Abstract
Human essential hypertension and rodent genetic hypertension are associated with increased sodium transport in the renal proximal tubule and medullary thick ascending limb of Henle. The proximal tubule, which secretes endothelin, expresses the endothelin type B (ETB) receptor. Low (nM) concentrations of endothelin, via the ETB receptor, inhibit sodium and water transport and ATP-driven drug secretion in the proximal tubule. In contrast, very low (pM) and high nM concentrations of endothelin increase renal proximal sodium transport, the receptor involved remains to be determined. The natriuretic effect of ETB receptor stimulation is impaired in spontaneously hypertensive rats, due in part to a defective interaction with D3 dopamine and angiotensin II type 1 receptors. Impaired ETB receptor function in the renal proximal tubule may be important in the pathogenesis of genetic hypertension.
Introduction
The kidney is important in the long-term regulation of blood pressure and is the major organ involved in the regulation of body sodium homeostasis1–3. The proximal tubule and medullary thick ascending limb of Henle are pre-eminent in the overall regulation of sodium balance in essential hypertension4. Indeed, several studies have shown that human essential hypertension and rodent genetic hypertension are associated with increased sodium transport in the renal proximal tubule (RPT) and medullary thick ascending limb of Henle4.
Endothelin (ET) was initially identified as an endothelial cell-derived peptide, with the greatest vasoconstrictor potency of any known endogenous compound5. Endothelins are a family of isopeptides (ET-1, ET-2, and ET-3), with at least two receptors subtypes (ETA and ETB). Renal tissue expresses both endothelin receptor subtypes6, and endothelin is secreted by renal tubules, including the renal proximal tubule7,8, where it can regulate sodium transport in an autocrine/paracrine manner9. In the RPTs, ET-1, principally through the ETB receptor, the major ET receptor expressed in the RPT, inhibits ion transport10, an effect opposite that of the ETA receptor. This review focuses on the regulation of endothelin on sodium transport in the renal proximal tubule and its role in the pathogenesis of essential hypertension.
Endothelin and it metabolism
In 1985, Hickey and co-workers11 described the existence of a trypsin-sensitive endothelium-derived constricting factor in cultured bovine endothelial cells, which they named as endothelin. Subsequently, more endothelin family members were found. These members include ET-1, ET-2, ET-3 and ET-4 (analogue of human ET-2 in rat and mouse, also named as vasoactive intestinal contractor), and three isoforms of 31-amino-acid ETs (ET-11–31, ET-21–31 and ET-31–31)12. Owing to the similarity of actions between ET-11–31 and ET-1 in vivo, it is possible that some of the effects of ET1–31 result from their partial bioconversion into ETs13. Among these isoforms, ET-1 is the principal isoform and is the most potent and long-lasting constrictor of human vessels known to date5.
ET-1 is produced in many cell types in the renal and cardiovascular systems, such as endothelial cells, smooth muscle cells, cardiomyocytes, leucocytes, macrophages, and renal tubular and mesangial cells14,15. Bioactive ETs are the product of post-translational processing of the parent pre-pro-ET peptide. The transcription and translation of pre-pro-ET result in the formation of a 203-amino-acid peptide which is subsequently cleaved by a furin convertase to the 38-amino-acid peptide big ET1–38. Big ET is processed further into ET1–31 by different isoforms of endothelin converting enzymes (ECEs), a group of proteases that belong to the metalloprotease family16, including ECE-1a, ECE-1b, ECE-1c and ECE-1d, derived from a single gene by the action of alternative promoters.
ET synthesis is regulated by many factors. It is enhanced in response to low-shear stress, turbulent blood flow, hypoxia, cytokines, angiotensin II, epinephrine, and low-density lipoproteins17. In contrast, high-shear stress, nitric oxide, vasodilating prostaglandins, and natriuretic peptides suppress ET production18. Endothelin synthesis is regulated by sodium diet; a high sodium diet, independent of blood pressure status, increases renal synthesis of endothelin19. Distal nephron segments synthesize ET-1 to a greater extent than the proximal tubule8.
The synthesized ET-1 is released in two ways. One way is via a constitutive pathway, producing intense constriction of the underlying smooth muscle, which contributes to the maintenance of endogenous vascular tone. The other way is via release from endothelial cell-specific storage granules (Weibel-Palade bodies) in response to external physiological stimuli, producing further vasoconstriction20. Although plasma ET-1 is present in the highest concentration in blood/plasma, compared with ET-2 and ET-3, ET-1 concentrations are still lower in plasma than in endothelial and other cells. It is accepted that ET-1 functions as a locally released, rather than a circulating, hormone.
Endothelin receptors
Endothelin receptor classification
ET receptors are classified as ETA and ETB by the International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification21. Both ETA and ETB receptors belong to the seven-transmembrane domain or G-protein-coupled rhodopsin-type receptor superfamily. Pharmacologically heterogeneous responses seem to be related to the existence of alternatively spliced variants of ETA and ETB receptors. A third receptor, named ETC, which is specific for ET-3 binding, was cloned from dermal melanophores from the amphibian Xenopus laevis, but a mammalian homologue has yet to be identified21 (see below).
The three ET receptors have different affinities to the three isoforms of ET (ET-1, ET-2 and ET-3). ET receptors can bind all endothelin isoforms, but the ETA receptor has a much higher affinity for ET-1, the most abundant ET in human plasma, than for ET-3, while the ETC receptor has a higher affinity for ET-3 than ET-1. In contrast, the ETB receptor binds to all three ET isoforms with equal affinity.
Localization of endothelin receptors
In all species studied, including humans, endothelin binding is greater in the renal inner medulla than in the inner cortex8,22,,23.
In the rat, there is faint immunostaining of the ETA receptor in the proximal convoluted and straight tubules, restricted to the basal side and intense staining in distal tubule collecting duct24,25; ETA receptor is also expressed in mesangial cells, pericytes of descending vasa recta and vascular smooth muscle cells (VSMCs) of veins and arteries, specifically the interlobar, arcuate, and interlobular arteries, as well as efferent and afferent arterioles25. The ETB receptor is the major endothelin receptor in the kidney; 70–80% of endothelin receptors in kidney are ETB receptors6,8, which in the rat are expressed in proximal tubule, inner medullary collecting duct, glomerular capillaries, vasa recta endothelial cells, and VSMCs of interlobular, efferent and afferent arteries25–28.
In the mouse, the ETA receptor has been shown to be expressed in some proximal tubules and vessels, but not in glomeruli. In agreement with the rat studies, the ETB receptor is expressed in the proximal tubule and collecting duct29. Faint staining of ETA and ETB receptors has also been reported in human glomeruli, and proximal and distal tubules. In agreement with the rat studies, the distal nephron expresses more ET receptors that the proximal nephron30,31.
Signal transduction of ET receptors in the RPTs
Both ETA and ETB receptors are linked to Gq/11, GαS and Gαi/O32,33. In some cells (e.g., Chinese hamster ovary cells), the ETA receptor is linked to GαS while the ETB receptor is linked to Gαi 34. However, in hepatocytes, the ETB receptor is also linked to GαS35. The ETB, but not the ETA receptor, also activates Gα1336.
In rat renal brush border membranes, the endothelin-mediated increase in phospholipase C activity and protein kinase C translocation to the brush border membrane are mediated by the ETB but not by the ETA receptor. In contrast, the ETC receptor is involved in endothelin-mediated signaling in basolateral membranes37. Both ETA and ETB receptors belong to the Class A receptors of the GPCR family because they bind to β arrestin 1 with a higher affinity than β arrestin 2 and do not bind to visual arrestin38,39.
Effect of endothelin on RPT transport
In-vivo studies
Low-dose infusion of endothelin in anesthetized rats has been reported to decrease sodium transport in proximal and distal nephron segments, assessed by lithium clearance, which is associated with an increase in renal blood flow but not glomerular filtration rate40. At a dose that does not alter renal plasma flow, endothelin also decreases proximal but not distal tubule sodium reabsorption, also assessed by lithium clearance8,41,42, showing that endothelin infusion causes natriuresis without altering glomerular filtration rate or renal blood flow, and suggesting a direct inhibitory effect of endothelin-1 on Na+ transport along the nephron. However, the endothelin-mediated natriuresis has not been consistently observed43,44. One group reported that the natriuretic effect of exogenous ET-1 is due solely to an increase in blood pressure since renal decapsulation or maintaining renal perfusion pressure at baseline values (ET agonists often increase arterial pressure) with an aortic clamp prevents ET-1-induced natriuresis43. The possible explanation for the inconsistent findings on endothelin agonist-induced natriuresis may be due, at least partly, to differential activation of ETA and ETB receptors. For example, when the ETA receptor, but not the ETB receptor, is blocked, a natriuretic effect of ET-1 is detected45. One study showed that low doses of ET causes a natriuresis by decreasing renal proximal tubular reabsorption that is not due to ETB receptors 46.
In contrast to the studies on sodium excretion, there is unanimous agreement that systemically administered endothelin increases urinary water excretion. Even when given at doses that markedly decreased renal blood flow, the renal arterial infusion of ET-1 increased urine volume and free water clearance47. This effect may be mediated by the ETB receptor, because the infusion of ETB-specific agonists (IRL1620 or sarafotoxin 6c) increases urine flow10,46.
The ETB receptor also works as a clearance receptor. The ETB receptor in endothelial cells removes ET-1 from the circulation48,49. Blockade of the ETB receptor increases circulating immunoreactive ETs (ET-1 and ET-3), and mice with genetic ablation of the ETB receptor in endothelial cells have elevated plasma concentrations of ET-148,49.
In-vitro studies
Endothelin has diverse effects throughout the nephron. In the rat proximal tubule, endothelin has a biphasic effect on ion and fluid transport50. Low concentrations (pM) increase whereas a high concentration (low nM) of ET-1, decreases fluid transport through protein kinase C-, cyclooxygenase- and lipoxygenase-dependent mechanisms42,50. However, higher concentrations (high nM) of endothelin also increase renal proximal tubule transport7. In agreement with the data on sodium transport, low nM concentrations of endothelin, via the ETB receptor, have also been shown to inhibit the secretion of fluorescent substrates in renal proximal tubules of the killifish51.
Endothelin-1 inhibits fluid and bicarbonate absorption in the isolated-perfused rat proximal straight tubule52 and fluid transport in the mid-proximal convoluted tubule measured by the split-drop micropuncture method53. A preliminary report also indicates that endothelin decreases sodium-phosphate cotransport in rat renal brush border membranes53. Garvin and Sanders showed that the ability of endothelin to inhibit fluid and bicarbonate transport in the rat proximal straight tubule is mediated by a reduction in Na+-K+ ATPase activity52. These effects are probably mediated by the ETB receptor because stimulation of the ETB receptor also inhibits Na+-K+ ATPase activity in immortalized rat S1 renal proximal tubule cells55, similar to that observed in proximal tubules, about 20–40% reduction52,53. The inhibitory effect of endothelin, via the ETB receptor, on Na+-K+ ATPase activity in human and rat renal proximal tubule cells is mediated by an increase in intracellular calcium via phosphatidyl inositol-3 kinase55. However, in suspensions of rabbit proximal tubule cells endothelin was not found to reduce oxygen consumption, used as an index of Na+-K+ ATPase activity56. It remains to be determined whether or not the differential effect of endothelin on Na+-K+ ATPase activity between rats and rabbits is related to species differences.
The effect of endothelin on NHE3 activity in renal proximal tubule cells is still controversial. Short-term (<1 hr) incubation of rat renal cortical cortical slices with endothelin increases NHE3 activity that is mediated by protein kinase C and inhibition of cAMP production57. Short-term (< 1hr) stimulation with endothelin of opossum kidney cells which express ETB but not ETA receptors, also increases NHE3 that is mediated equally by Ca2+-dependent and tyrosine kinase-dependent pathways58,59. However, long-term (≥6 hr) stimulation of the ETB receptor in the same opossum kidney cells leads to inhibition of NHE3 activity and expression60. One may conclude from these studies that in the renal proximal tubule, endothelin, via the ETB receptor, inhibits sodium transport by decreasing Na+-K+ ATPase activity. In contrast, endothelin, via the ETB receptor, acutely stimulates but chronically inhibits NHE3 activity. However, the end-result of ETB receptor stimulation should still be a decrease in renal proximal tubule transport because of the inhibition of Na+-K+ ATPase activity.
Interaction with other GPCRs
Interaction between ETA and ETB receptors
ETA and ETB receptors may exist as constitutive homodimers61. They may also exist as constitutive heterodimers62 and therefore, there may be a “cross-talk” between these two ET receptors. The ETB receptor may be essential in regulating the development or expression of the ETA receptor because ETA receptor expression is decreased in central or peripheral tissues of ETB receptor deficient mice63. ETA and ETB receptor heterodimers may be responsible for a sustained Ca2+ signaling by delaying their internalization64. Both the ETA and ETB receptors are required for the full diuretic and natriuretic actions of endothelin associated with the intramedulary hyperosmotic saline infusion65. An increased ratio of ETA and ETB receptor activity is important in the development and progression of DOCA-salt-induced hypertension and organ damage66. Indeed, blockade of ETB receptors in this model of hypertension increases the severity of vascular and renal proximal tubular damage66.
Interaction with the D3 dopamine receptor
Dopamine, an endogenous catecholamine, is an important regulator of sodium balance and blood pressure via renal and non-renal mechanisms, including the regulation of appetite centers in the brain, secretion/release of hormones and humoral agents and interaction with hormones that regulate renal ion transport. Dopamine receptors are divided into D1- and D2-like subtypes based on their interaction with the effector enzyme, adenylyl cyclase. Among D2-like receptor subtypes, the D3 receptor, along with the D4 receptor, has the highest affinity for dopamine. Stimulation of the D3 receptor increases sodium and water excretion in normotensive rats67,68. The natriuresis caused by D3 receptor stimulation is due, in part, to inhibition of Na+-K+ ATPase activity, via a cooperative interaction with ETB receptors28. The D3 and ETB receptors co-localize and physically interact in rat renal proximal tubules and increase each other’s expression (Figure 1). In immortalized renal proximal tubule cells, D3 receptor stimulation increases ETB receptor expression by a calcium-mediated process which is absent in renal proximal tubule cells from spontaneously hypertensive rats69,70.
Figure 1. Interactions among ETB (ETBR), D3 dopamine (D3R), and AT1 (AT1R) receptors in the regulation of sodium transport in the renal proximal tubule.
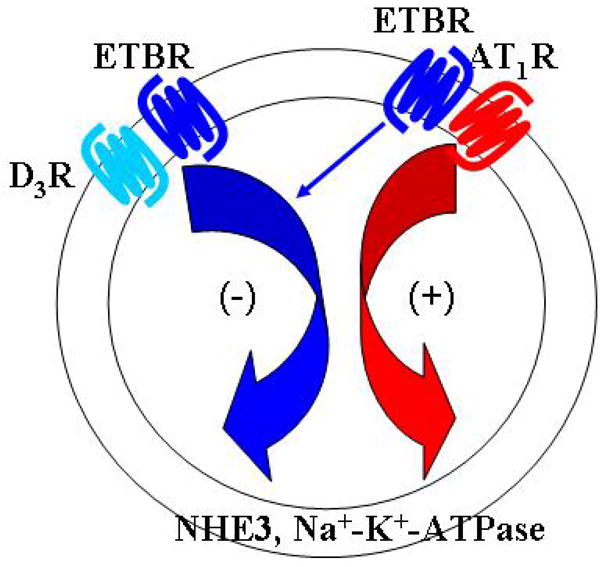
Stimulation of the AT1R increases the activity of sodium transporters, sodium exchangers, and the sodium pump (e.g., NaHCO3 cotransporter, Na+H+exchanger, and Na+-K+-ATPase), while their activity is decreased by the stimulation of ETBR and dopamine receptors (e.g., D1R and D3R). The ETBR, D3R, and AT1R physically interact with each other. Stimulation of the ETBR increases D3R expression and function but the opposite effect occurs with AT1R expression and function. The D3R also negatively regulates AT1R expression. In contrast, stimulation of the AT1R increases ETBR expression, in the long-term, and ETBR cell surface expression, in the short-term. Thus, the ETBR synergistically interacts with the D3R to counterbalance the stimulatory effect of the AT1R on sodium transport in the renal proximal tubule.
Interaction with the AT1 receptor
In the basal state on normal sodium intake and especially during sodium deficit, angiotensin II, via the AT1 receptor, is pre-eminent in renal sodium conservation. The AT1 and ETB receptors physically interact and regulate each other’s expression (Figure 1). Long-term activation of the AT1 receptor increases ETB receptor expression whereas short-term activation increases cell surface ETB receptor expression in rat renal proximal tubule cells, effects that are not observed in renal proximal tubule cells from spontaneously hypertensive rats71,72.
Endothelin and hypertension
Increased ET-1 plasma levels, relative to normotensive subjects, regardless of renal function, have been reported in hypertensive patients in some studies73–75. Most studies, however, have found no differences in circulating endothelin levels between hypertensive and normotensive subjects76. However, the ETB receptor may be involved in the pathogenesis of hypertension. High-sodium diet or deoxycorticosterone-salt-treatment increases the blood pressure of ETB receptor-deficient rats. The increased renal sodium transport in these rats probably occurs at a distal tubular level because the increased blood pressure caused by increased sodium diet is normalized by blockade of the epithelial sodium channel with amiloride77. ETB receptor blockade produces hypertension that is exaggerated by salt intake or deoxycorticosterone78,79. Systemic ETB receptor blockade also produces hypertension in mice that is maintained by the ETA receptor80. These findings strongly suggest that the ETB receptor, by itself, or in conjunction with the ETA receptor, can regulate blood pressure and decreased expression or activity of ETB receptors increases blood pressure. However, these studies have not determined the involvement of the ETB receptor expressed in the proximal tubule. As indicated above, the natriuretic effect of ETB receptor agonists is impaired in spontaneously hypertensive rats. Although ETB receptor expression is not different in renal proximal tubules between normotensive and spontaneously hypertensive rats, angiotensin II increases total ETB receptor expression or plasma membrane expression in cells from normotensive but not from hypertensive rats71. The physical interaction between ETB and AT1 receptors is also impaired in renal proximal tubule cells from spontaneously hypertensive rats72. The blunted natriuretic effect of dopamine in spontaneously hypertensive rats may also be due to an impaired physical interaction between D3 and ETB receptors in the proximal tubule28, resulting in the impaired inhibition of Na+-K+ ATPase activity70.
Conclusion
The renal proximal tubule in all studied species expresses the ETB receptor. Endothelin has a U-shaped effect on ion and fluid absorption in the renal proximal tubule. The natriuretic effect of ETB receptor agonists and low nM concentrations of endothelin is, in part, due to inhibition of sodium and water transport in the proximal tubule. This is due mainly to inhibition of Na+-K+ ATPase activity and NHE3 activity, the latter occurring only in the long-term, as acute stimulation of ETB receptors increases NHE3 activity. The inhibition of Na+-K+ ATPase activity in renal proximal tubules by stimulation of the ETB receptor that results in natriuresis is probably abetted by increased positive interaction with the D3 receptor and negative interaction with the AT1 receptor. The impaired natriuretic effect of ETB receptor stimulation in spontaneously hypertensive rats may be caused by impaired ETB receptor function that may be related to impaired interaction with D3 and AT1 receptors.
Acknowledgments
Source of Funding
These studies were supported in part by grants from the National Institutes of Health, USA (HL023081, HL074940, DK039308, HL068686, HL092196), the National Natural Science Foundation of China (30470728, 30672199), Natural Science Foundation Project of CQ CSTC (CSTC, 2009BA5044), and the grants for Distinguished Young Scholars of China from the National Natural Science Foundation of China (30925018).
Footnotes
Disclosures
None.
References
- 1.Crowley SD, Coffman TM. In hypertension, the kidney rules. Curr Hypertens Rep. 2007;9:148–53. doi: 10.1007/s11906-007-0026-2. [DOI] [PubMed] [Google Scholar]
- 2.Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 3.Asico L, Zhang X, Jiang J, Cabrera D, Escano CS, Sibley DR, Wang X, Yang Y, Mannon R, Jones JE, Armando I, Jose PA. Lack of renal dopamine D5 receptors promotes hypertension. J Am Soc Nephrol. 2011;22:82–9. doi: 10.1681/ASN.2010050533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doris PA. Renal proximal tubule sodium transport and genetic mechanisms of essential hypertension. J Hypertens. 2000;18:509–19. doi: 10.1097/00004872-200018050-00002. [DOI] [PubMed] [Google Scholar]
- 5.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–5. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 6.Schneider MP, Boesen EI, Pollock DM. Contrasting actions of endothelin ETA and ETB receptors in cardiovascular disease. Annu Rev Pharmacol Toxicol. 2007;47:731–59. doi: 10.1146/annurev.pharmtox.47.120505.105134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romano G, Giagu P, Favret G, Bartoli E. Effect of endothelin 1 on proximal reabsorption and tubuloglomerular feedback. Kidney Blood Press Res. 2000;23:360–5. doi: 10.1159/000025984. [DOI] [PubMed] [Google Scholar]
- 8.Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev. 2011;91:1–77. doi: 10.1152/physrev.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Licht C, Laghmani K, Yanagisawa M, Preisig PA, Alpern RJ. An autocrine role for endothelin-1 in the regulation of proximal tubule NHE3. Kidney Int. 2004;65:1320–6. doi: 10.1111/j.1523-1755.2004.00506.x. [DOI] [PubMed] [Google Scholar]
- 10.Matsuo G, Matsumura Y, Tadano K, Hashimoto T, Morimoto S. Effects of sarafotoxin S6c on renal haemodynamics and urine formation in anaesthetized dogs. Clin Exp Pharmacol Physiol. 1997;24:487–91. doi: 10.1111/j.1440-1681.1997.tb01232.x. [DOI] [PubMed] [Google Scholar]
- 11.Hickey KA, Rubanyi G, Paul RJ, Highsmith RF. Characterization of a coronary vasoconstrictor produced by cultured endothelial cells. Am J Physiol. 1985;248:C550–6. doi: 10.1152/ajpcell.1985.248.5.C550. [DOI] [PubMed] [Google Scholar]
- 12.Kishi F, Minami K, Okishima N, Murakami M, Mori S, Yano M, Niwa Y, Nakaya Y, Kido H. Novel 31-amino-acid-length endothelins cause constriction of vascular smooth muscle. Biochem Biophys Res Commun. 1998;248:387–90. doi: 10.1006/bbrc.1998.8980. [DOI] [PubMed] [Google Scholar]
- 13.Fecteau MH, Honoré JC, Plante M, Labonté J, Rae GA, D’Orléans-Juste P. Endothelin-1 (1–31) is an intermediate in the production of endothelin-1 after big endothelin-1 administration in vivo. Hypertension. 2005;46:87–92. doi: 10.1161/01.HYP.0000170460.24604.23. [DOI] [PubMed] [Google Scholar]
- 14.Brunner F, Brás-Silva C, Cerdeira AS, Leite-Moreira AF. Cardiovascular endothelins: essential regulators of cardiovascular homeostasis. Pharmacol Ther. 2006;111:508–31. doi: 10.1016/j.pharmthera.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Granger JP, Abram S, Stec D, Chandler D, LaMarca B. Endothelin, the kidney, and hypertension. Curr Hypertens Rep. 2006;8:298–303. doi: 10.1007/s11906-006-0068-x. [DOI] [PubMed] [Google Scholar]
- 16.Turner AJ, Barnes K, Schweizer A, Valdenaire O. Isoforms of endothelin-converting enzyme: why and where? Trends Pharmacol Sci. 1998;19:483–6. doi: 10.1016/s0165-6147(98)01251-6. [DOI] [PubMed] [Google Scholar]
- 17.Martínez-Miguel P, Raoch V, Zaragoza C, Valdivielso JM, Rodríguez-Puyol M, Rodríguez-Puyol D, López-Ongil S. Endothellin-converting enzyme-1 increases in atherosclerotic mice: potential role of oxidized low density lipoproteins. J Lipid Res. 2009;50:364–75. doi: 10.1194/jlr.M800215-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Papachristou E, Papadimitropoulos A, Kotsantis P, Goumenos DS, Katsoris PG, Vlachojannis JG. Interaction of endothelin-1 and nitric oxide pathways in human tubular epithelial cells under the influence of cyclosporine A. Ren Fail. 2010;32:727–32. doi: 10.3109/0886022X.2010.486487. [DOI] [PubMed] [Google Scholar]
- 19.Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol. 2001;281:F144–50. doi: 10.1152/ajprenal.2001.281.1.F144. [DOI] [PubMed] [Google Scholar]
- 20.Russell FD, Skepper JN, Davenport AP. Human endothelial cell storage granules: a novel intracellular site for isoforms of the endothelin-converting enzyme. Circ Res. 1998;83:314–21. doi: 10.1161/01.res.83.3.314. [DOI] [PubMed] [Google Scholar]
- 21.Davenport AP International Union of Pharmacology. XXIX. Update on endothelin receptor nomenclature. Pharmacol Rev. 2002;54:219–26. doi: 10.1124/pr.54.2.219. [DOI] [PubMed] [Google Scholar]
- 22.Lattmann T, Shaw S, Münter K, Vetter W, Barton M. Anatomically distinct activation of endothelin-3 and the L-arginine/nitric oxide pathway in the kidney with advanced aging. Biochem Biophys Res Commun. 2005;327:234–41. doi: 10.1016/j.bbrc.2004.11.160. [DOI] [PubMed] [Google Scholar]
- 23.Goettsch W, Lattmann T, Amann K, Szibor M, Morawietz H, Münter K, Müller SP, Shaw S, Barton M. Increased expression of endothelin-1 and inducible nitric oxide synthase isoform II in aging arteries in vivo: implications for atherosclerosis. Biochem Biophys Res Commun. 2001;280:908–13. doi: 10.1006/bbrc.2000.4180. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto T, Suzuki H, Kubo Y, Matsumoto A, Uemura H. Endothelin A receptor-like immunoreactivity on the basal infoldings of rat renal tubules and collecting ducts. Arch Histol Cytol. 2008;71:77–87. doi: 10.1679/aohc.71.77. [DOI] [PubMed] [Google Scholar]
- 25.Wendel M, Knels L, Kummer W, Koch T. Distribution of endothelin receptor subtypes ETA and ETB in the rat kidney. J Histochem Cytochem. 2006;54:1193–203. doi: 10.1369/jhc.5A6888.2006. [DOI] [PubMed] [Google Scholar]
- 26.Francis BN, Abassi Z, Heyman S, Winaver J, Hoffman A. Differential regulation of ETA and ETB in the renal tissue of rats with compensated and decompensated heart failure. J Cardiovasc Pharmacol. 2004;44 (Suppl 1):S362–5. doi: 10.1097/01.fjc.0000166302.56184.fa. [DOI] [PubMed] [Google Scholar]
- 27.Schiffrin EL. Vascular endothelin in hypertension. Vascul Pharmacol. 2005;43:19–29. doi: 10.1016/j.vph.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Zeng C, Asico LD, Yu C, Villar VA, Shi W, Luo Y, Wang Z, He D, Liu Y, Huang L, Yang C, Wang X, Hopfer U, Eisner GM, Jose PA. Renal D3 dopamine receptor stimulation induces natriuresis by endothelin B receptor interactions. Kidney Int. 2008;74:750–9. doi: 10.1038/ki.2008.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S, Ahn D. Expression of endothelin-1 and its receptors in Cisplatin-induced acute renal failure in mice. Korean J Physiol Pharmacol. 2008;12:149–53. doi: 10.4196/kjpp.2008.12.4.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frank K, Zeier M, Gross ML, Waldherr R, Ritz E, Amann K. Comprehensive immunohistological analysis of the endothelin system in human kidney grafts. Nephrol Dial Transplant. 2006;21:1365–72. doi: 10.1093/ndt/gfk087. [DOI] [PubMed] [Google Scholar]
- 31.Karet FE. Endothelin peptides and receptors in human kidney. Clin Sci (Lond) 1996;91:267–73. doi: 10.1042/cs0910267. [DOI] [PubMed] [Google Scholar]
- 32.Takigawa M, Sakurai T, Kasuya Y, Abe Y, Masaki T, Goto K. Molecular identification of guanine-nucleotide-binding regulatory proteins which couple to endothelin receptors. Eur J Biochem. 1995;228:102–8. doi: 10.1111/j.1432-1033.1995.tb20236.x. [DOI] [PubMed] [Google Scholar]
- 33.Doi T, Sugimoto H, Arimoto I, Hiroaki Y, Fujiyoshi Y. Interactions of endothelin receptor subtypes A and B with Gi, Go, and Gq in reconstituted phospholipid vesicles. Biochemistry. 1999;38:3090–9. doi: 10.1021/bi981919m. [DOI] [PubMed] [Google Scholar]
- 34.Aramori I, Nakanishi S. Coupling of two endothelin receptor subtypes to differing signal transduction in transfected Chinese hamster ovary cells. J Biol Chem. 1992;267:12468–74. [PubMed] [Google Scholar]
- 35.Jouneaux C, Mallat A, Serradeil-Le Gal C, Goldsmith P, Hanoune J, Lotersztajn S. Coupling of endothelin B receptors to the calcium pump and phospholipase C via Gs and Gq in rat liver. J Biol Chem. 1994;269:1845–51. [PubMed] [Google Scholar]
- 36.Kitamura K, Shiraishi N, Singer WD, Handlogten ME, Tomita K, Miller RT. Endothelin-B receptors activate Gα13. Am J Physiol. 1999;276:C930–7. doi: 10.1152/ajpcell.1999.276.4.C930. [DOI] [PubMed] [Google Scholar]
- 37.Knotek M, Jaksić O, Selmani R, Skorić B, Banfić H. Different endothelin receptor subtypes are involved in phospholipid signalling in the proximal tubule of rat kidney. Pflugers Arch. 1996;432:165–73. doi: 10.1007/s004240050120. [DOI] [PubMed] [Google Scholar]
- 38.Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–10. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 39.Hamdan FF, Rochdi MD, Breton B, Fessart D, Michaud DE, Charest PG, Laporte SA, Bouvier M. Unraveling G protein-coupled receptor endocytosis pathways using real-time monitoring of agonist-promoted interaction between β-arrestins and AP-2. J Biol Chem. 2007;282:29089–100. doi: 10.1074/jbc.M700577200. [DOI] [PubMed] [Google Scholar]
- 40.Harris PJ, Zhuo J, Mendelsohn FA, Skinner SL. Haemodynamic and renal tubular effects of low doses of endothelin in anaesthetized rats. J Physiol. 1991;433:25–39. doi: 10.1113/jphysiol.1991.sp018412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perico N, Cornejo RP, Benigni A, Malanchini B, Ladny JR, Remuzzi G. Endothelin induces diuresis and natriuresis in the rat by acting on proximal tubular cells through a mechanism mediated by lipoxygenase products. J Am Soc Nephrol. 1991;2:57–69. doi: 10.1681/ASN.V2157. [DOI] [PubMed] [Google Scholar]
- 42.Garvin JL, Herrera M, Ortiz PA. Regulation of renal NaCl transport by nitric oxide, endothelin, and ATP: clinical implications. Annu Rev Physiol. 2011;73:359–76. doi: 10.1146/annurev-physiol-012110-142247. [DOI] [PubMed] [Google Scholar]
- 43.Uzuner K, Banks RO. Endothelin-induced natriuresis and diuresis are pressure-dependent events in the rat. Am J Physiol. 1993;265:R90–6. doi: 10.1152/ajpregu.1993.265.1.R90. [DOI] [PubMed] [Google Scholar]
- 44.Freed MI, Thompson KA, Wilson DE, Etheredge R, Jorkasky DK. Endothelin receptor antagonism does not alter renal hemodynamic responses or urinary sodium excretion in healthy humans. J Am Soc Nephrol. 1996;7:1580. (abstract) [Google Scholar]
- 45.Brooks DP, DePalma PD, Pullen M, Gellai M, Nambi P. Identification and function of putative ETB receptor subtypes in the dog kidney. J Cardiovasc Pharmacol. 1995;26:S322–5. [PubMed] [Google Scholar]
- 46.Clavell AL, Stingo AJ, Margulies KB, Brandt RR, Burnett JC., Jr Role of endothelin receptor subtypes in the in vivo regulation of renal function. Am J Physiol. 1995;268:F455–60. doi: 10.1152/ajprenal.1995.268.3.F455. [DOI] [PubMed] [Google Scholar]
- 47.Kamphuis C, Yates NA, McDougall JG. Differential blockade of the renal vasoconstrictor and diuretic responses to endothelin-1 by endothelin antagonist. Clin Exp Pharmacol Physiol. 1994;21:329–33. doi: 10.1111/j.1440-1681.1994.tb02522.x. [DOI] [PubMed] [Google Scholar]
- 48.Bagnall AJ, Kelland NF, Gulliver-Sloan F, Davenport AP, Gray GA, Yanagisawa M, Webb DJ, Kotelevtsev YV. Deletion of endothelial cell endothelin B receptors does not affect blood pressure or sensitivity to salt. Hypertension. 2006;48:286–93. doi: 10.1161/01.HYP.0000229907.58470.4c. [DOI] [PubMed] [Google Scholar]
- 49.D’Orléans-Juste P, Labonté J, Bkaily G, Choufani S, Plante M, Honoré JC. Function of the endothelinB receptor in cardiovascular physiology and pathophysiology. Pharmacol Ther. 2002;95:221–38. doi: 10.1016/s0163-7258(02)00235-8. [DOI] [PubMed] [Google Scholar]
- 50.Garcia NH, Garvin JL. Endothelin’s biphasic effect on fluid absorption in the proximal straight tubule and its inhibitory cascade. J Clin Invest. 1994;93:2572–7. doi: 10.1172/JCI117268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masereeuw R, Terlouw SA, van Aubel RA, Russel FG, Miller DS. Endothelin B receptor-mediated regulation of ATP-driven drug secretion in renal proximal tubule. Mol Pharmacol. 2000;57:59–67. [PubMed] [Google Scholar]
- 52.Garvin J, Sanders K. Endothelin inhibits fluid and bicarbonate transport in part by reducing Na+/K+ATPase activity in the rat proximal straight tubule. J Am Soc Nephrol. 1991;2:976–82. doi: 10.1681/ASN.V25976. [DOI] [PubMed] [Google Scholar]
- 53.Harris PJ, Thomas D. Effects of endothelin- 1 (ET) on proximal tubule fluid reabsorption. J Am Soc Nephrol. 1990;1:416. (abstract) [Google Scholar]
- 54.Phelps R, Guntupalli J. Effects of endothelin on Na-P1 cotransport in rat renal brush border membranes (BBM) J Am Soc Nephrol. 1990;1:580. (abstract) [Google Scholar]
- 55.Liu Y, Yang J, Ren H, He D, Pascua A, Armando MI, Yang C, Zhou L, Felder RA, Jose PA, Zeng C. Inhibitory effect of ETB receptor on Na+-K+ ATPase activity by extracellular Ca2+ entry and Ca2+ release from the endoplasmic reticulum in renal proximal tubule cells. Hypertens Res. 2009;32:846–52. doi: 10.1038/hr.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeidel ML, Brady HR, Kone BC, Gullans SR, Brenner BM. Endothelin, a peptide inhibitor of Na-K-ATPase in intact renal tubular epithelial cells. Am J Physiol. 1989;257:C1101–7. doi: 10.1152/ajpcell.1989.257.6.C1101. [DOI] [PubMed] [Google Scholar]
- 57.Guntupalli J, DuBose TD., Jr Effects of endothelin on rat renal proximal tubule Na+-Pi cotransport and Na+/H+ exchange. Am J Physiol. 1994;266:F658–66. doi: 10.1152/ajprenal.1994.266.4.F658. [DOI] [PubMed] [Google Scholar]
- 58.Peng Y, Moe OW, Chu T, Preisig PA, Yanagisawa M, Alpern RJ. ETB receptor activation leads to activation and phosphorylation of NHE3. Am J Physiol. 1999;276:C938–45. doi: 10.1152/ajpcell.1999.276.4.C938. [DOI] [PubMed] [Google Scholar]
- 59.Chu TS, Tsuganezawa H, Peng Y, Cano A, Yanagisawa M, Alpern RJ. Role of tyrosine kinase pathways in ETB receptor activation of NHE3. Am J Physiol. 1996;271:C763–71. doi: 10.1152/ajpcell.1996.271.3.C763. [DOI] [PubMed] [Google Scholar]
- 60.Chu TS, Wu KD, Wu MS, Hsieh BS. Endothelin-1 chronically inhibits Na/H exchanger-3 in ETB-overexpressing OKP cells. Biochem Biophys Res Commun. 2000;271:807–11. doi: 10.1006/bbrc.2000.2724. [DOI] [PubMed] [Google Scholar]
- 61.Gregan B, Schaefer M, Rosenthal W, Oksche A. Fluorescence resonance energy transfer analysis reveals the existence of endothelin-A and endothelin-B receptor homodimers. J Cardiovasc Pharmacol. 2004;44 (Suppl 1):S30–3. doi: 10.1097/01.fjc.0000166218.35168.79. [DOI] [PubMed] [Google Scholar]
- 62.Gregan B, Jürgensen J, Papsdorf G, Furkert J, Schaefer M, Beyermann M, Rosenthal W, Oksche A. Ligand-dependent differences in the internalization of endothelin A and endothelin B receptor heterodimers. J Biol Chem. 2004;279:27679–87. doi: 10.1074/jbc.M403601200. [DOI] [PubMed] [Google Scholar]
- 63.Davenport AP, Kuc RE. Down-regulation of ETA receptors in ETB receptor-deficient mice. J Cardiovasc Pharmacol. 2004;44 (Suppl 1):S276–8. doi: 10.1097/01.fjc.0000166284.08657.64. [DOI] [PubMed] [Google Scholar]
- 64.Evans NJ, Walker JW. Sustained Ca2+ signaling and delayed internalization associated with endothelin receptor heterodimers linked through a PDZ finger. Can J Physiol Pharmacol. 2008;86:526–35. doi: 10.1139/Y08-050. [DOI] [PubMed] [Google Scholar]
- 65.Boesen EI, Pollock DM. Cooperative role of ETA and ETB receptors in mediating the diuretic response to intramedullary hyperosmotic NaCl infusion. Am J Physiol Renal Physiol. 2010;299:F1424–32. doi: 10.1152/ajprenal.00015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsumura Y, Hashimoto N, Taira S, Kuro T, Kitano R, Ohkita M, Opgenorth TJ, Takaoka M. Different contributions of endothelin-A and endothelin-B receptors in the pathogenesis of deoxycorticosterone acetate-salt-induced hypertension in rats. Hypertension. 1999;33:759–65. doi: 10.1161/01.hyp.33.2.759. [DOI] [PubMed] [Google Scholar]
- 67.Jose PA, Asico LD, Eisner GM, Pocchiari F, Semeraro C, Felder RA. Effects of costimulation of dopamine D1- and D2-like receptors on renal function. Am J Physiol. 1998;275:R986–94. doi: 10.1152/ajpregu.1998.275.4.R986. [DOI] [PubMed] [Google Scholar]
- 68.Luippold G, Küster E, Joos TO, Mühlbauer B. Dopamine D3 receptor activation modulates renal function in anesthetized rats. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:690–3. doi: 10.1007/pl00005314. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Fu C, Ren H, He D, Wang X, Asico LD, Jose PA, Zeng C. Impaired stimulatory effect of ETB receptor on D3 receptor in immortalized renal proximal tbule cells of spontaneously hypertensive rats. Kidney Blood Press Res. 2011;34:75–82. doi: 10.1159/000323135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu C, Yang Z, Ren H, Zhang Y, Han Y, He D, Lu Q, Wang X, Wang X, Yang C, Asico LD, Hopfer U, Eisner GM, Jose PA, Zeng C. D3 dopamine receptor regulation of ETB receptors in renal proximal tubule cells from WKY and SHRs. Am J Hypertens. 2009;22:877–83. doi: 10.1038/ajh.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeng C, Hopfer U, Eisner GM, Felder RA, Jose PA. Altered AT1 receptor regulation of ETB receptors in renal proximal tubule cells of spontaneously hypertensive rats. Hypertension. 2005;46:926–31. doi: 10.1161/01.HYP.0000174595.41637.13. [DOI] [PubMed] [Google Scholar]
- 72.Zeng C, Wang Z, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Aberrant ETB receptor regulation of AT receptors in immortalized renal proximal tubule cells of spontaneously hypertensive rats. Kidney Int. 2005;68:623–31. doi: 10.1111/j.1523-1755.2005.00440.x. [DOI] [PubMed] [Google Scholar]
- 73.Shichiri M, Hirata Y, Ando K, Emori T, Ohta K, Kimoto S, Ogura M, Inoue A, Marumo F. Plasma endothelin levels in hypertension and chronic renal failure. Hypertension. 1990;15:493–6. doi: 10.1161/01.hyp.15.5.493. [DOI] [PubMed] [Google Scholar]
- 74.Veglio F, Bertello P, Pinna G, Mulatero P, Rossi A, Gurioli L, Panarelli M, Chiandussi L. Plasma endothelin in essential hypertension and diabetes mellitus. J Hum Hypertens. 1993;7:321–5. [PubMed] [Google Scholar]
- 75.Schiffrin EL, Thibault G. Plasma endothelin in human essential hypertension. Am J Hypertens. 1991;4:303–8. doi: 10.1093/ajh/4.4.303. [DOI] [PubMed] [Google Scholar]
- 76.Davenport AP, Ashby MJ, Easton P, Ella S, Bedford J, Dickerson C. A sensitive radioimmunoassay measuring endothelin-like immunoreactivity in human plasma: comparison of levels in patients with essential hypertension and normotensive control subjects. Clin Sci (Lond) 1990;78:261–4. doi: 10.1042/cs0780261. [DOI] [PubMed] [Google Scholar]
- 77.Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest. 2000;105:925–33. doi: 10.1172/JCI8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Williams JM, Pollock JS, Pollock DM. Arterial pressure response to the antioxidant tempol and ETB receptor blockade in rats on a high-salt diet. Hypertension. 2004;44:770–5. doi: 10.1161/01.HYP.0000144073.42537.06. [DOI] [PubMed] [Google Scholar]
- 79.Pollock DM, Allcock GH, Krishnan A, Dayton BD, Pollock JS. Upregulation of endothelin B receptors in kidneys of DOCA-salt hypertensive rats. Am J Physiol Renal Physiol. 2000;278:F279–86. doi: 10.1152/ajprenal.2000.278.2.F279. [DOI] [PubMed] [Google Scholar]
- 80.Fryer RM, Rakestraw PA, Banfor PN, Cox BF, Opgenorth TJ, Reinhart GA. Blood pressure regulation by ETA and ETB receptors in conscious, telemetry-instrumented mice and role of ETA in hypertension produced by selective ETB blockade. Am J Physiol Heart Circ Physiol. 2006;290:H2554–9. doi: 10.1152/ajpheart.01221.2005. [DOI] [PubMed] [Google Scholar]


