Abstract
Aim
To gauge patient interest in receiving long-acting injectable nanoformulated antiretroviral therapy.
Methods
Four hundred adult HIV-infected patients currently prescribed antiretroviral therapy were surveyed. χ2 tests were used for comparisons of interest across groups.
Results
Respondents were 68% male and 53% African–American, with a mean age of 47 years. Overall, 73% of patients indicated that they would definitely or probably try injectable nanoformulated antiretroviral therapy; 61% with weekly dosing; 72% every 2 weekly; and 84% monthly. In total, 48% indicated that they were very concerned about the possible side effects and 35% were very concerned about needle use.
Conclusion
The majority of respondents indicated that they definitely or probably would try parenteral nanoformulated antiretroviral therapy.
Keywords: AIDS, antiretroviral therapy, HIV, intravenous drug abuse, nanoformulated antiretroviral therapy, nonadherence, substance abuse
Combination antiretroviral therapy (ART) when taken consistently is effective at inhibiting HIV replication, and preventing viral mutation and the development of drug resistance, resulting in substantive reductions in morbidity and mortality for infected individuals [101]. However, there are still a number of limitations associated with traditional oral ART. Most significantly, currently available therapies necessitate lifelong, daily dosing, and suboptimal adherence places the patient at risk of treatment failure and viral resistance [1]. Moreover, only zidovudine and enfuvirtide are available in a parenteral formulation [2]. This lack of parenteral formulations is particularly relevant to patients who are critically ill, hospitalized, or in the perioperative period and unable to take oral medications.
Preliminary investigations are underway to determine whether nanomedicines could optimize antiretroviral drug adherence and decrease adverse effects [3]. Nanomedicines contain crystalline drug particles of small diameter, coated with low-molecular-weight excipients to produce specific sizes, charges and shapes that optimize cell and tissue penetrance. The process of developing a nanomedicine is referred to as nanoformulation. The term ‘nanoformulated ART’ (nano-ART) has been coined by our group to describe nano-ART where hydrophobic drugs are made to target immune cells contained in sustained-release carriers [4]. Nano-ART formulations are in development through modifications of existing atazanavir, ritonavir and efavirenz suspensions [5]. The ability of nano-ART to affect immune and antiviral responses before or following HIV-1 infection was tested in immune-deficient mice reconstituted with human peripheral blood lymphocytes. Here, weekly subcutaneous injections of nanoformulated drugs 1 day before, and/or 1 and 7 days after viral exposure produced systemic drug concentrations that paralleled those reported in humans, and produced reductions in virus load and preservation of CD4 cells with limited toxicities [4]. In this mouse model, nano-ART proved more effective than orally administered ART, and studies are ongoing in nonhuman primates, making the availability of such formulations for human use a potential next step. Another example is the parenteral administration of long-acting nanoformulated rilpivirine, which has been evaluated in animal and human studies [6]. Following single-dose administration, the plasma concentration profiles showed sustained release of rilpivirine over 3 months in dogs and 3 weeks in mice. More recently, pharmacokinetics in plasma, genital tract in females and rectal tissue in males were explored [7]. Long-acting nanoformulated rilpivirine by single intramuscular injection exhibited prolonged plasma and genital tract exposure, suggesting potential as an agent for use in pre-exposure prophylaxis.
Patient acceptance of, and interest in, this method of drug delivery is currently unknown. This study was to gauge patient interest in the new drug-delivery system, as well as to determine what patient characteristic(s) contribute to such interest. For example, substance-abuse disorders are both highly correlated with non-adherence to oral regimens and common among HIV-1-infected individuals, and have long been recognized as a risk factor for poor adherence to treatment [8]. Therefore, HIV-infected patients with concomitant drug-abuse disorders often have poor outcomes and, as a result, healthcare providers may be reluctant to prescribe ART to patients actively using drugs, owing to potential promotion of virologic resistance and viral transmission to uninfected contacts [9]. For this reason, we were particularly interested in patient acceptance of chronically administered parenteral ART, and the influence of current or prior drug use on the level of interest.
Methods
English-speaking adult men and women with HIV infection who were prescribed ART at the time were surveyed. Performance sites were the HIV clinics of the University of Nebraska Medical Center (USA) and Johns Hopkins University (MD, USA). Eligible patients were invited to participate as they attended clinic visits; there was no formal selection process. The study was approved by the institutional review board of each institution.
A standardized five-page survey was developed (Supplementary Material; see online at www.futuremedicine.com/doi/suppl/10.2217/NNM.12.214). The purpose of the survey was to gauge patient interest in a new drug-delivery system and included questions about patients’ current ART, adherence to the current regimen, and reasons for their interest in, or concerns about, nano-ART. The survey also included patient demographics, source of funding for healthcare and current, or prior, substance use. Adherence to ART was assessed using 4-day recall, adapted from the validated questionnaire that was developed by the AIDS Clinical Trials Group [10]. Enthusiasm for receiving nano-ART by subcutaneous injection was graded on a four-point scale and by intervals between injection (once weekly, every 2 weeks and every month). Respondents were also queried about their level of enthusiasm regarding the cost of nano-ART and about possible concerns with the new system, such as side effects, use of needles and change in daily routine.
The sample size was based on an estimation of the proportion of respondents who indicated that they definitely or probably would try the new drug-delivery system. We used a conservative, estimated, observed proportion of 0.50, which required 371 completed surveys to produce a 95% confidence interval with a precision of 0.05. In order to allow for incomplete surveys, 200 surveys were collected from each institution, giving a total of 400 surveys. The primary outcomes of interest in the new method overall, by frequency of injection and by cost, along with the demographic characteristics of respondents and drug-use history, were examined for the entire sample and by subgroups within the sample. Subgroups included males and females, age groups, drug users and nonusers, those who reported adherence to their medication schedule (i.e., never missed doses) and those who did not.
The population was divided into three age groups: 18–39, 40–54 and more than 55 years old. For the purposes of gender-based outcome comparisons, gender was limited to persons who entered only ‘male’ or ‘female’ for the gender question. Drug users and nonusers were defined in several ways. The first groups of users and nonusers were defined as those who had used marijuana, cocaine, heroin, amphetamines or intravenous (iv.) drugs in the past 6 months versus those who had not. In the second grouping, drug users were limited to those who had used cocaine, heroin, amphetamines or iv. drugs in the past 6 months (excluding those who only used marijuana). The final group of users was limited only to persons who had used iv. drugs in the past 6 months. For purposes of the analyses, medication adherence was based on two questions from the survey. The first asked how many days out of the last 4 days a respondent had missed all of the doses. Responses were dichotomized to values of 0 and 1–4 days. The second question asked for the last time a respondent missed a medication dose. Responses were dichotomized to values of never and something other than never. Comparisons of interest levels across groups were conducted using Mantel–Haenszel χ2 tests, with exact tests used where expected cell sizes were less than five.
Prior to the analyses, responses to some questions regarding drug use were imputed. The first set of questions about drug use asked respondents to indicate for each drug in a list whether they had used it within the past 6 months, had used it but not within the past 6 months, or had never used it. If a respondent answered for one or more drugs but left the other drugs blank, ‘never used’ was assigned as a response to the blank responses. The second area where imputation was applied was a pair of questions about iv. drug use: the first asked whether the respondent had ever used iv. drugs, and the follow-up for those who answered in the affirmative asked about use within the past 6 months. A few respondents answered the iv. drug use in the past 6 months question without answering the iv. drug use ever question. The ‘past 6 months’ question was intended to be a follow-up for those respondents who answered ‘Yes’ to the ‘ever use’ question. In these cases, the 6-month response (‘Yes’ or ‘No’) was imputed as the ever-use response. Finally, some respondents reported use in the past 6 months after reporting that they had never used. No changes were made in these instances, so that in all of the analyses, usage within the past 6 months is based upon the actual response to that question.
Results
Four hundred respondents completed the survey between May and July 2011; one was ineligible because the respondent was under 18 years of age and was excluded from the analysis. Demographics of the 399 eligible respondents are given in Table 1. A total of 68% of respondents were male, 53% were African–American and the mean age was 47 years old (range: 18–71 years). Of respondents, 80% indicated ‘Government Funding’ as a source of payment for their healthcare. The proportion of respondents who indicated that they had never used drugs was 52% for cocaine, 76% for heroin and 77% for amphetamines; however, 64% indicated marijuana use in the past and 23% in the last 6 months (Table 2). Of respondents, 27% indicated iv. drug use ‘ever’, and 5% during the last 6 months. Twenty-four (6%) respondents were currently in a methadone-maintenance program. Regarding antiretroviral medication adherence, the majority of respondents (75%) stated that they had not missed any doses in the past 4 days and 134 (35%) respondents answered that they never skipped medications.
Table 1.
Respondent demographics.
| Parameter | n (%); or n (range) |
|---|---|
| Mean age in years | 47 (18–71) |
| Gender | |
| Male | 269 (68) |
| Female | 123 (31) |
| Transgender | 4 (1) |
| Race/ethnicity | |
| African–American | 210 (53) |
| Asian/Pacific Islander | 1 (<1) |
| Native American | 4 (1) |
| Other | 2 (1) |
| White | 157 (40) |
| Hispanic | 13 (3) |
| How healthcare is paid for | |
| Government-funded healthcare | 316 (80) |
| Private health insurance | 87 (22) |
| Self-pay | 67 (24) |
| Self-reported adherence | |
| Not missed in last 4 days | 298 (75) |
| Missed in last 4 days | 98 (25) |
| Ever missed | 254 (66) |
| Never missed | 134 (35) |
| Intravenous drug use | |
| Ever used | 104 (27) |
| Never used | 289 (74) |
| Used in past 6 months | 20 (5) |
| Not used in past 6 months | 358 (95) |
| Methadone treatment | |
| Currently in treatment | 24 (6) |
| Not currently in treatment | 357 (94) |
| Ever in treatment | 29 (11) |
| Never in treatment | 243 (89) |
Table 2.
Respondent drug use.
| Drug | Never used; n (%) |
Past use ≥6 months ago; n (%) |
Used in past 6 months; n (%) |
|---|---|---|---|
| Marijuana | 140 (36) | 162 (42) | 88 (23) |
| Cocaine | 203 (52) | 162 (42) | 25 (6) |
| Heroin | 295 (76) | 81 (21) | 14 (4) |
| Amphetamines | 302 (77) | 82 (21) | 6 (2) |
In response to questions about the likelihood of trying nano-ART by injection, 73% of respondents stated that they would definitely or probably try the new method (95% CI: 68–77) (Figure 1A). Breaking the results down by frequency of dosing, 61% of respondents would still try the new method if it was as frequently as once per week (95% CI: 55–66), 72% for 2-week intervals (95% CI: 67–76) and 84% with once-monthly dosing (95% CI: 80–89). Younger people indicated more willingness to try nano-ART compared with older individuals (p = 0.03) (Figure 1B). There were no statistical differences by race or gender (Figure 1C). Respondents who had missed ART doses in the past 4 days were more likely to try the new method when dosed once per week (p = 0.03), once every 2 weeks (p < 0.01) and every month (p = 0.04) (Figure 1D). Those respondents who had ever missed a dose were increasingly likely to try the new method as dosing became less frequent (p = 0.04 for every 2 weeks and p = 0.01 for every month).
Figure 1. Patient interest by timing of injections, respondent demographics and self-reported adherence.
Percentages of respondents who indicated that they definitely would, probably would, probably would not or definitely would not be interested in trying nanoformulated parenteral antiretroviral therapy (A) overall, (B) by age, (C) by race or gender and (D) by level of self-reported adherence. The actual number of respondents to each specific question is included in parentheses.
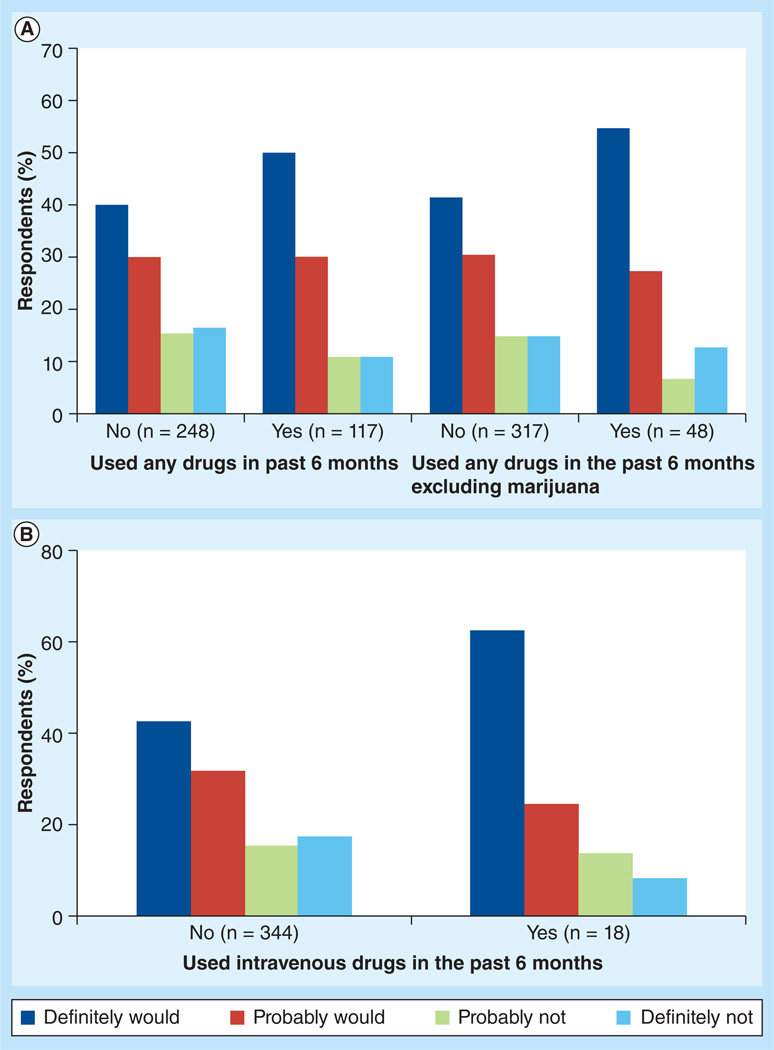
When cost was taken into consideration, respondents indicated that they probably would try the new method if the cost was much less (82.3%), a little less (77.4%), the same amount (73.2%), a little more (47%) or much more (28.4%) compared with their current regimen. Of the 365 respondents who answered questions about drug use in the last 6 months, 171 out of 248 (69.0%) who indicated no drug use were definitely or probably interested in trying the new method, which was significantly less than the proportion (93 out of 117; 79.5%) of those who indicated drug use in the last 6 months who were definitely or probably interested (p = 0.03) (Figure 2A). When marijuana was excluded, 225 out of 317 respondents (71.0%) who indicated no drug use, and 39 out of 48 respondents (81.3%) who indicated drug use in the last 6 months were definitely or probably interested in trying the new method (Figure 2A). Of iv. drug users who had used in the past 6 months, 100% would definitely try the new method if dosed monthly, compared with 62% of nonusers (p = 0.04) (Figure 2B).
Figure 2. Patient interest by drug use.
Percentages of respondents who indicated that they definitely would, probably would, probably would not or definitely would not be interested in trying nanoformulated parenteral antiretroviral therapy by (A) drug use in the previous 6 months and by (B) intravenous drug use in the previous 6 months. The actual number of respondents to each specific question is included in parentheses.
Overall, 177 respondents (48%) indicated that they were very concerned about possible side effects and 122 (35%) were very concerned about needle use. Changing routine was only a serious concern for 83 (24%) respondents. Details of respondents’ concerns about side effects are shown in Table 3. When compared with males, female respondents were more concerned about side effects (p < 0.01), about using needles (p = 0.01) and about change in daily routine (p < 0.01), as were African–Americans compared with whites (p = 0.01, p < 0.01 and p < 0.01, respectively). Iv. drug users who had used in the past 6 months were less concerned about using needles than respondents who had not (p = 0.02). Regarding adherence, those who had never missed a dose of medication were more concerned about changing their daily routine than those who had missed a dose of medication (p = 0.01).
Table 3.
Concern about side effects of nanoformulated antiretroviral therapy.
| Parameter | Not at all concerned; n (%) |
A little concerned; n (%) |
Somewhat concerned; n (%) |
Very concerned; n (%) |
Total (n) |
p-value |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| 18–39 | 11 (13) | 12 (14) | 18 (21) | 44 (52) | 370 | 0.89 |
| 40–54 | 34 (17) | 34 (17) | 42 (21) | 86 (44) | ||
| 55+ | 11 (12) | 14 (16) | 20 (22) | 44 (49) | ||
| Gender | ||||||
| Male | 46 (18) | 48 (19) | 58 (23) | 99 (39) | 367 | <0.01 |
| Female | 9 (8) | 12 (10) | 22 (19) | 73 (63) | ||
| Race | ||||||
| African–American | 29 (16) | 21 (11) | 26 (14) | 109 (59) | 342 | 0.01 |
| White | 22 (14) | 32 (20) | 48 (31) | 55 (35) | ||
| Drug use | ||||||
| Used in past 6 months | 16 (14) | 19 (17) | 24 (21) | 55 (48) | 321 | 0.81 |
| Not used in past 6 months | 31 (15) | 31 (15) | 51 (25) | 94 (45) | ||
| Adherence | ||||||
| Missed doses in last 4 days | 7 (8) | 16 (18) | 20 (22) | 46 (52) | 374 | 0.11 |
| No missed doses in last 4 days | 49 (17) | 44 (15) | 60 (21) | 131 (46) | ||
| Ever missed a dose | 33 (14) | 37 (14) | 55 (23) | 118 (49) | 365 | 0.37 |
| Never missed a dose | 21 (17) | 20 (16) | 25 (20) | 56 (46) | ||
Differences between the 399 overall number of participants and the values listed are due to missing data for individual questions. Exceptions to this are for gender and race where, for purposes of the analyses, gender was limited to those who reported only ‘male’ or ‘female’, and race was limited only to those who reported ‘African–American’ or ‘white’.
Discussion
In this first reported survey of patient interest in long-acting parenteral nano-ART, the majority of respondents indicated that they definitely or probably would try it. Longer dosing intervals attracted greater interest than shorter intervals. Patients who reported missed ART doses and iv. drug users indicated increased interest, and may represent those who would benefit most from this strategy to optimize adherence to ART. Respondents expressed several concerns over trying the new method. Concerns included potential side effects of the drugs, using needles and changing routine. These concerns varied somewhat among groups, with females being most concerned, in general, and current or previous iv. drug users being less concerned about the use of needles.
ART can be packaged into nanoformulated delivery vehicles. These systems have served to improve clinical efficacy based on inherent abilities to extend drug circulating half-lives and limit toxicity profiles [11,12]. The potential clinical applications for nanomedicines are quite extensive, and range from tissue regeneration and repair to antimicrobial therapy and correction of metabolic disorders. For nano-ART, preserved sites for drug delivery are in the reticuloendothelial system and the CNS: potential reservoirs for viral replication [13].
The promise of long-acting nano-ART is both extensive and substantive for treating HIV disease. The potential to reduce side effects is yet another attraction for such therapeutic delivery schemes. To realize the potential of nano-ART and make the transition from bench to bedside, we must focus research efforts on using the cell-based carriage of drug nanoparticles to improve clinical outcomes. Extensive works performed in both our own and other laboratories have seen the realization of mononuclear phagocytes as depots and as Trojan horses for drug nanoparticle delivery. Success was recently achieved in small animal models of HIV/AIDS using subcutaneous delivery, although the volumes required and potential injection-site reactions necessitate alternative injection schemes for human translation [14,15]. However, one obstacle to realizing this goal rests in assessing the interest in this new method of administration among our patients.
Despite limited familiarity with nanomedicine and concerns over potential adverse effects, community survey recipients are, in general, optimistic and interested in the potential of nanomedicine and nanotechnologies [16,17]. Novel applications of nanotechnology may impact medical care in diagnostics, vaccine strategies and therapeutics. Issues in the development of these approaches include an uncertain regulatory pathway, which may lead to confusion in consumers who are unable to judge the relative risks and benefits of nano-containing products. Inclusion of key stakeholders in the process is critical. The EU has an action plan for nanosciences and nanotechnologies, including a recommendation for public deliberation on the societal context [102]. Engagement and input from the community is essential in the research and development of this rapidly growing field.
There are some limitations to this study. Of the population surveyed, the majority of respondents were not drug abusers; however, the statistically significant difference in responses between drug abusers and nonusers, as well as adherent and nonadherent individuals, suggests that these populations would be interested in nano-ART. In relation to data collection, we did not obtain recipient CD4 count or viral-load data, which may have been useful when comparing interest between adherent and non-adherent individuals. It may also be interesting to examine the attitudes of those not on ART.
Conclusion
Our survey gauged patient interest in receiving parenteral nano-ART and what patient characteristics contributed to that interest. Not only were the majority of surveyed patients interested in trying nano-ART, but interest increased among iv. drug users and patients with suboptimal self-reported adherence, two important target populations for nano-ART.
Future perspective
In the absence of a cure for HIV infection, lifelong ART will probably remain the optimal treatment strategy. An alternative to daily oral therapy is an urgent need. Nano-ART holds great promise for improving adherence to treatment and minimizing adverse effects. A safe implementation of this emerging technology will probably pave the way, but community input, confidence and support will prove vital for success.
Supplementary Material
Executive summary.
Patient interest
-
▪
In the first reported survey of patient interest in long-acting parenteral nanoformulated antiretroviral therapy, the majority of respondents indicated that they definitely or probably would try it.
Dosing intervals
-
▪
Longer dosing intervals attracted greater interest than shorter intervals.
Targeted populations
-
▪
Patients who reported missed antiretroviral therapy doses and intravenous drug users indicated increased interest, and may represent those who would benefit most from this strategy to optimize adherence to therapy.
Acknowledgements
The authors wish to thank D Hansen for outstanding administrative support.
U Sandkovsky and S Swindells report receiving grant support to the University of Nebraska Medical Center (USA) from GlaxoSmithKline and Pfizer for research unrelated to this study. C Flexner reports receiving grant support from GlaxoSmithKline for research unrelated to this study, and has served as a consultant to Bristol-Myers Squibb, Glaxo-SmithKline, Merck, Roche, Vertex and ViiV Healthcare. U Sandkovsky has served as a Consultant for Merck. This work was supported by the National Institute of Drug Abuse P01 DA028555-01A1 (to S Swindells and HE Gendel-man).
No writing assistance was utilized in the production of this manuscript.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann. Intern. Med. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 2. Swindells S, Flexner C, Fletcher CV, Jacobson JM. The critical need for alternative antiretroviral formulations, and obstacles to their development. J. Infect. Dis. 2011;204(5):669–674. doi: 10.1093/infdis/jir370. Recent position paper highlighting the need for alternative methods of delivering antiretroviral therapy.
- 3.Pautler M, Brenner S. Nanomedicine: promises and challenges for the future of public health. Int. J. Nanomedicine. 2010;5:803–809. doi: 10.2147/IJN.S13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roy U, Mcmillan J, Alnouti Y, et al. Pharmacodynamic and antiretroviral activities of combination nanoformulated antiretrovirals in HIV-1-infected human peripheral blood lymphocyte-reconstituted mice. J. Infect. Dis. 2012;206(10):1577–1588. doi: 10.1093/infdis/jis395. Results of experiments with nanoformulated antiretroviral agents in mice, demonstrating prolonged antiviral activity.
- 5. Balkundi S, Nowacek AS, Veerubhotla RS, et al. Comparative manufacture and cell-based delivery of antiretroviral nanoformulations. Int. J. Nanomedicine. 2011;6:3393–3404. doi: 10.2147/IJN.S27830. Evaluation of candidate nanoformulated antiretroviral therapy agents, in vitro, outlining the issues concerning drug formulation and composition.
- 6.Baert L, Van ’t Klooster G, Dries W, et al. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur. J. Pharm. Biopharm. 2009;72(3):502–508. doi: 10.1016/j.ejpb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 7. Jackson Akil EL, Tjai J, Seymour N, et al. Rilpivirine-LA formulation: pharmacokinetics in plasma, genital tract in HIV-females and rectum in males. Presented at: The 19th Conference on Retroviruses and Opportunistic Infections; 5–8 March 2012; Seattle, WA USA. First presentation of data from a study in uninfected volunteers evaluating pharmacokinetics of nanoformulated rilpivirine.
- 8.Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376(9738):367–387. doi: 10.1016/S0140-6736(10)60829-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozal MJ, Amico KR, Chiarella J, et al. HIV drug resistance and HIV transmission risk behaviors among active injection drug users. J. Acquir. Immune Defic. Syndr. 2005;40(1):106–109. doi: 10.1097/01.qai.0000159666.95455.d2. [DOI] [PubMed] [Google Scholar]
- 10.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 11.Tang MF, Lei L, Guo SR, Huang WL. Recent progress in nanotechnology for cancer therapy. Chin. J. Cancer. 2010;29(9):775–780. doi: 10.5732/cjc.010.10075. [DOI] [PubMed] [Google Scholar]
- 12.Hu CM, Zhang L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem. Pharmacol. 2012;83(8):1104–1111. doi: 10.1016/j.bcp.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 13. Trono D, Van Lint C, Rouzioux C, et al. HIV persistence and the prospect of long-term drug-free remissions for HIV-infected individuals. Science. 2010;329(5988):174–180. doi: 10.1126/science.1191047. Review from experts in the field discussing the current understanding of HIV persistence and the limitations of available treatment approaches, including the critical role of tissue reservoirs.
- 14.Dash P, Gorantla S, Roy U, et al. Long-acting nanoformulated antiretroviral therapy elicits potent antiretroviral and neuroprotective responses in HIV-1-infected humanized mice. AIDS. 2012;26(17):2135–2144. doi: 10.1097/QAD.0b013e328357f5ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanmogne GD, Singh S, Roy U, et al. Mononuclear phagocyte intercellular crosstalk facilites transmission of cell targeted nanoformulated antiretroviral drugs to human brain endothelial cells. Int. J. Nanomedicine. 2012;7:2373–2388. doi: 10.2147/IJN.S29454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bainbridge WS. Public attitudes toward nanotechnology. J. Nanopart. Res. 2002;4(6):561–570. [Google Scholar]
- 17.Bottini M, Rosato N, Gloria F, et al. Public optimism towards nanomedicine. Int. J. Nanomedicine. 2011;6:3473–3485. doi: 10.2147/IJN.S26340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101. Panel on Antiretroviral Guidelines for Adults and Adolescents. Department of Health and Human Services; Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. www.aidsinfo.nih.gov/guidelines. Current US treatment guidelines outlining principles of antiretroviral therapy, preferred and alternative treatment regimens, and rationale for their selection.
- 102.Second Implementation Report 2007–2009. Communication from the Commission to the Council, the European Parliament and the European Economic and Social Committee; 2009. Nanosciences and nanotechnologies: an action plan for Europe 2005–2009. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2009:0607:FIN:EN:PDF. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.