Abstract
Background
K-complexes (KC) are evoked delta frequency EEG responses during sleep that occur when large numbers of healthy cortical cells burst fire in a synchronized manner. KC amplitude and incidence are sensitive measures of normal healthy brain aging. Given the known neurodegenerative consequences of alcohol abuse it was hypothesized that alcoholism would be associated with further KC amplitude and incidence reductions.
Methods
84 subjects (42 alcoholics) screened for medical, psychiatric and sleep problems, participated. The protocol involved the presentation of auditory stimuli during stage 2 sleep throughout a night in the laboratory. KCs were identified and averaged, to enable measurement of the P2, N550, and P900 peaks.
Results
Compared with controls, alcoholic men and women had lower KC incidence (p < .001) and P2 (P < .001), N550 (p < .05) and P900 (p < .05) amplitudes. There was a significant diagnosis by site interaction (p < .001) indicating the group difference was largest at frontal sites. Longer sobriety correlated with increased N550 amplitude (p < .01).
Conclusions
KC incidence and amplitude were negatively impacted in alcoholic men and women with exacerbation of the normal aging effects, particularly over frontal scalp regions. The observed relationship between improvements in KC measures and increased time of abstinence suggests that these measures may provide a useful marker of brain recovery with continued abstinence from alcohol.
Keywords: alcoholism, sleep, K-complex, N550, Delta, sex
Introduction
Acute and chronic alcohol consumption cause sleep disturbances (1) which in alcoholics may persist even after abstinence. The most consistently reported finding in detoxified uncomplicated alcoholism is a reduction in slow wave sleep (SWS) (2), defined by the presence of delta EEG activity with amplitude greater than 75 microvolts (3).. Alcoholism also disrupts functional aspects of SWS with reduced SWS rebound following recovery from sleep deprivation (4) and reduced SWS has been shown to be a predictor of relapse (5).
The expression of the high amplitude delta EEG that defines SWS requires the synchronized burst firing of large numbers of healthy neurons (6). The reduction in SWS and in delta power seen in alcoholics (7) and in animal models of alcoholism (8) may provide a functional measure of the neuronal loss produced by alcohol abuse seen in post mortem pathology (9; 10) and in the reduced gray matter volumes from in vivo magnetic resonance imaging (MRI) studies (11)(12).
K-complexes (KCs) are high-voltage delta frequency EEG events seen in non-Rapid Eye Movement (NREM) sleep that occur spontaneously and can be evoked by external stimuli without awakening the subject (13). First reported by Loomis et al. (14), they are defined as “EEG waveforms having a well delineated negative sharp wave which is immediately followed by a positive component. The total duration of the complex should exceed 0.5 sec.” (3). The 0.5 second minimum duration corresponds to a frequency of 2Hz or less, and thus KCs are delta frequency waveforms, produced by the same generator mechanisms as those responsible for SWS delta EEG activity (15).
When evoked KCs are averaged, the resulting event related potential (ERP) waveform contains a series of components (16), with the most prominent being P2, N550 and P900. P2 is the positive deflection at the start of the KC, N550 represents the negative KC peak and P900, the terminating positive peak, or repolarization (17). It has been argued (18; 19) that averaging provides a cleaner estimate of the “true” KC response than individual KC events, which occur randomly with respect to other ongoing EEG phenomena. Thus, assessment of components in the averaged KC ERP may provide a more reliable experimental method to measure the ability of the sleeping brain to develop delta activity than that provided by observational measures of spontaneous SWS.
In adults, the scalp distribution of the KC ERP N550 shows a bilaterally symmetrical, distribution, with the amplitude typically maximal at Fz and then reducing systematically as electrodes are placed at more posterior locations (17; 20; 21). Similar scalp distributions have been reported for spontaneous KCs (22) and SWS delta activity (23). The frontal distribution supports positron emission tomography (PET) (24) and high density EEG array (25) studies, implying a role for frontal cortex in the generation of delta activity during sleep. Given the differential sensitivity of frontal cortical regions to alcoholism-related gray matter volume reduction (26), (26), study of frontally-dominant KCs is of clear relevance to the study of alcoholism.
The pattern of SWS change seen in alcoholism reflects an acceleration of the impact of normal aging on SWS (27; 28) and other measures of delta activity during sleep (29; 30). The incidence of spontaneous KCs (31) or evoked KCs (31; 32) and KC ERP N550 amplitude (31; 32) are all reduced in older subjects, and N550 amplitude shows a highly significant linear reduction across the adult lifespan, with linear regression equations accounting for approximately 60% of the age-related variance. The slope of the relationship is steepest at Fz, with progressive flattening of the slope of the regression function at more posterior sites (33).
In the only study to date of KCs in alcoholism (34), we reported data from seven alcoholic men and eight age-matched control men. The alcoholics had significantly reduced N550 amplitudes at a frontal scalp site (Fz) and a lower rate of KC elicitation than controls. There were a number of limitations with that paper, including the lack of an adaptation night to the laboratory and the absence of polysomnographic screening of subjects for sleep disorders. The small numbers did not provide sufficient statistical power to permit an analysis of the N550 across the multiple scalp sites and thus could not inform the question as to whether the impact of alcoholism on this sleep delta EEG measure shows any regional specificity. The exclusive use of male subjects obviously prohibited any evaluation of possible sex differences in the response.
The present study is designed to test the hypothesis that recently detoxified, chronic alcoholic men and women have smaller KC ERP N550 amplitudes, and lower evoked KC incidence rates compared to sex- and age-matched controls; and that the alcoholic-control differences will be greater over frontal scalp regions when compared to more posterior scalp sites.
Methods
Participants
Potential participants underwent medical and psychiatric screening that included a structured alcohol history (35) and structured clinical interview (SCID) (36). All alcoholic participants and none of the controls met DSM – IV criteria for alcohol dependence. Twenty five potential participants were excluded because they exhibited evidence of another DSM – IV Axis I disorder or other substance abuse or dependence. However, a past history of depression was allowed (seven alcoholics and one control subject). Thirty nine subjects who passed the SCID were excluded due to reasons such as deafness or were lost to contact. One hundred and five subjects underwent a screening polysomnogram (PSG), and 21 were excluded due to the presence of a clinically significant sleep disorder (obstructive sleep apnea or periodic leg movements). Eighty four subjects then completed the study, 42 abstinent long-term alcoholics (27 men) and 42 controls (19 men).
Stimuli
Auditory stimuli consisted of 1000Hz pure tones presented for 50ms (2ms rise time) at 80 dB(A). These stimuli are optimal for KC elicitation (18). The tones were presented binaurally via E-A-RTONE 3A insert earphones, with a random inter stimulus interval of between 15 and 30 seconds. A minimum of 200 stimuli were presented in stable stage 2 sleep across a night in the laboratory. When subjects entered SWS or REM sleep or the EEG showed signs of arousal or motor artifact, stimulus presentation was paused. Data were not collected during REM due to the absence of KCs from this sleep stage. SWS was avoided due to the low proportions of SWS typically seen with normal aging and in alcoholism. Sleep staging was verified after data collection according to standard criteria (3). In subjects over the age of 65 years hearing was verified using an audiometer to be within 15dB ISO at 1000Hz (a 20 dB decrease in stimulus intensity has no impact on N550 amplitude (18)). All subjects were tested during wakefulness to ensure that they could hear the tones that would be presented to them during sleep.
EEG data collection
EEG and evoked potential data were recorded from 7 scalp sites adapted from the international 10/20 system (FP1, FP2, Fz, FCz, Cz, CPz, Pz), using Grass gold-plated 10mm electrodes. Two channels of electro-oculogram (EOG) and a submental electromyogram (EMG) were also recorded. All EEG channels were referenced to linked earlobes. Raw data were acquired with a sampling rate of 1kHz using Neuroscan Synamps® amplifiers and stored for offline analysis using Neuroscan Scan ™ software. Signals were continuously displayed in real-time and filtered optimally for visual recognition of sleep stages (3) (30sec per page, EEG & EOG: band pass filter, 0.3 – 30Hz; EMG: band pass filter, 10 – 100Hz.). Raw EEG data were epoched time-locked to tone stimuli, and corrected for baseline differences across the pre-stimulus period.
Evoked KCs were defined using a modification of the standard (3) criteria, using data from Cz and Fz, with the additional parameter that the negative peak of the KC had to occur between 400 and 900 ms after the tone. No amplitude criterion was used. Epochs containing movement artifacts observed in EOG or EMG channels were discarded. All KC responses within stage 2 sleep were then averaged for each subject to produce an averaged KC evoked potential at all sites. The proportion of stimuli producing a KC was recorded as the KC incidence value for each subject.
Component identification was achieved using Scan ™ software. The P2 component was defined as the most positive peak between 100 and 300ms post stimulus, the N550 as the most negative value between 400 and 900ms post stimulus and the P900 as the most positive peak between 800 and 1200ms post stimulus. Amplitudes were then determined relative to the average of the pre-stimulus baseline. All peaks were determined within all electrode sites.
Statistical Analysis
Data are shown as mean (SD). Univariate ANOVAs with age as a covariate were used to investigate changes in KC incidence and demographic and alcohol use data according to diagnosis and sex. Where data did not meet parametric assumptions they were compared using appropriate non-parametric tests. Amplitude and latency of each peak component was subjected to a mixed model ANOVA, with diagnosis and sex as between-group factors, electrode site as a repeated measures, and age as a covariate. When the assumption of sphericity was violated, degrees of freedom were adjusted using the Greenhouse-Geisser correction, but original degrees of freedom are reported. Relationships between KC variables at Fz with age and alcohol consumption variables were explored with Pearson's Correlation.
Results
Subject Characteristics
Characteristics of alcoholics and controls are presented in Table 1. There were no significant effects of alcoholism diagnosis or sex on age, BMI, Dementia rating Scale (DRS) ratings or Mini Mental State Examination (MMSE). There was a trend for the alcoholics to be sleepier as measured by the Epworth Sleepiness Scale (ESS) [F(1,80) = 3.66, p = .06]. As expected, estimated lifetime alcohol consumption was significantly higher in alcoholics than in controls [ F(1,80) = 99.59, p < .001] and significantly higher in men than in women [F(1,80) = 11.06, p < .01] with the sex difference more pronounced in the alcoholics [F(1,80) = 7.83, p < .01]. Alcoholic women had significantly longer sobriety (271 ± 200 days) before testing than alcoholic men (131 ± 146 days); Mann-Whitney test [Z=-2.475, p = .013], and there was a trend for the duration of alcoholism to be longer in the alcoholic men (28 ± 27 years) than in the alcoholic women (14 ± 21years) [Z=-1.804, p = .07].
Table 1.
Participant characteristics presented as means (standard deviations) for all controls and alcoholics and then separately for men and women in each diagnostic group. Data are presented for age in years, body mass index (BMI) in Kg/m2, Epworth Sleepiness Scale (ESS) (61), Mattis Dementia Rating Scale (DRS) (62), mini mental state examination (MMSE) (63) and lifetime estimated alcohol consumption (EAC) in kg.
| Age | BMI | ESS | DRS | MMSE | EAC* | ||
|---|---|---|---|---|---|---|---|
| All | Control | 50.8 (9.8) | 25.3 (4.6) | 4.5 (2.9) | 140.2 (2.3) | 28.0 (1.4) | 54.4 (84.7) |
| Alcoholic | 48.8 (8.3) | 26.2 (4.7) | 6.2 (3.6) | 138.8 (5.3) | 27.5 (1.8) | 1294.7 (784.8) | |
| Women | Control | 51.0 (9.8) | 24.2 (3.5) | 4.1 (3.1) | 140.3 (1.9) | 27.8 (1.3) | 27.2 (36.7) |
| Alcoholic | 47.7 (9.6) | 26.1 (4.4) | 5.6 (2.6) | 140.9 (1.2) | 27.9 (1.2) | 845.9 (326.9) | |
| Men | Control | 50.5 (9.9) | 26.7 (5.6) | 5.1 (2.6) | 140.0 (2.7) | 28.1 (1.5) | 87.4 (112.3) |
| Alcoholic | 49.3 (7.6) | 26.3 (5.4) | 6.5 (4.1) | 137.6 (6.3) | 27.3 (2.0) | 1544.0 (856.2) | |
Alcoholics vs. Controls, p < .001; men vs. women p < .01; diagnosis by sex interaction p < .01.
KC incidence
The probability of eliciting a KC was 0.62 (0.20) in control women, 0.54 (0.19) in control men, 0.46 (0.20) in alcoholic women, and 0.35 (0.16) in alcoholic men. Alcoholics were significantly less likely to produce KCs than controls [F(1,42) = 7.89, p < .01] no significant sex or age effects and no significant interactions.
Evoked Potential Components
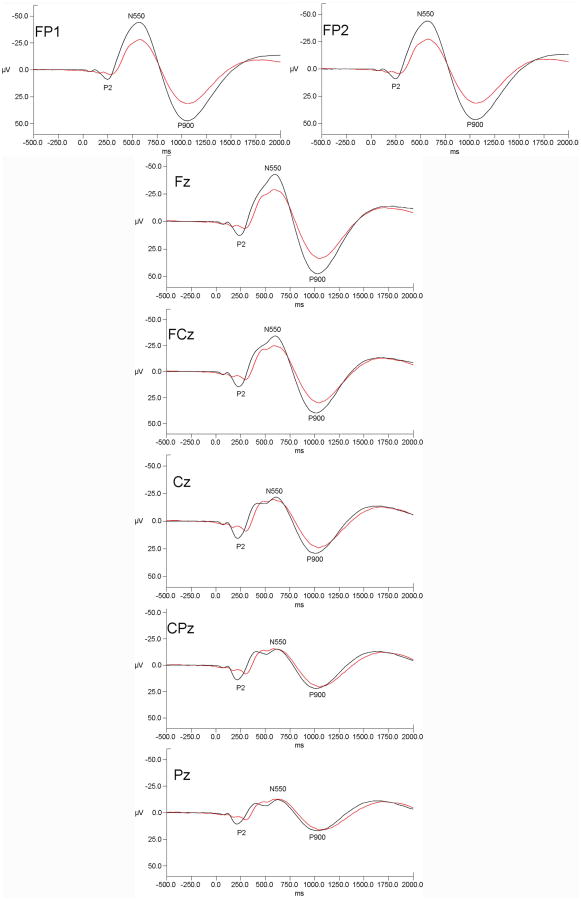
Grand mean evoked potentials for alcoholic and controls at all electrode sites are presented in Figure 1.
Figure 1.
Grand mean evoked potential waveforms for alcoholics (red lines) and controls (black lines) for the seven measured electrode sites. Data are presented with negative voltages up the Y axis. The pattern of results indicates that alcoholic-control differences were prominent over frontal and frontal-polar sites but were diminished at more posterior scalp locations.
P2
P2 amplitude and latency values are presented in Table 2. There was a significant effect of diagnosis [F(1,77) = 28.39, p < .001] for P2 amplitude, which was smaller in alcoholics than controls. There was a significant effect of electrode site [F(6,462) = 14.01, p < .001] with P2 amplitude being largest at Cz and becoming smaller progressively at more anterior and posterior sites. There was a significant diagnosis by site interaction [F(6,462) = 7.04, p < .01] with the group difference being largest at Cz and diminishing with distance from Cz. There was a significant effect of diagnosis [F(1,77) = 5.46, p < .05 ] for P2 latency, which was longer in alcoholics than controls. Women tended to have a shorter P2 latency than men [F(1,77) = 3.84, p = .054]. There was no significant effect of electrode site and none of the interaction effects approached significance.
Table 2.
P2 amplitude and latency means (standard deviations) derived from elicitation of KCs following auditory tones during stage 2 sleep for each electrode site for 42 alcoholics and 42 controls. (Diag = the main effect of diagnosis; Site = the main effect of site, Diag × site = the diagnosis by site interaction terms in the ANOVA model)
| P2 Amplitude (uV) | FP1 | FP2 | Fz | FCz | Cz | CPz | Pz | |
|---|---|---|---|---|---|---|---|---|
| All | Control | 11.70 (4.06) | 11.79 (4.17) | 18.52 (5.82) | 22.48 (6.92) | 25.29 (7.74) | 23.30 (7.84) | 17.94 (6.60) |
| Alcoholic | 7.61 (3.69) | 7.84 (3.59) | 11.78 (5.14) | 14.30 (6.34) | 15.79 (7.89) | 14.63 (7.35) | 11.49 (6.02) | |
| Women | Control | 12.12 (4.04) | 12.11 (4.23) | 18.63 (6.11) | 22.37 (7.37) | 24.88 (7.92) | 23.23 (7.46) | 17.97 (6.09) |
| Alcoholic | 8.73 (3.37) | 8.88 (2.57) | 14.34 (5.23) | 17.62 (7.06) | 19.73 (8.64) | 18.41 (8.21) | 14.96 (6.25) | |
| Men | Control | 11.21 (4.14) | 11.39 (4.17) | 18.37 (5.61) | 22.61 (6.53) | 25.79 (7.71) | 23.39 (8.47) | 17.90 (7.33) |
| Alcoholic | 7.00 (3.77) | 7.28 (3.97) | 10.41 (4.61) | 12.52 (5.23) | 13.67 (6.70) | 12.59 (6.08) | 9.62 (5.08) | |
| Diag p < .001 | Site p < .001 | Diag × Site p < .01 | ||||||
| P2 Latency (ms) | FP1 | FP2 | Fz | FCz | Cz | CPz | Pz | |
| All | Control | 262.17 (47.42) | 261.33 (45.42) | 262.31(63.18) | 259.55 (64.90) | 258.62 (65.49) | 258.05 (65.32) | 255.74 (65.90) |
| Alcoholic | 295.45 (49.49) | 291.75 (56.23) | 289.42 (55.66) | 292.78 (5.36) | 291.65 (57.74) | 288.73 (56.08) | 283.35 (60.21) | |
| Women | Control | 258.78 (44.18) | 258.26 (40.66) | 252.17 (56.70) | 249.78 (57.39) | 247.87 (57.32) | 247.17 (56.00) | 243.87 (56.97) |
| Alcoholic | 277.29 (50.83) | 264.21 (61.32) | 270.86 (55.41) | 270.21 (55.25) | 272.29 (55.50) | 272.14 (57.69) | 270.93 (55.58) | |
| Men | Control | 266.26 (52.00) | 265.05 (51.50) | 274.58 (69.80) | 271.37 (72.78) | 271.63 (73.68) | 271.21 (74.51) | 270.11 (74.35) |
| Alcoholic | 305.23 (46.83) | 306.58 (48.21) | 299.42 (54.22) | 304.00 (51.06) | 302.08 (57.24) | 297.65 (54.21) | 290.04 (62.58) | |
| Diag p <.05 | ||||||||
N550
N550 amplitude and latency values are presented in Table 3. There was a significant effect of diagnosis [F(1,77) = 12.83, p < .001] for N550 amplitude, which was smaller in alcoholics than controls. There was a significant effect of sex [F(1,77) = 4.76, p < .05] with the men having smaller N550 amplitudes, but there was no diagnosis by sex interaction. There was a significant effect of electrode site [F(6,462) = 8.55, p < .01] with the amplitude being largest at Fz and becoming smaller progressively at more posterior sites and at lateral sites, FP1 and FP2. There was a significant diagnosis by site interaction [F(6,462) = 16.05, p < .001] indicating the group difference was present at frontal sites but not at more posterior locations (Figure 1). The age covariate effect was significant [F(1,77) = 26.01, p < .001], with amplitude decreasing with age. N550 latency displayed no significant main effects or interactions, other than the age covariate effect [F(1,77) = 18.52, p < .001], with latency increasing with age.
Table 3.
N550 amplitude and latency means (standard deviations) derived from elicitation of KCs following auditory tones during stage 2 sleep for each electrode site for 42 alcoholics and 42 controls. (Diag = the main effect of diagnosis; Site = the main effect of site, Age = the main effect of age, Sex = the main effect of sex, Diag × site = the diagnosis by site interaction terms in the ANOVA model)
| N550 Amplitude (uV) | FP1 | FP2 | Fz | FCz | Cz | CPz | Pz | |
|---|---|---|---|---|---|---|---|---|
| All | Control | -51.96 (21.07) | -51.86 (21.61) | -52.60 (22.01) | -43.47 (19.84) | -29.56 (16.72) | -22.77 (14.39) | -18.49 (12.30) |
| Alcoholic | -33.41 (16.16) | -32.86 (15.23) | -36.71 (15.51) | -33.12 (14.90) | -27.23 (12.48) | -22.51 (11.06) | -18.66 (10.27) | |
| Women | Control | -55.14 (22.40) | -54.48 (22.33) | -54.49 (23.85) | -44.67 (21.12) | -30.69 (16.34) | -23.19 (14.51) | -18.91 (12.18) |
| Alcoholic | -39.09 (20.30) | -39.48 (18.26) | -44.21 (19.55) | -41.57 (18.11) | -33.08 (13.97) | -26.74 (10.67) | -16.30 (9.37) | |
| Men | Control | -48.10 (19.21) | -48.68 (20.84) | -50.31 (19.95) | -42.02 (18.63) | -28.18 (17.51) | -22.26 (14.62) | -17.98 (12.75) |
| Alcoholic | -30.36 (12.86) | -29.30 (12.28) | -32.67 (11.32) | -28.57 (10.69) | -24.07 (10.59) | -20.23 (10.78) | -16.30 (9.37) | |
| Diag p < .001 | Site p < .01 | Diag × Site p < .001 | Age p < .001 | Sex p < .05 | ||||
| N550 Latency (ms) | FP1 | FP2 | Fz | FCz | Cz | CPz | Pz | |
| All | Control | 587.40 (73.21) | 588.00 (70.61) | 604.64 (73.60) | 604.55 (87.80) | 624.55 (105.57) | 630.76 (105.50) | 635.33 (119.35) |
| Alcoholic | 594.65 (60.83) | 594.40 (64.39) | 606.12 (77.96) | 610.05 (79.32) | 610.62 (85.49) | 621.75 (87.35) | 633.15 (94.23) | |
| Women | Control | 569.91 (64.00) | 569.91 (58.55) | 595.78 (64.68) | 612.13 (77.47) | 616.57 (83.66) | 622.22 (81.99) | 626.83 (98.71) |
| Alcoholic | 585.64 (67.92) | 583.36 (68.61) | 575.50 (89.66) | 582.50 (91.96) | 584.93 (92.55) | 594.36 (97.34) | 594.50 (95.74) | |
| Men | Control | 608.58 (79.62) | 609.89 (79.01) | 615.37 (83.69) | 595.37 (100.30) | 634.21 (129.05) | 641.11 (130.11) | 645.63 (142.59) |
| Alcoholic | 599.50 (57.48) | 600.35 (62.57) | 622.62 (67.03) | 624.88 (69.03) | 624.36 (79.86) | 636.50 (79.56) | 653.96 (88.31) | |
| Age p < .001 | ||||||||
P900
P900 amplitude and latency values are presented in Table 4. There was a significant effect of diagnosis [F(1,77) = 8.22, p < .01] for P900 amplitude, which was smaller in alcoholics than controls. There was a significant effect of electrode site [F(6,462) = 5.87, p < .01] with amplitude being largest at Fz and becoming smaller progressively at more posterior sites and at FP1 and FP2. There was a significant diagnosis by site interaction [F(6,462) = 13.91, p < .001] indicating the group difference was present at frontal sites and not at more posterior locations. There was a trend for an effect of sex [F(1,77) = 3.50, p = .065) with women tending to have larger P900 amplitudes. The age covariate effect was significant [F(1,77) = 13.43, p < .001], with amplitude decreasing with age. No other interactions in the ANOVA model approached significance. There was a significant effect of electrode site [F(6,462) = 4.43, p < .05] for P900 latency, which was longer at FP sites. There were no other significant main effects or interactions.
Table 4.
P900 amplitude and latency means (standard deviations) derived from elicitation of KCs following auditory tones during stage 2 sleep for each electrode site for 42 alcoholics and 42 controls. (Diag = the main effect of diagnosis; Site = the main effect of site, Age = the main effect of age, Diag × site = the diagnosis by site interaction terms in the ANOVA model)
| P900 Amplitude (uV) | FP1 | FP2 | Fz | FCz | Cz | CPz | Pz | |
|---|---|---|---|---|---|---|---|---|
| All | Control | 51.86 (18.48) | 51.25 (18.46) | 52.29 (20.03) | 42.43 (21.57) | 31.74 (17.65) | 25.44 (15.84) | 19.32 (12.92) |
| Alcoholic | 35.73 (14.08) | 35.11 (13.57) | 37.83 (14.57) | 33.76 (14.57) | 28.39 (12.83) | 24.37 (12.51) | 20.35 (12.22) | |
| Women | Control | 53.93 (16.00) | 53.06 (16.26) | 52.60 (17.77) | 43.01 (17.45) | 31.58 (14.36) | 24.88 (14.43) | 19.32 (12.20) |
| Alcoholic | 41.39 (14.52) | 40.02 (13.32) | 44.56 (14.30) | 41.91 (15.21) | 35.87 (12.48) | 30.90 (12.26) | 27.13 (12.76) | |
| Men | Control | 49.35 (21.29) | 49.07 (21.07) | 51.92 (22.97) | 41.72 (26.21) | 31.93 (21.39) | 26.11 (17.78) | 19.33 (14.09) |
| Alcoholic | 32.68 (13.11) | 32.46 (13.20) | 34.20 (13.63) | 29.38 (12.40) | 24.36 (11.30) | 20.85. (11.36) | 16.71 (10.43) | |
| Diag p <. 01 | Site p < .01 | Diag x Site p < .001 | Age p < .001 | |||||
| P900 Latency (ms) | FP1 | FP2 | Fz | FCz | Cz | CPz | Pz | |
| All | Control | 1057.83 (82.91) | 1065.31 (86.22) | 1049.19 (91.13) | 1027.48 (108.39) | 1013.79 (105.22) | 1029.26 (119.63) | 1045.29 (127.13) |
| Alcoholic | 1051.02 (82.67) | 1053.08 (82.84) | 1052.23 (78.15) | 1029.13 (80.60) | 1030.73 (92.63) | 1048.80 (102.78) | 1065.30 (111.57) | |
| Latency | ||||||||
| Women | Control | 1057.74 (76.52) | 1065.04 (78.71) | 1053.52 (90.36) | 1042.91 (101.28) | 1025.22 (104.99) | 1035.91 (113.79) | 1052.74 (127.68) |
| Alcoholic | 1051.79 (101.78) | 1062.36 (103.87) | 1051.21 (101.29) | 1045.71 (96.18) | 1024.93 (104.45) | 1049.50 (111.54) | 1070.14 (127.76) | |
| Men | Control | 1057.95 (92.21) | 1065.63 (96.76) | 1043.95 (94.25) | 1008.79 (116.41) | 999.95 (106.67) | 1021.21 (129.01) | 1036.26 (129.35) |
| Alcoholic | 1050.62 (72.62) | 1048.08 (70.85) | 1052.77 (64.74) | 1020.19 (71.33) | 1033.85 (87.65) | 1048.42 (100.04) | 1062.69 (104.45) | |
| Site p < .05 | ||||||||
Relationships between age, alcohol consumption variables and KC variables
In control subjects, increasing age was significantly correlated with a reduced KC incidence (r = -.277, p = .038), and reduced N550 (r = .551, p < .001) and P900 (r = -.360, p = .010) amplitudes. There were no significant correlations with estimated total alcohol consumption or days since last alcoholic drink.
In alcoholics, increasing age was significantly correlated with reduced KC incidence (r = -.305, p = .025), and reduced N550 (r = .369, p = .008) and P900 (r = -.404, p = .004) amplitudes. Estimated total alcohol consumption was not significantly correlated with any variables however there was a trend for a decrease in N550 amplitude with more alcohol consumed (r = .224, p = .077). Longer sobriety correlated with increased N550 (r = -.44, p = .002) and P900 Fz (r = .369, p = .008) amplitudes, and a trend for more KCs to be elicited (r = .222, p = .079). After controlling for age and estimated total alcohol consumption, the correlations increase to r = =0.541, p < 0.001 for N550 amplitude, r = 0.475, p = 0.001 for P900 amplitude and r = 0.282, p < 0.05 for KC incidence.
Alcoholism effects as an indicator of accelerated aging
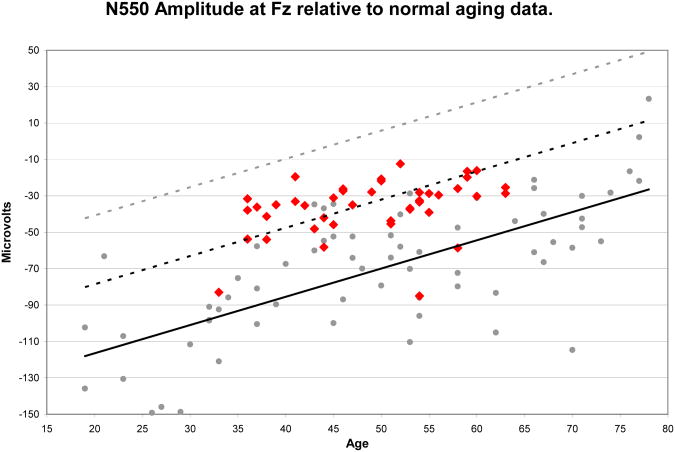
Figure 2 displays the normal aging regression relationship for N550 amplitude at Fz from (33), plotted with the regression lines representing one and two standard deviations below the observed relationship. Data from alcoholics in the present study are placed on the figure showing that most of the alcoholics are at least one standard deviation below where they should be based on the normal aging data. Application of the regression equation from our previous study (33) to the alcoholic's data reveals that their N550 amplitudes predict ages that are 23.0 ±10.35 years older than true chronological age.
Figure 2.
N550 amplitude values measured at Fz for alcoholic subjects (red diamonds) presented with the normative aging data (gray dots) and linear regression equation (black line) from Colrain et al. (34) along with regression lines representing 1 SD (dotted black line) and 2 SD (dotted gray line) below the age-regressed means. All but two of the 42 alcoholics had amplitudes that were smaller than that predicted for age by the normal-regression line. NOTE: Smaller negative values indicate reduced N550 amplitudes.
Discussion
Uncomplicated alcoholism has a clear impact on evoked delta frequency responses during NREM sleep. The general pattern of amplitude reduction of the three major components of the KC ERP represents an exacerbation of the normal aging effects previously reported (31-33). The amplitude effects in alcoholics are more pronounced at frontal scalp sites with reduced differences at posterior locations and are equally present in alcoholic men and women. The exacerbation of the aging effect is highlighted by the application of the normal aging regression equation to the alcoholic data indicating that alcoholics have EEG consistent with them being aged by an additional two decades.
The reduced KC incidence in alcoholics presumably reflects some negative impact(s) of alcohol abuse on some aspect(s) of the KC triggering process. There may be an effect on the slow frequency oscillation such that basal forebrain cells are less often in the hyperpolarized state necessary for burst firing (37), possibly due to alcohol-mediated alterations in T-type Ca++ channels (38) or modification of glial cells (39). Alternatively alcohol abuse might directly impact the basal forebrain cells involved in KC triggering. Many animal studies have shown that alcohol has neurotoxic effects upon the basal forebrain cholinergic projections (40-42), and there is substantial evidence of allosteric modification of GABA receptors following alcohol abuse (43). Studies have shown elevated GABAα1 receptor subunit mRNA expression (44) and GABAA receptor binding (45) in the frontal cortex of alcoholics, and both PET (46) and fMRI (47) studies have demonstrated decreased activation in frontal regions following challenges to the GABAergic system.
In addition to the impact of alcoholism on evoked KC incidence, the present data also show a marked impact on KC (delta) amplitude. Bastien and Campbell (18) reported that when stimulus properties are held constant, the KC appears as an “all or none” phenomenon with little variation in the baseline to peak N550 amplitude in the KC ERP. Thus once triggered, the burst firing should propagate along the relevant pathways engaging as many neurons as are available. The impact of alcohol abuse on KC amplitude is, therefore, presumably via a reduction in available neurons in the target regions of the basal forebrain projecting cells. Uncomplicated alcoholism has been shown to produce reduced numbers of superior prefrontal cortex (PFC) neurons as measured in post-mortem cells counts (9; 10; 48; 49) and to preferentially reduce gray matter volume in PFC as measured with in vivo MRI (26). There is also evidence of diminished metabolism (50) and perfusion (51) in frontal cortex of alcoholics. In the present study the alcoholics had significantly smaller N550 amplitudes at Fz and FP sites but the difference between alcoholics and controls was diminished at FCz, and was not present at Cz or more posterior sites. The reduced P2 and P900 amplitudes provide further evidence of the overall reduction in activation, and the increased P2 latency may reflect a delay in the start of the KC possibly due to degradation of white matter (52). P900 demonstrated a very similar pattern of scalp topography to that of N550 in terms of alcoholic-control differences. These data reinforce the hypothesis of P900 reflecting a repolarization of tissue following the burst firing associated with KC production that is manifest in the N550 (17). The frontally predominant alcoholic-control differences in the ERP data indicate that the KC represents a functional correlate of the post-mortem cell-count and in vivo MRI data.
A number of sex differences were apparent. Alcoholic women had longer sobriety and a trend for a shorter duration of abusive drinking, and an overall reduced level of alcohol consumption. In this sample, women overall had larger N550 amplitudes than men, with no significant diagnosis by sex interaction for either measure. However, the male-female differences were smaller in the controls than in the alcoholics and our previously published larger study of controls showed no sex differences in N550 amplitude (33) The alcoholism impact on N550 amplitude was substantially greater in men (17.6 μV at Fz) than in women (10.3 μV at Fz). The lack of a significant sex by diagnosis interaction could be a function of reduced power for this term in the ANOVA model given the unequal subject numbers between groups. Certainly, alcoholic women may have less cortical loss relative to that seen in men (53; 54) (but see (55)) and possibly, unlike alcoholic men, have brain metabolism levels that are not reduced relative to controls (56). What is clear from the present data is that regardless of the possible difference in magnitude, women show the same general pattern of alcoholism-related change in the evoked KC measures.
As reviewed by Sullivan and Pfefferbaum (11) many aspects of alcoholism-related cognitive and motor decline have been shown to recover with abstinence, although to varying extents and with variable trajectories. White matter volume has been shown to increase with abstinence (57; 58) although differences between abstinent and relapsed alcoholics can be driven largely by the negative changes in the relapsed group rather than or in addition to improvements in the abstinent group (59). There is less evidence for improvements in gray matter volume although Pfefferbaum et al. (59) reported a trend for increased gray matter volume at 30 days of abstinence. In the present data there was a significant relationship between N550 amplitude and length of sobriety, with longer periods of abstinence being associated with increased N550 amplitude. This relationship may be a reflection of some functional recovery in brain tissue however confirmation of this hypothesis would require longitudinal data collection of MRI and KC measures from the same subjects.
The application of the regression equation for the impact of normal aging on N550 amplitude (33) to the data from alcoholics in the present study reveals a pattern of accelerated aging in the alcoholic subjects as has previously been shown with SWS (60) and gray matter volume (26). The lack of an age by diagnosis interaction in the ANOVA model would argue for alcoholism and aging having additive effects.
Conclusion
The evoked KC measures previously shown to be a sensitive measure of normal aging clearly demonstrate reduced delta generation capacity in men and women suffering from uncomplicated alcoholism. The frontal scalp topography of the alcoholic-control differences is consistent with both post-mortem and in-vivo brain data measures and thus can be argued, provide a functional correlate of these anatomical measures. The observed relationship between improvements in KC measures and increased time of abstinence allows for the speculation that these measures may also provide a useful marker of brain recovery with continued abstinence from alcohol.
Acknowledgments
The work was funded by NIH (NIAAA) grant AA14211. The authors wish to thank Dolf Pfefferbaum, Edie Sullivan and John Trinder for their contributions to the work.
Footnotes
Financial Disclosures: The authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ehlers CL. Alcohol and Sleep. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAA's Neuroscience and Behavioral Research Portfolio. Research Monograph 34. Bethesda, MD: U.S. Department of Health and Human Services; 2000. pp. 417–436. [Google Scholar]
- 2.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 3.Rechtschaffen A, Kales A. A Manual of Standardised Terminology, Techniques and Scoring Systems for Sleep Stages of Human Subjects. Washington D.C: U.S. Government Printing Office; 1968. [Google Scholar]
- 4.Irwin M, Gillin JC, Dang J, Weissman J, Phillips E, Ehlers CL. Sleep deprivation as a probe of homeostatic sleep regulation in primary alcoholics. Biol Psychiatry. 2002;51:632–641. doi: 10.1016/s0006-3223(01)01304-x. [DOI] [PubMed] [Google Scholar]
- 5.Brower KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. 2003;7:523–539. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- 6.Destexhe A, Contreras D, Steriade M. Spatiotemporal analysis of local field potentials and unit discharges in cat cerebral cortex during natural wake and sleep states. J Neurosci. 1999;19:4595–4608. doi: 10.1523/JNEUROSCI.19-11-04595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irwin M, Miller C, Gillin JC, Demodena A, Ehlers CL. Polysomnographic and spectral sleep EEG in primary alcoholics: an interaction between alcohol dependence and African-American ethnicity. Alcohol Clin Exp Res. 2000;24:1376–1384. [PubMed] [Google Scholar]
- 8.Ehlers C, Slawecki C. Effects of chronic ethanol exposure on sleep in rats. Alcohol. 2000;20:173–179. doi: 10.1016/s0741-8329(99)00077-4. [DOI] [PubMed] [Google Scholar]
- 9.Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- 10.Harper C, Dixon G, Sheedy D, Garrick T. Neuropathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:951–961. doi: 10.1016/S0278-5846(03)00155-6. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- 12.Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcoholism Clinical and Experimental Research. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- 13.Colrain IM. The K-complex: A seven-decade history. Sleep. 2005;28:255–273. doi: 10.1093/sleep/28.2.255. [DOI] [PubMed] [Google Scholar]
- 14.Loomis A, Harvey E, Hobart G. Distribution of disturbance-patterns in the human electroencephalogram, with special reference to sleep. Journal of Neurophysiology. 1938;1:413–430. [Google Scholar]
- 15.Amzica F, Steriade M. The K-complex: its slow (<1-Hz) rhythmicity and relation to delta waves. Neurology. 1997;49:952–959. doi: 10.1212/wnl.49.4.952. [DOI] [PubMed] [Google Scholar]
- 16.Bastien CH, Crowley KE, Colrain IM. Evoked potential components unique to non-REM sleep: relationship to evoked K-complexes and vertex sharp waves. Int J Psychophysiol. 2002;46:257–274. doi: 10.1016/s0167-8760(02)00117-4. [DOI] [PubMed] [Google Scholar]
- 17.Cote KA, de Lugt DR, Langley SD, Campbell KB. Scalp topography of the auditory evoked K-complex in stage 2 and slow wave sleep. J Sleep Res. 1999;8:263–272. doi: 10.1046/j.1365-2869.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- 18.Bastien C, Campbell K. The evoked K-complex: All-or-none phenomenon? Sleep. 1992;15:236–245. doi: 10.1093/sleep/15.3.236. [DOI] [PubMed] [Google Scholar]
- 19.Bastien C, Campbell K. Effects of rate of tone-pip stimulation on the evoked K-Complex. J Sleep Res. 1994;3:65–72. doi: 10.1111/j.1365-2869.1994.tb00109.x. [DOI] [PubMed] [Google Scholar]
- 20.Colrain IM, Webster KE, Hirst G. The N550 component of the evoked K-complex: a modality non-specific response? J Sleep Res. 1999;8:273–280. doi: 10.1046/j.1365-2869.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- 21.Niiyama Y, Fushimi M, Sekine A, Hishikawa Y. K-complex evoked in NREM sleep is accompanied by a slow negative potential related to cognitive process. Electroencephalogr Clin Neurophysiol. 1995;95:27–33. doi: 10.1016/0013-4694(95)00021-p. [DOI] [PubMed] [Google Scholar]
- 22.Happe S, Anderer P, Gruber G, Klosch G, Saletu B, Zeitlhofer J. Scalp topography of the spontaneous K-complex and of delta-waves in human sleep. Brain Topogr. 2002;15:43–49. doi: 10.1023/a:1019992523246. [DOI] [PubMed] [Google Scholar]
- 23.Finelli LA, Borbely AA, Achermann P. Functional topography of the human nonREM sleep electroencephalogram. Eur J Neurosci. 2001;13:2282–2290. doi: 10.1046/j.0953-816x.2001.01597.x. [DOI] [PubMed] [Google Scholar]
- 24.Dang-Vu TT, Desseilles M, Laureys S, Degueldre C, Perrin F, Phillips C, et al. Cerebral correlates of delta waves during non-REM sleep revisited. Neuroimage. 2005;28:14–21. doi: 10.1016/j.neuroimage.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 25.Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcoholism: Clinical and Experimental Research. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- 27.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 28.Bonnet MH, Arand DL. EEG arousal norms by age. J Clin Sleep Med. 2007;3:271–274. [PMC free article] [PubMed] [Google Scholar]
- 29.Ehlers C, Kupfer D. Effects of age on delta and REM sleep parameters. Electroencephalography and Clinical Neurophysiology. 1989;72:118–125. doi: 10.1016/0013-4694(89)90172-7. [DOI] [PubMed] [Google Scholar]
- 30.Feinberg I, Campbell IG. Kinetics of non-rapid eye movement delta production across sleep and waking in young and elderly normal subjects: theoretical implications. Sleep. 2003;26:192–200. doi: 10.1093/sleep/26.2.192. [DOI] [PubMed] [Google Scholar]
- 31.Crowley KE, Trinder J, Colrain IM. An examination of evoked K-complex amplitude and frequency of occurrence in the elderly. J Sleep Res. 2002;11:129–140. doi: 10.1046/j.1365-2869.2002.00293.x. [DOI] [PubMed] [Google Scholar]
- 32.Crowley KE, Trinder J, Colrain IM. Evoked K-Complex Generation: The Impact of Sleep Spindles and Age. Clinical Neurophysiology. 2004;115:471–476. doi: 10.1016/j.clinph.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Colrain IM, Crowley KE, Nicholas CL, Afifi L, Baker FC, Padilla M, et al. Sleep evoked delta frequency responses show a linear decline in amplitude across the adult lifespan. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.06.003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholas CL, Sullivan EV, Pfefferbaum A, Trinder J, Colrain IM. The effects of alcoholism on auditory evoked potentials during sleep. J Sleep Res. 2002;11:247–253. doi: 10.1046/j.1365-2869.2002.00298.x. [DOI] [PubMed] [Google Scholar]
- 35.Pfefferbaum A, Rosenbloom M, Crusan K, Jernigan TL. Brain CT changes in alcoholics: effects of age and alcohol consumption. Alcohol Clin Exp Res. 1988;12:81–87. doi: 10.1111/j.1530-0277.1988.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 36.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for Axis I DSM IV disorders. New York: New York State Psychiatric Institute; 1994. [Google Scholar]
- 37.Steriade M, Nunez A, Amzica F. A novel slow (1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. Journal of Neuroscience. 1993;13:3852–3865. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carden WB, Alexander GM, Friedman DP, Daunais JB, Grant KA, Mu J, et al. Chronic ethanol drinking reduces native T-type calcium current in the thalamus of nonhuman primates. Brain Res. 2006;1089:92–100. doi: 10.1016/j.brainres.2006.02.135. [DOI] [PubMed] [Google Scholar]
- 39.Adermark L, Lovinger DM. Ethanol effects on electrophysiological properties of astrocytes in striatal brain slices. Neuropharmacology. 2006;51:1099–1108. doi: 10.1016/j.neuropharm.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 40.Arendt T, Hennig D, Gray JA, Marchbanks RM. Loss of neurons in the rat basal forebrain cholinergic projection system after prolonged intake of ethanol. Brain Research Bulletin. 1988;21:563–570. doi: 10.1016/0361-9230(88)90193-1. [DOI] [PubMed] [Google Scholar]
- 41.Arendt T, Bruckner MK, Pagliusi S, Krell T. Degeneration of rat cholinergic basal forebrain neurons and reactive changes in nerve growth factor expression after chronic neurotoxic injury: I. Degeneration and plastic response of basal forebrain neurons. Neuroscience. 1995;65:633–645. doi: 10.1016/0306-4522(94)00526-b. [DOI] [PubMed] [Google Scholar]
- 42.Floyd EA, Young-Seigler AC, Ford BD, Reasor JD, Moore EL, Townsel JG, et al. Chronic ethanol ingestion produces cholinergic hypofunction in rat brain. Alcohol. 1997;14:93–98. doi: 10.1016/s0741-8329(97)86147-2. [DOI] [PubMed] [Google Scholar]
- 43.Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 44.Lewohl JM, Crane DI, Dodd PR. Expression of the alpha 1, alpha 2 and alpha 3 isoforms of the GABAA receptor in human alcoholic brain. Brain Res. 1997;751:102–112. doi: 10.1016/s0006-8993(96)01396-0. [DOI] [PubMed] [Google Scholar]
- 45.Dodd PR, Thomas GJ, Harper CG, Kril JJ. Amino acid neurotransmitter receptor changes in cerebral cortex in alcoholism: effect of cirrhosis of the liver. J Neurochem. 1992;59:1506–1515. doi: 10.1111/j.1471-4159.1992.tb08467.x. [DOI] [PubMed] [Google Scholar]
- 46.Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Wolf AP, Pappas N, et al. Decreased cerebral response to inhibitory neurotransmission in alcoholics. Am J Psychiatry. 1993;150:417–422. doi: 10.1176/ajp.150.3.417. [DOI] [PubMed] [Google Scholar]
- 47.Schlosser RG, Gesierich T, Wagner G, Bolz M, Grunder G, Dielentheis TF, et al. Altered benzodiazepine receptor sensitivity in alcoholism: a study with fMRI and acute lorazepam challenge. Psychiatry Res. 2007;154:241–251. doi: 10.1016/j.pscychresns.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Harper C, Kril JJ. Patterns of neuronal loss in the cerebral cortex in chronic alcoholic patients. Journal of Neurological Science. 1989;92:81–89. doi: 10.1016/0022-510x(89)90177-9. [DOI] [PubMed] [Google Scholar]
- 49.Harper CG, Kril JJ, Daly JM. Are we drinking our neurones away? British Medical Journal. 1987;294:534–536. doi: 10.1136/bmj.294.6571.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samson Y, Baron JC, Feline A, Bories J, Crouzel C. Local cerebral glucose utilisation in chronic alcoholics: a positron tomographic study. J Neurol Neurosurg Psychiatry. 1986;49:1165–1170. doi: 10.1136/jnnp.49.10.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hata T, Meyer JS, Tanahashi N, Ishikawa Y, Imai A, Shinohara T, et al. Three-dimensional mapping of local cerebral perfusion in alcoholic encephalopathy with and without Wernicke-Korsakoff syndrome. J Cereb Blood Flow Metab. 1987;7:35–44. doi: 10.1038/jcbfm.1987.6. [DOI] [PubMed] [Google Scholar]
- 52.Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: Evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30:423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- 53.Kroft C, Gescuk B, Woods B, Mello N, Weiss R, Mendelson J. Brain ventricular size in female alcoholics: An MRI study. Alcohol. 1991;8:31–34. doi: 10.1016/0741-8329(91)91200-l. [DOI] [PubMed] [Google Scholar]
- 54.Pfefferbaum A, Rosenbloom M, Deshmukh A, Sullivan E. Sex differences in the effects of alcohol on brain structure. Am J Psychiatry. 2001;158:188–197. doi: 10.1176/appi.ajp.158.2.188. [DOI] [PubMed] [Google Scholar]
- 55.Hommer DW, Momenan R, Kaiser E, Rawlings RR. Evidence for a gender-related effect of alcoholism on brain volumes. American Journal of Psychiatry. 2001;158:198–204. doi: 10.1176/appi.ajp.158.2.198. [DOI] [PubMed] [Google Scholar]
- 56.Wang G, Volkow N, Fowler J, Pappas N, Wong C, Pascani K, et al. Regional cerebral metabolism in female alcoholics of moderate severity does not differ from that of controls. Alcoholism: Clinical and Experimental Research. 1998;22:1850–1854. [PubMed] [Google Scholar]
- 57.O'Neill J, Cardenas VA, Meyerhoff DJ. Effects of abstinence on the brain: quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcohol Clin Exp Res. 2001;25:1673–1682. [PubMed] [Google Scholar]
- 58.Shear PK, Jernigan TL, Butters N. Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics. Alcohol Clin Exp Res. 1994;18:172–176. doi: 10.1111/j.1530-0277.1994.tb00899.x. [DOI] [PubMed] [Google Scholar]
- 59.Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- 60.Brower KJ, Hall JM. Effects of age and alcoholism on sleep: a controlled study. J Stud Alcohol. 2001;62:335–343. doi: 10.15288/jsa.2001.62.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 62.Mattis S. Dementia Rating Scale (DRS) Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc; 1988. [Google Scholar]
- 63.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatry Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]