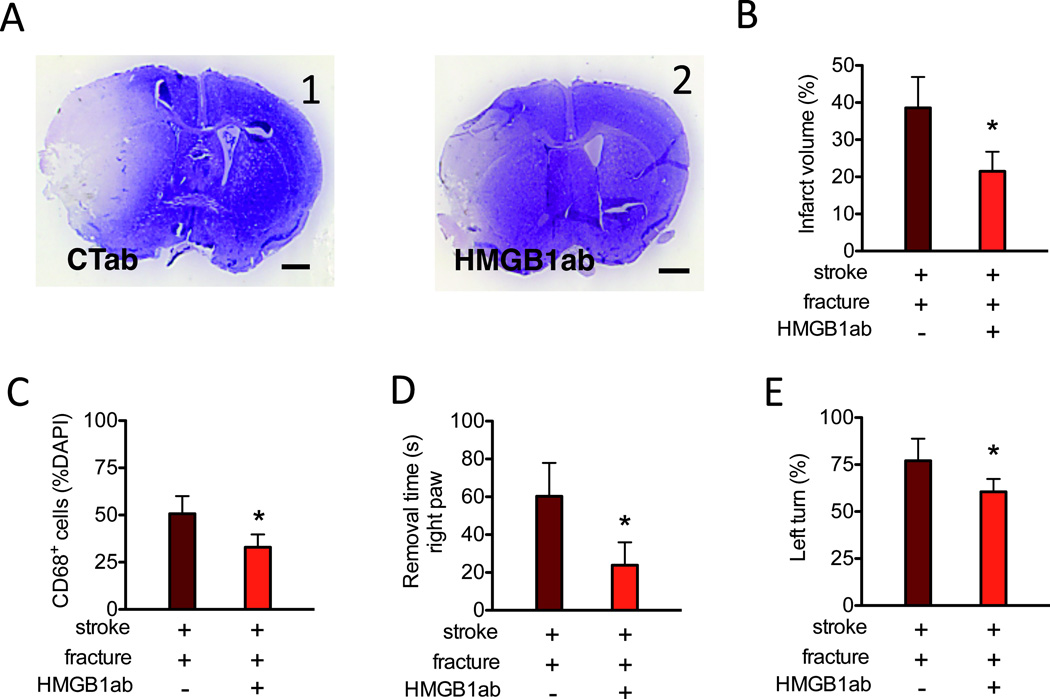
Intraperitoneal administration of HMGB1 neutralizing antibodies (10 mg/kg) immediately before bone fracture attenuated fracture-enhanced infarct volume (Fig 5A). HMGB1 neutralizing antibodies also attenuated TUNEL positive neurons in the infarct area (47±9% vs. 17±4%, P<0.001, n=6), the number of CD68+ cells in the peri-infarct region (Fig 5B) as well as the behavioral dysfunction (Fig 5C-D).
Figure 5. Neutralized HMGB1 antibodies attenuate the negative impact of bone fracture on stroke injury.
A. Representative images of cresyl violet stained brain sections (bregma 1.3 mm, scale bar: 1 mm) of stroke mice with bone fracture procedure receiving control antibodies (A1, CTab) or anti-HMGB1 antibodies (A2, HMGB1ab). B. The bar graph shows quantification of the infarct volumes (n=7, *: P<0.001). C. Quantification of CD68+ cells in the peri-infarct region (n=6, *: P<0.001). D. Adhesive removal time of the right paw of mice treated with CTab or HMGB1ab (n=10, *: P<0.001). E. Percentage of left turn in the corner test of mice treated with CTab or HMGB1ab (n=10, *: P<0.001). CTab: Control antibody; DAPI: 4',6-Diamidino-2-Phenylindole; HMGB1ab:High-mobility-group box chromosomal protein-1 neutralized antibody.
Systemic Macrophages Play a Causative Role in the Exacerbating Effects of Bone Fracture on Stroke Injury
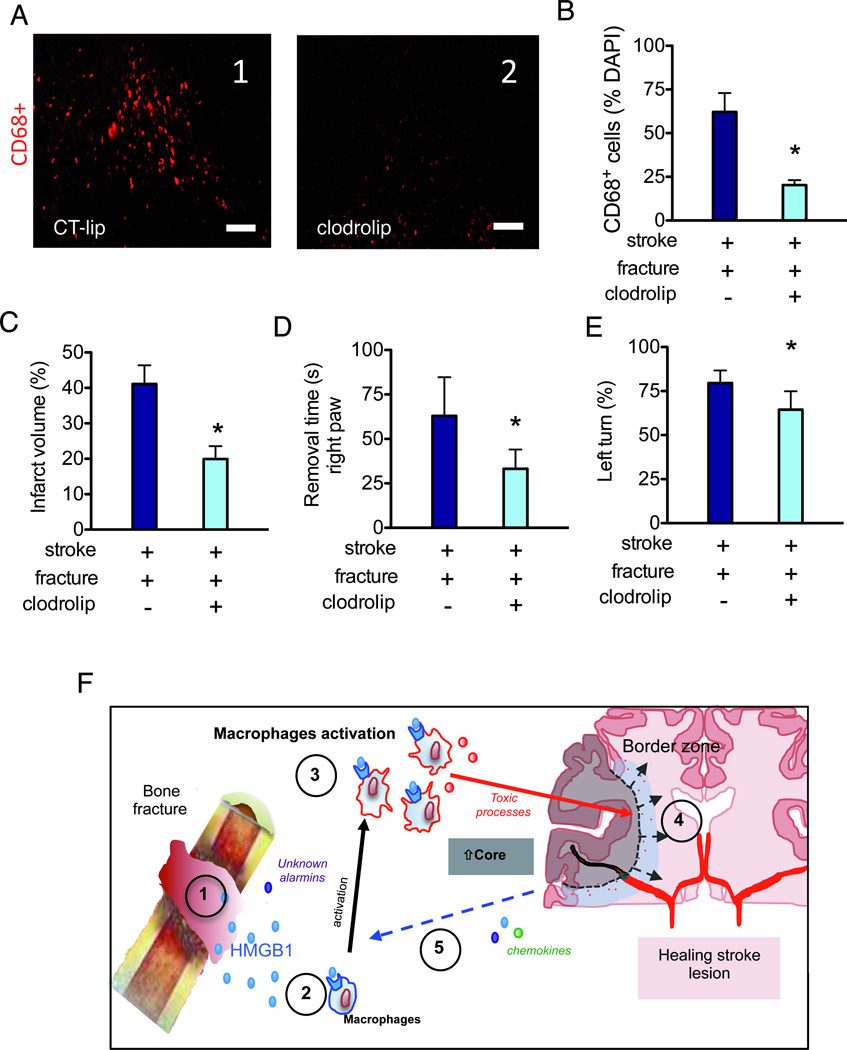
We selectively depleted macrophages16 through intraperitoneal injection of liposomal formulation of clodronate (100 mg/kg) (clodrolip) immediately before bone fracture. This intervention drastically reduced the number of CD68+ cells in the spleen (data not shown) and decreased by three times the number of CD68+ cells in the peri-infarct region (P<0.001, Fig 6A). Clodrolip treatment prior to the bone fracture significantly reduced infarct volume (Fig 6B), neuronal injury (NeuN-TUNEL positive cells of 37±6% vs. 17±4%, P=0.001, n=6), and neurobehavioral dysfunction (Fig 6C-D) in mice subjected to stroke and bone fracture.
Figure 6. Depletion of macrophage reduces the negative impact of bone fracture on stroke injury.
A. Representative picture taken in the peri-infarct region (bregma 1.3 mm) of mice treated with control liposome (A1, CT-lip) and clodrolip (A2). Scale bar: 100 µm. B. A bar graph shows the quantification of CD68+ cells in the peri-infarct region C. Quantification of infarct volume (n=7, *: P<0.001). D. Quantification of adhesive removal time of the right paw (n=10, *: P<0.001). E. Quantification of the percentage of left turn (n=10, *: P<0.001). F. Synthesis of possible mechanisms underlying the negative impact of bone fracture on stroke injury: 1-Alarmins including HMGB1 is released into blood after bone fracture. 2-HMGB1 interacts with its receptors on innate immune cells including macrophages. 3-Systemic macrophages are recruited to the stroke lesion site in the brain; together with activated microglia, they release neurotoxic molecules 4-Exacerbation of neuro-inflammation, neuronal cell death and behavior dysfunction. 5-Increased neuronal death further increases the release of alarmins and chemokines, triggering a vicious cycle through HMGB1-macrophage activation. CT-lip: control liposome; HMGB1:High-mobility-group box chromosomal protein-1.
Discussion
Sterile tissue injury, caused by surgery (Fig 4A-B), ischemia-reperfusion23 or hemorrhagic shock, 24 provokes release of the alarmin HMGB1 that can initiate systemic inflammation and cause remote organ injury.25 Previously, we showed that bone fracture increased CD11b positive cells in the hippocampus.6, 8 Using CCR2RFP/+CX3CR1GFP/+ mice, we found that a majority of the infiltrated CD11b positive cells are bone marrow-derived macrophages.8 In our study, both species of monocytes increased in the peri-infarct region of stroke mice with tibia fracture; additionally, some of the monocytic cells were positive for both CCR2 and CX3CR1.
We postulate that after bone fracture, HMGB1 is released into the blood and interacts with pattern recognition receptors (Toll-like receptors 2 and 4 as well as the receptor for advanced glycation end-products) on immunocytes, including macrophages. Together with activated microglia, systemic macrophages are recruited to the site of the stroke lesion where they are capable of releasing pro-inflammatory cytokines, which then exacerbate neuroinflammation that causes neuronal cell death and worsening behavior dysfunction in a feed-forward manner. Supporting these interpretations are data from the ‘sufficiency/necessity’ experiments involving HMGB1 (Fig 4–5) and the necessity experiments involving systemic macrophages (Fig 6). Thus, our data demonstrate that bone fracture aggravates the functional and morphological consequences of stroke, and further suggest that this occurs through engagement of the innate immune response by the alarmin HMGB1 that likely enhances neuroinflammation in the peri-infact region (Fig 4D).
Stroke is associated with increased risk of severe fall-related bone fracture,26, 27 with 4–7% of patients suffering from bone fracture within the first year of their stroke.4 Previously, we reported that circulating levels of HMGB1 increase after aseptic tibia fracture in mice, and that this is accompanied by an influx of macrophages and expression of pro-inflammatory cytokines in the hippocampus;6, 7 others have shown that inflammation is an important modulatory factor in stroke-related injuries and post-stroke recovery.11–13, 28, 29 Circulating levels of IL-6 in humans correlated positively with imaged brain infarct volume assessed by imaging analysis, and negatively with 1-year survival.30 In our study, IL-6 transcript did not significantly increase in the ipsilateral hemisphere of stroke mice. However, subsequent bone fracture significantly increased messenger RNA expression of IL-6 in the ipsilateral hemisphere of stroke mice, which was accompanied by increased infarct volume and more severe neurobehavioral dysfunction. Our data support enhanced inflammation for mediating the negative impact of bone fracture on the functional and morphological consequences of stroke.
We acknowledge that the direct link between the number of CD68+ cells and the number of TUNEL+ neurons could not be determined by this study. However, we found that the depletion of the CD68+ cells with clodrolip reduced the ratio of TUNEL+ neurons to the total number of neurons. We have also found a significant correlation between the number of CD68+ cells and the TUNEL+ neurons (Fig 3C). Moreover, in vitro studies about macrophage/microglia’s activation showed that excitotoxic and/or inflammation stressors can induce neuronal toxicity.31, 32 All together, the evidence suggests that the activation of macrophages/microglia can be involved in early neuronal cell death and thus, exacerbate lesions and behavioral dysfunction.
We found that both CCR2-RFP+ bone marrow-derived macrophages and CX3CR1-GFP+ microglia increased in the peri-infarct region of stroke mice with bone fracture. In addition, some of the cells were positive for both CCR2 and CX3CR1, suggesting that some of the bone marrow-derived macrophages acquired microglia phenotype. This phenomenon was previously observed in an experimental autoimmune encephalomyelitis model.14 Depleting the systemic macrophages with clodrolip prior to bone fracture reduced CD68+ cells infiltration, infarct volume and neurobehavioral dysfunction. We do not know if intraperitoneal injection of clodrolip affects resident microglia in the brain. However, our data show that both macrophages and activated microglia are capable of playing important roles in bone fracture-induced exacerbation of stroke injury.
Limitations of the Study
We showed that bone fracture shortly after stroke enhances stroke injury via augmented inflammation through HMGB1 and macrophage/microglia infiltration. This experimental study mimics a relevant rare scenario, in which ischemic stroke patients suffer bone fracture within the first 24 hours of the ischemic insult.3, 4 Kanis et al4 showed that ≈ 9% of stroke patients experienced a bone fracture after stroke and that 10–15% of the fracture occurs on the first day of stroke, which means that 1–1.5% of stroke patients will have a fracture on the first day of stroke. Interestingly, more than 80% of them would be older than 60. Because the elderly patient subgroup is on the rise in Western countries, this incidence should increase in the next decades.
These results give rise to questions and issues that need to be addressed in future studies. Because we report on only one time point, is it possible that bone fracture occurring at different time points, both before or after stroke, would have a different impact on stroke outcome? Could our neutralizing strategies regarding HMGB1 and systemic macrophage have blocked the alarmin and inflammatory response of the stroke itself? Given that HMGB1 has not only a neurotoxic effect in the acute stage of ischemic stroke33 but also a protective effect in the latter stages of stroke,11, 12 could an alternative timing of the administration of the neutralizing antibody have produced a different response? Shichita et al recently showed that blocking the release of HMGB1 with neutralized antibody was only protective when it was given during the MCAO procedure but not 6 hours after the ischemic injury.34 Even if these data argue against the use of neutralizing HMGB1 as a neuroprotective strategy for delayed stroke lesion, our data show that inhibiting HMGB1 appears to be effective in preventing a second increase of HMGB1 induced by a bone fracture surgery 24 hours after the stroke lesion. Further studies are needed to evaluate the effect of bone fracture occurring at different time points (before the stroke or a longer time period after).
In this study, we found that two treatments (neutralization of HMGB1 and systemic macrophage depletion) were neuroprotective in our model of pMCAO+bone fracture, and our data suggest that this neuroprotection was induced by the reduction of the macrophage recruitment. However, these two strategies can also indirectly affect other pro-inflammatory pathways, such as trauma-induced hyperthermia, that could provide neuroprotection as well.
We measured the ratio of the immune cells (CD68, CX3CR1 and CCR2) in the peri-infarct region just outside the infarct border, as shown in Fig 1C and 3A. These measures were performed at the same coordinates for all the mice (bregma +1.3 mm) for consistency. It is important to note that the variation of the infarct size can affect the location of the infarct border. We cannot exclude the possibility that the quantification of the macrophages was influenced by the location of the infarct border.
Using CX3CR1-GFP; CCR2-RFP mice, we found that bone fracture increased the numbers of both CCR2+ and CX3CR1+ cells in the brain. For this reason, we considered that the increase in the two cell populations was associated with the phenotype, and thus decided to focus on the CD68+ cells. CD68 antibody stains for a lysosomal protein that is mainly expressed in the activated phagocytosis cells. In the normal brain, there are few CD68+ cells, whereas a significant number of CD68+ cells is present in the peri-infarct side of the border zone of pMCAO.21 Although we cannot distinguish CCR2+ and CX3CR1+ cells by CD68 antibody staining, we were able to show that the neutralized antibody and the clodrolip treatments reduced CD68+ cells in the peri-infarct side of the border zone.
We selected two well-established behavior tests that exhibit reproducibility in a pMCAO model.18, 19 As shown in Fig 1, tibia fracture alone did not significantly influence mouse performance in these tests. However, we could not completely rule out the influence of bone fracture on this function in mice with pMCAO and bone fracture, as these mice may have more severe hind-limb dysfunction due to increased inflammation in the fracture. Furthermore, even if we show that the frequency of neuronal cell death increases in mice with pMCAO and bone fracture, we cannot rule out that cortical edema induced by the activation of the neuroinflammation does not play a role in increased behavioral dysfunction.
In summary, we demonstrated that bone fracture shortly after ischemic stroke increases stroke-related neuronal injury and neurobehavioral dysfunction in mice. HMGB1 and macrophage/microglia play a causal role in the negative impact of bone fracture on stroke outcomes. Our findings regarding modulation of HMGB1 level and macrophage/microglia activities pose possible intervention opportunities for patients with both stroke and bone fracture.
Summary Statement.
Stroke is a risk factor for fracture and 1–1.5% of stroke patients suffer a fracture within 24 hours of a stroke. We showed that fracture enhances stroke injury via HMGB1 release and macrophage/microglia brain infiltration.
Acknowledgments
Supported by grant nos. R01NS0027713, P01NS044155 and R21NS070153 from the National Institutes of Health, Bethesda, Maryland; AHA10GRNT3130004 from the American Heart Association, Dallas, Texas; the Department of Anesthesia and Perioperative Care, University of California, San Francisco; INSERM (Paris, France) and Société Française d’Anesthésie Réanimation (Paris, France).
The authors thank members of the Center for Cerebrovascular Research (UCSF, San Francisco, California) and the Maze laboratory (UCSF, San Francisco, California) for their support, as well as Katerina Akassoglou, PhD, Israel F. Charo, MD, PhD, and Kim Baeten, PhD (Associate Investigator, Associate Director, and Postdoctoral Fellow, respectively, UCSF Gladstone Institute, San Francisco, California), for providing the CCR2RFP/+CX3CR1GFP/+ mice.
Footnotes
This work is presented by the Department of Anesthesia and Perioperative Care, University of California, San Francisco, USA.
References
- 1.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.Sennerby U, Melhus H, Gedeborg R, Byberg L, Garmo H, Ahlbom A, Pedersen NL, Michaelsson K. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302:1666–1673. doi: 10.1001/jama.2009.1463. [DOI] [PubMed] [Google Scholar]
- 3.Lakshminarayan K, Schissel C, Anderson DC, Vazquez G, Jacobs DR, Jr, Ezzeddine M, Luepker RV, Virnig BA. Five-year rehospitalization outcomes in a cohort of patients with acute ischemic stroke: Medicare linkage study. Stroke. 2011;42:1556–1562. doi: 10.1161/STROKEAHA.110.605600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanis J, Oden A, Johnell O. Acute and long-term increase in fracture risk after hospitalization for stroke. Stroke. 2001;32:702–706. doi: 10.1161/01.str.32.3.702. [DOI] [PubMed] [Google Scholar]
- 5.Sanders RD, Bottle A, Jameson SS, Mozid A, Aylin P, Edger L, Ma D, Reed M, Walters M, Lees KR, Maze M. Independent preoperative predictors of outcomes in orthopedic and vascular surgery. Ann Surg. 2012;255:901–907. doi: 10.1097/SLA.0b013e31824c438d. [DOI] [PubMed] [Google Scholar]
- 6.Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS, Maze M. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107:20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terrando N, Brzezinski M, Degos V, Eriksson LI, Kramer JH, Leung JM, Miller BL, Mucke L, Seeley WW, Vacas S, Weiner MW, Yaffe K, Young WL, Xie Z, Maze M. Perioperative cognitive decline with the aging population. Mayo Clin Proc. 2011;86:885–893. doi: 10.4065/mcp.2011.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange SS, Mitchell DL, Vasquez KM. High mobility group protein B1 enhances DNA repair and chromatin modification after DNA damage. Proc Natl Acad Sci U S A. 2008;105:10320–10325. doi: 10.1073/pnas.0803181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 11.Hayakawa K, Qiu J, Lo EH. Biphasic actions of HMGB1 signaling in inflammation and recovery after stroke. Ann N Y Acad Sci. 2010;1207:50–57. doi: 10.1111/j.1749-6632.2010.05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo EH. T time in the brain. Nat Med. 2009;15:844–846. doi: 10.1038/nm0809-844. [DOI] [PubMed] [Google Scholar]
- 13.Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke. 2006;37:291–293. doi: 10.1161/01.STR.0000200561.69611.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saederup N, Cardona AE, Croft K, Mizutani M, Cotleur AC, Tsou CL, Ransohoff RM, Charo IF. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One. 2010;5:e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tada Y, Kanematsu Y, Kanematsu M, Nuki Y, Liang EI, Wada K, Makino H, Hashimoto T. A mouse model of intracranial aneurysm: Technical considerations. Acta Neurochir Suppl. 2011;111:31–35. doi: 10.1007/978-3-7091-0693-8_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: Mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 17.van Rooijen N, Bakker J, Sanders A. Transient suppression of macrophage functions by liposome-encapsulated drugs. Trends Biotechnol. 1997;15:178–185. doi: 10.1016/s0167-7799(97)01019-6. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Schallert T, Zhang ZG, Jiang Q, Arniego P, Li Q, Lu M, Chopp M. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods. 2002;117:207–214. doi: 10.1016/s0165-0270(02)00114-0. [DOI] [PubMed] [Google Scholar]
- 19.Bouet V, Freret T, Toutain J, Divoux D, Boulouard M, Schumann-Bard P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp Neurol. 2007;203:555–667. doi: 10.1016/j.expneurol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Shen F, Walker EJ, Jiang L, Degos V, Li J, Sun B, Heriyanto F, Young WL, Su H. Coexpression of angiopoietin1 with VEGF increases the structural integrity of the blood-brain barrier and reduces atrophy volume. J Cereb Blood Flow Metab. 2011;31:2343–2351. doi: 10.1038/jcbfm.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inacio AR, Ruscher K, Leng L, Bucala R, Deierborg T. Macrophage migration inhibitory factor promotes cell death and aggravates neurologic deficits after experimental stroke. J Cereb Blood Flow Metab. 2011;31:1093–1106. doi: 10.1038/jcbfm.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: Relationship to alpha-synuclein deposition. J Neuroinflammation. 2005;2:14. doi: 10.1186/1742-2094-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JY, Park JS, Strassheim D, Douglas I, Diaz del Valle F, Asehnoune K, Mitra S, Kwak SH, Yamada S, Maruyama I, Ishizaka A, Abraham E. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2005;288:L958–L965. doi: 10.1152/ajplung.00359.2004. [DOI] [PubMed] [Google Scholar]
- 25.Levy RM, Mollen KP, Prince JM, Kaczorowski DJ, Vallabhaneni R, Liu S, Tracey KJ, Lotze MT, Hackam DJ, Fink MP, Vodovotz Y, Billiar TR. Systemic inflammation and remote organ injury following trauma require HMGB1. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1538–R1544. doi: 10.1152/ajpregu.00272.2007. [DOI] [PubMed] [Google Scholar]
- 26.Coutinho ES, Fletcher A, Bloch KV, Rodrigues LC. Risk factors for falls with severe fracture in elderly people living in a middle-income country: A case control study. BMC Geriatr. 2008;8:21. doi: 10.1186/1471-2318-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myint PK, Poole KE, Warburton EA. Hip fractures after stroke and their prevention. QJM. 2007;100:539–545. doi: 10.1093/qjmed/hcm067. [DOI] [PubMed] [Google Scholar]
- 28.Alberti A, Agnelli G, Caso V, Venti M, Acciarresi M, D'Amore C, Paciaroni M. Non-neurological complications of acute stroke: Frequency and influence on clinical outcome. Intern Emerg Med. 2011;6 Suppl 1:119–123. doi: 10.1007/s11739-011-0675-7. [DOI] [PubMed] [Google Scholar]
- 29.Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: Recent and emerging concepts. Lancet Neurol. 2008;7:341–353. doi: 10.1016/S1474-4422(08)70061-9. [DOI] [PubMed] [Google Scholar]
- 30.Smith CJ, Emsley HC, Gavin CM, Georgiou RF, Vail A, Barberan EM, del Zoppo GJ, Hallenbeck JM, Rothwell NJ, Hopkins SJ, Tyrrell PJ. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4:2. doi: 10.1186/1471-2377-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Byrnes KR, Stoica B, Loane DJ, Riccio A, Davis MI, Faden AI. Metabotropic glutamate receptor 5 activation inhibits microglial associated inflammation and neurotoxicity. Glia. 2009;57:550–560. doi: 10.1002/glia.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaindl AM, Degos V, Peineau S, Gouadon E, Chhor V, Loron G, Le Charpentier T, Josserand J, Ali C, Vivien D, Collingridge GL, Lombet A, Issa L, Rene F, Loeffler JP, Kavelaars A, Verney C, Mantz J, Gressens P. Activation of microglial NMDA receptors triggers inflammation and neuronal cell death in the developing and mature brain. Ann Neurol. 2012 doi: 10.1002/ana.23626. [DOI] [PubMed] [Google Scholar]
- 33.Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, Bendszus M, Rossetti G, Nawroth PP, Bierhaus A, Schwaninger M. The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci. 2008;28:12023–12031. doi: 10.1523/JNEUROSCI.2435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shichita T, Hasegawa E, Kimura A, Morita R, Sakaguchi R, Takada I, Sekiya T, Ooboshi H, Kitazono T, Yanagawa T, Ishii T, Takahashi H, Mori S, Nishibori M, Kuroda K, Akira S, Miyake K, Yoshimura A. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat Med. 2012;18:911–918. doi: 10.1038/nm.2749. [DOI] [PubMed] [Google Scholar]