Abstract
Hyperalgesic priming is a model of the transition from acute to chronic pain, in which previous activation of cell surface receptors or direct activation of protein kinase C epsilon (PKCε) markedly prolongs mechanical hyperalgesia induced by pronociceptive cytokines. We recently demonstrated a role of peripheral protein translation, alpha-calmodulin-dependent protein kinase II (αCaMKII) activation and the ryanodine receptor, in the induction of hyperalgesic priming. In the present study we tested if they also mediate the prolonged phase of PGE2-induced hyperalgesia. We found that inhibition of αCaMKII and local protein translation eliminates the prolonged phase of PGE2 hyperalgesia. While priming induced by receptor agonists or direct activation of PKCε occurs in male but not female rats, activation of αCaMKII and the ryanodine receptor also produces priming in females. As in males, the prolonged phase of PGE2–induced hyperalgesia in female rats is also PKCε-, αCaMKII- and protein translation-dependent. In addition, in both male and female primed rats the prolonged PGE2-induced hyperalgesia was significantly attenuated by inhibition of MEK/ERK. Based on these data we suggest that the mechanisms previously shown to be involved in the induction of the neuroplastic state of hyperalgesic priming also mediate the prolongation of hyperalgesia.
Keywords: Second messengers, hyperalgesic priming, sensory neuron, mechanical hyperalgesia, rat
Introduction
Hyperalgesic priming, a model of the transition from acute to chronic pain produced by a prior inflammatory insult, is expressed as a long-lasting neuroplastic state in which there is enhanced hyperalgesia induced by agents such as prostaglangin E2 (PGE2), adenosine and serotonin 3,38,41. The induction of hyperalgesic priming is triggered by a transient activation of protein kinase C epsilon (PKCε) 3,39 or molecules downstream of PKCε, including alpha calmodulin-dependent protein kinase II (αCaMKII) and the ryanodine receptor 20, inducing translation of mRNAs in the peripheral terminal of the nociceptor 19,20 that are responsible for the chronic maintenance of this state 6,19.
In the naïve rat, PGE2-induced hyperalgesia is of short duration (~1 h) and dependent on activation of stimulatory G-protein (Gs) and protein kinase A (PKA)1. However, in the primed state, the hyperalgesia induced by PGE2 is markedly prolonged, lasting more than 24 h3, due to a novel linkage of prostaglandin receptor activation to an additional signaling pathway involving an inhibitory G-protein (Gi), phospholipase C beta 3 (PLCβ3) and PKCε during the prolonged phase of the PGE2-induced hyperalgesia 3,15,22,28,32,37,41.
In this study we show that the prolonged phase of PGE2-induced hyperalgesia also involves αCaMKII, local protein translation, and the MEK/ERK pathway, mechanisms not involved in signaling for PGE2-induced hyperalgesia in the naïve state2. We also show that these mechanisms contribute to the prolonged phase of PGE2-induced hyperalgesia in female rats in which hyperalgesic priming can be induced by αCaMKII or ryanodine receptor activation.
Materials and Methods
Animals
All experiments were performed on adult male and female Sprague Dawley rats (220–400 g; Charles River Laboratories). Animals were housed, 3 per cage, under a 12-h light/dark cycle in a temperature- and humidity-controlled room in the animal care facility of the University of California, San Francisco. Food and water were available ad libitum. All nociceptive testing was done between 10:00 am and 5:00 pm and the experimental protocols were approved by the Institutional Animal Care and Use Committee at University of California at San Francisco and adhered to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All effort was made to minimize the number of animals used and their suffering.
Mechanical nociceptive threshold testing
Mechanical nociceptive threshold was quantified using an Ugo Basile Analgesymeter® (Randall-Selitto paw-withdrawal test, Stoelting, Chicago, IL), which applies a linearly increasing mechanical force to the dorsum of the rat’s hind paw, as previously described 41,48,50. The nociceptive threshold was defined as the force in grams at which the rat withdrew its paw, and baseline paw-pressure threshold defined as the mean of the three readings taken before the test agents were injected. Each paw was treated as an independent measure and each experiment performed on a separate group of rats. Data are presented as mean change from baseline mechanical nociceptive threshold.
Drugs
The following drugs were used in this study: prostaglandin E2 (PGE2), the ryanodine receptor activator ryanodine, and the specific inhibitor of MEK 1/2 U0126 ([1,4-Diamino-2, 3-dicyano-1, 4-bis (2-aminophenylthio)butadiene] 13, all from Sigma-Aldrich (St. Louis, MO); alpha calcium/calmodulin-dependent protein kinase II recombinant (activated αCaMKII, New England Biolabs, Ipswich, MA), the CaMKII inhibitor peptide CaM2INtide (GenScript, Piscataway, NJ), PKCεV1–2, a PKCε specific translocation inhibitor peptide (PKCε-I) 27,33 (Calbiochem, La Jolla, CA), the PKCε activator ψεRACK (Biomatik, Delaware, USA), and the protein translation inhibitors cordycepin 5′-triphosphate sodium salt [Sigma-Aldrich (St. Louis, MO)] and rapamycin [EMD Chemicals (Gibbstown, NJ)]. The selection of the drug doses used in this study was based on our previous studies 3,19,33,36,37,49.
Stock solutions of PGE2 in absolute ethanol (1 μg/μl) were further diluted in 0.9% NaCl (1:50, Cfinal=0.2 μg/μl) immediately before injection. The ethanol concentration of the final PGE2 solution was ~2% and the injection volume 5 μl. Stock solutions of cordycepin (10 μg/μl, dissolved in a 1:1 mixture of 0.9% NaCl and absolute ethanol) or rapamycin (20 μg/μl, dissolved in absolute DMSO) were further diluted in 0.9% NaCl or distilled water, respectively, immediately before injection. The ethanol or DMSO concentration in the final solutions was ~2%.
Activation of αCaMKII was performed in vitro and a dose of 25 ng, in a volume of 2.5 μl, of the activated αCaMKII was injected intradermally on the dorsum of the rat hind paw. αCaMKII was diluted in 1X NEBuffer for PK (50 mM Tris-HCl, 10 mM MgCl2, 0.1 mM EDTA, 2mM DTT, 0.01% Brij 35, pH 7.5 at 25°C) supplemented with 200 μM ATP, 1.2 μM calmodulin and 2 mM CaCl2, and incubated for 10 min at 30°C before injection.
Drugs were administered intradermally on the dorsum of the hind paw via a beveled 30-gauge hypodermic needle attached to a Hamilton® microsyringe (Reno, NV, USA) by a short length of polyethylene (PE-10) tubing. The administration of all drugs, except PGE2, was preceded by a hypotonic shock to facilitate cell permeability to these agents (2 μl of distilled water, separated by a bubble of air to avoid mixing in the same syringe), to get compounds into the nerve terminal 7,9.
Oligodeoxynucleotide antisense to αCaMKII
The oligodeoxynucleotide (ODN) antisense sequence for the α-subunit of CaMKII, 5′-GGT AGC CAT CCT GGC ACT-3′ (Invitrogen), was directed against a unique region of the rat mRNA sequence. The corresponding NCBI GenBank accession number and ODN position within the mRNA-sequence are NM_012920 and 33 to 50, respectively. That this antisense can be used to downregulate the expression of αCaMKII has been shown previously 11. The ODN mismatch 10 sequence 5′-GGT AGC CAT AAG GGC ACT-3′ corresponds to the antisense sequence with 3 bases mismatched (denoted in bold).
Before use, the ODNs were lyophilized and reconstituted in 0.9% NaCl to a concentration of 2 μg/μl. During each injection, rats were briefly anesthetized with 2.5% isoflurane in 95% O2. A 30-gauge hypodermic needle was inserted into the subarachnoid space on the midline, between the L4 and L5 vertebrae. A total of 40 μg ODN in a volume of 20 μl was slowly injected. Proper intrathecal injections were systematically confirmed by checking for a sudden flicking of the tail, a reflex that is evoked by subarachnoid space access and bolus injection 35. The animals regained consciousness approximately 1 minute after the injection. The use of antisense to manipulate the expression of proteins in nociceptors, important for their role in nociceptor sensitization, is well supported by previous studies by others 40,45,46,47 as well as our group 6,18,21,39.
Prolonged phase of PGE2-induced mechanical hyperalgesia
To evaluate signaling mechanisms involved in the prolongation phase of the PGE2-induced mechanical hyperalgesia observed in our model of chronic pain, we injected one of 3 agents that induce priming, ψεRACK, activated αCaMKII or ryanodine, intradermally on the dorsum of the rat’s hind paw 3,20. These agents induce mechanical hyperalgesia that resolves in 3–5 days (ψεRACK), ~10 days (activated αCaMKII) or less than 24 h (ryanodine), and after the return of the mechanical thresholds to baseline values, the intradermal injection of PGE2 at the same site produces enhanced and prolonged mechanical hyperalgesia that lasts more than 4 h and is still significant at 24 h, as opposed to the effect of intradermal injection of PGE2 in control animals, that lasts ~1 h 3,37,39. In this study we performed the injection of PGE2 one (ψεRACK- and ryanodine-treated rats) or two (αCaMKII-treated rats) weeks after the priming stimulus. Of note, while activation of PKCε was shown to produce hyperalgesic priming only in male rats 29, the activation of αCaMKII or ryanodine receptors is able to induce priming in female as well as male rats 20.
Statistics
In all experiments, the dependent variable was paw-withdrawal threshold, expressed as the percentage change from baseline. The average paw withdrawal thresholds before and after (1 or 2 weeks, depending on the stimulus) the injection of the priming stimuli was 116.0 ± 1.4 g and 115.8 ± 1.2 g, respectively (n = 210 paws). Of note, no statistically significant difference between paw withdrawal threshold values was observed between groups of rats subsequently injected with ψεRACK (t11=0.08305, p = 0.9353), activated αCaMKII (t89=0.3258, p = 0.7453) or ryanodine (t107=0.8813, p = 0.3801), and in these groups immediately before the injection of PGE2 (paired Student’s t-test). In addition, the αCaMKII antisense treatment did not induce changes in the mechanical nociceptive threshold by itself (p = NS). To compare the hyperalgesia induced by PGE2 injection in different groups, two-way repeated-measures ANOVA, followed by Bonferroni post-test, was performed. Graph Pad Prism 5.0 (GraphPad Software, Inc., San Diego, CA) was used to plot the graphics and to perform the statistical analysis; P < 0.05 was considered statistically significant. Data are presented as mean ± SEM.
Results
Role of αCaMKII in the prolonged phase of PGE2-induced hyperalgesia in the primed animal
We investigated if αCaMKII participates in the signaling pathway involved in the prolonged phase of PGE2-induced hyperalgesia in states of hyperalgesic priming induced by ψεRACK, activated αCaMKII or ryanodine. Male rats previously primed by activation of PKCε in the hind paw, using ψεRACK (Fig. 1A), were treated with intrathecal injection of antisense or mismatch for αCaMKII mRNA for 3 consecutive days and, on the 4th day, the αCaMKII inhibitor CaM2INtide or its vehicle was injected on the dorsum of the hind paw at the site of nociceptive testing. This protocol, combining intrathecal antisense treatment and pharmacological inhibition of αCaMKII in the paw is based on our initial study of the role of αCaMKII in hyperalgesic priming 20, in which we attempted to achieve as complete inhibition of αCaMKII as possible. At the same site (on the dorsum of the hind paw), 15 min later, PGE2 was injected; mechanical hyperalgesia was evaluated 30 min and 4 h after PGE2. We observed that at 4 h but not 30 min the hyperalgesia induced by PGE2 was significantly attenuated in the rats treated with the αCaMKII antisense plus the inhibitor. In control rats treated with mismatch plus vehicle there was no significant attenuation (***p < 0.0001, when both groups are compared at the 4 h time point). Similarly, when the same protocol was applied to rats that had previously received intradermal injection of activated αCaMKII (two weeks before, Fig. 1B) or ryanodine (one week before, Fig. 1C) on the dorsum of the hind paw, the magnitude of the PGE2-induced mechanical hyperalgesia was significantly decreased at 4 h but not 30 min in the αCaMKII antisense plus inhibitor-treated groups, when compared to the control groups (***p < 0.0001, in both cases, when the αCaMKII antisense plus the inhibitor groups are compared to the mismatch plus vehicle groups at the 4 h time point), showing that αCaMKII plays a role in the prolonged phase of PGE2-induced hyperalgesia in the primed condition produced by previous activation, in the rat hind paw, of PKCε, αCaMKII or the ryanodine receptor.
Figure 1. The prolongation of PGE2-induced hyperalgesia in primed male rats is dependent on αCaMKII.

Male rats that received intradermal injection of the PKCε activator ψεRACK (1 μg, panel A), activated αCaMKII (25 ng, panel B) or the ryanodine receptor agonist (1 μg, panel C) on the dorsum of the hind paw one (panels A and C) or two (panel B) weeks before were treated with intrathecal injection of ODN mismatch (clear bars) or antisense (black bars) for αCaMKII for 3 consecutive days. On the 4th day, vehicle or the αCaMKII inhibitor CaM2INtide (1 μg) was administered, 15 min prior to the injection of PGE2 (100 ng), at the same site. Mechanical nociceptive thresholds were evaluated 30 min and 4 h after PGE2, by the Randall-Selitto paw withdrawal test. We observed, in all cases, significant attenuation of the hyperalgesia induced by PGE2 at the 4th h in the groups pretreated with the antisense/CaM2INtide (panel A, F1,10 = 52.07, ***p < 0.0001; panel B, F1,10 = 68.83, ***p < 0.0001; panel C, F1,10 = 46.14, ***p < 0.0001), when compared to the mismatch/vehicle groups (two-way repeated measures ANOVA followed by Bonferroni post-test, n = 6 paws per group), indicating a role of αCaMKII in the prolongation of PGE2 hyperalgesia in the primed condition.
Prolonged phase of PGE2-induced hyperalgesia in male rats primed with ryanodine depends on local protein translation
Local administration of protein translation inhibitors such as cordycepin or rapamycin at the site of nociceptive testing, on the dorsum of the rat hind paw, permanently reverses hyperalgesic priming previously produced by injection of ψεRACK 19 or αCaMKII 20 at the same site, suggesting that the maintenance of the primed state is dependent on ongoing protein translation in the peripheral terminal of the nociceptor. We tested if hyperalgesic priming induced by injection of ryanodine on the dorsum of the rat hind paw also depends on ongoing protein translation in the periphery. We observed (Fig. 2) that local injection of cordycepin or rapamycin reversed ryanodine-induced hyperalgesic priming, i.e., hyperalgesia induced by a PGE2 challenge delivered 15 min after local administration of either cordycepin or rapamycin in ryanodine-primed rats is not prolonged (***p < 0.001 for both cases, when compared to the vehicle group).
Figure 2. Prolonged phase of PGE2-induced hyperalgesia in male rats primed with ryanodine is dependent on local protein translation.
Male rats received an intradermal injection of ryanodine (1 μg). One week later cordycepin (1 μg), rapamycin (1 μg), or vehicle was injected on the dorsum of the hind paw and, 15 min later, PGE2 (100 ng) was injected in the same site. Mechanical nociceptive thresholds were evaluated 30 min and 4 h after PGE2, by the Randall-Selitto paw withdrawal test. In all cases, the pretreatment with the protein translation inhibitor significantly attenuated the hyperalgesia induced by PGE2 at 4th h (***p < 0.001), when compared to the vehicle group (F2,15 = 85.11, ***p < 0.0001, two-way repeated measures ANOVA followed by Bonferroni post-test, n = 6 paws per group).
Activated αCaMKII- and ryanodine-induced priming: role of PKCε
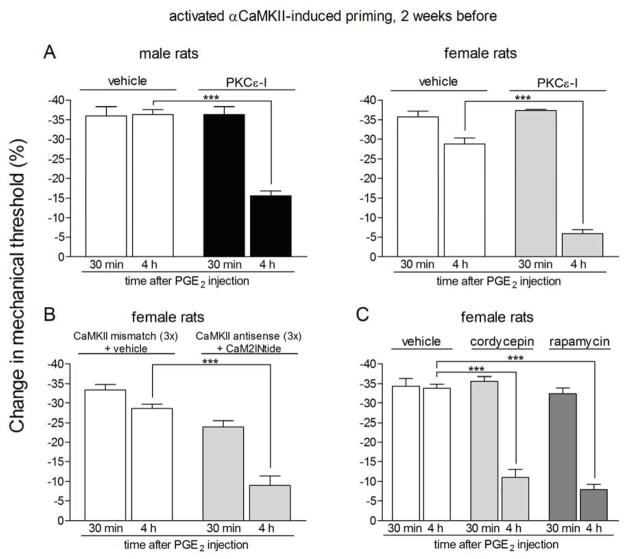
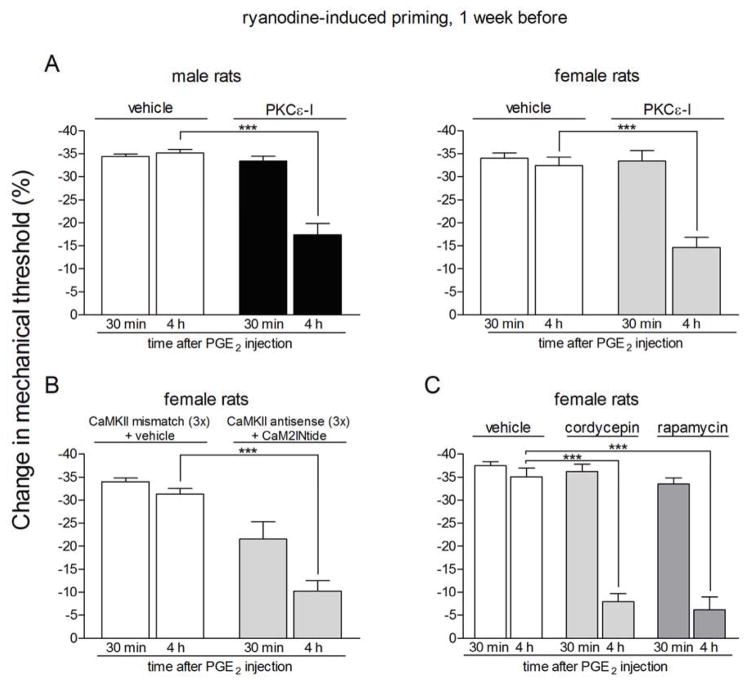
We tested if the prolonged phase of hyperalgesia induced by a PGE2 challenge in the primed state involves a similar PKCε-dependent signaling pathway to that which has been demonstrated in hyperalgesic priming induced by PKCε activation 3,14,16,38,39. Male and female rats previously treated with activated αCaMKII (Fig. 3A) or ryanodine (Fig. 4A) on the dorsum of the hind paw, received an intradermal injection of PGE2 5 min after the administration of vehicle or PKCε-I, at the same site. PKCε-I, but not vehicle, significantly attenuated the prolongation of PGE2 hyperalgesia, evaluated 4 h after PGE2 injection (Fig. 3A, male rats: p = 0.0004; female rats: p < 0.0001; Fig. 4A, male rats: p = 0.0001; female rats: p < 0.0015, when compared to the control groups).
Figure 3. Prolonged phase of PGE2-induced hyperalgesia in rats with αCaMKII-induced hyperalgesic priming is dependent on PKCε, αCaMKII and local protein translation.
Rats primed with intradermal injection of activated αCaMKII (25 ng) on the dorsum of the hind paw 2 weeks before received injection of PGE2 in the presence or absence of inhibitors of the seconds messengers PKCε (PKCε-I, panel A) or αCaMKII (ODN antisense or mismatch for αCaMKII and the αCaMKII inhibitor CaM2INtide, panel B), and the protein translation inhibitors cordycepin or rapamycin (panel C). Mechanical nociceptive thresholds were evaluated 30 min and 4 h after PGE2, by the Randall-Selitto paw withdrawal test. PGE2 was injected 5 min (panel A) or 15 min (panels B and C) after the inhibitors. We observed, in all cases, significant attenuation of the hyperalgesia induced by PGE2 at 4th h (***p < 0.001) in the groups treated with the inhibitors when compared to the ODN mismatch/vehicle groups (panel A, male rats: F1,10 = 27.36, p = 0.0004; female rats: F1,10 = 437.96, p < 0.0001; panel B, F1,10 = 57.90, p < 0.0001; panel C, F2,15 = 26.65, p < 0.0001, two-way repeated measures ANOVA followed by Bonferroni post-test, n = 6 paws per group), indicating a role of PKCε, αCaMKII and local protein translation in the prolongation of PGE2 hyperalgesia in the αCaMKII-induced hyperalgesic priming.
Figure 4. Prolonged phase of PGE2-induced hyperalgesia in rats previously treated with ryanodine is dependent on PKCε, αCaMKII and local protein translation.
Rats primed with intradermal injection of ryanodine (1 μg) on the dorsum of the hind paw one week prior received intradermal injection of PGE2 on the dorsum of the hind paw in the presence or absence of inhibitors of the seconds messengers PKCε (PKCε-I, panel A) or αCaMKII (ODN antisense or mismatch for αCaMKII and the αCaMKII inhibitor CaM2INtide, panel B), and the protein translation inhibitors cordycepin or rapamycin (panel C). Mechanical nociceptive thresholds were evaluated 30 min and 4 h after PGE2, by the Randall-Selitto paw withdrawal test. PGE2 was injected 5 min (panel A) or 15 min (panels B and C) after the inhibitors. We observed, in all cases, significant attenuation of the hyperalgesia induced by PGE2 at the 4th h (***p < 0.001) in the groups treated with the inhibitors when compared to the vehicle groups (panel A, male rats: F1,10 = 36.03, p = 0.0001; female rats: F1,10 = 18.76, p < 0.0015; panel B, F1,10 = 56.01, p < 0.0001; panel C, F2,15 = 39.30, p < 0.0001, two-way repeated measures ANOVA followed by Bonferroni post-test, n = 6 paws per group), indicating a role of PKCε, αCaMKII and local protein translation in the prolongation of PGE2 hyperalgesia in the ryanodine-induced hyperalgesic priming.
αCaMKII and local protein translation are also involved in the PGE2 signaling in female rats primed with activated αCaMKII or ryanodine
When female rats previously primed with activated αCaMKII (Fig. 3B) or ryanodine (Fig. 4B) on the dorsum of the hind paw and treated with intrathecal injections of antisense against αCaMKII for 3 days received intradermal injection of the αCaMKII inhibitor CaMINtide and, 10 min later, PGE2, both at the same site in the hind paw, a significant attenuation of the prolonged phase of hyperalgesia measured at the 4 h time point was also observed (Fig. 3B, p < 0.0001; Fig. 4B, p < 0.0001), compared to the primed rats treated with mismatch and CaMINtide vehicle plus PGE2. In addition, the chronic increased sensitivity to PGE2, induced by previous injection of activated αCaMKII or ryanodine, on the dorsum of the hind paw of female rats, was also attenuated by the injection of the protein translation inhibitors cordycepin or rapamycin at the same site (Fig. 3C, p < 0.0001; Fig. 4C, p < 0.0001, when compared to the control groups). These results indicate that, similarly to the hyperalgesic priming induced by activation of PKCε on the dorsum of the hind paw of male rats, the prolongation of PGE2 hyperalgesia induced by previous activation of αCaMKII or ryanodine receptors in male and female rats, is also dependent on a switch from PKA to the addition of PKCε and αCaMKII signaling, and local protein synthesis.
Prolongation of PGE2-induced hyperalgesia in primed rats is also dependent on the MEK/ERK pathway
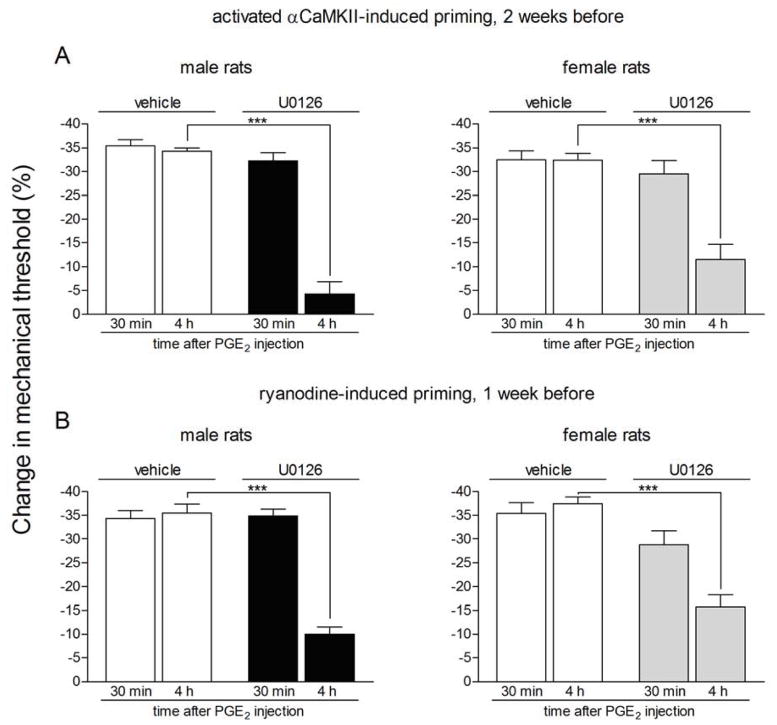
It has been demonstrated that the MEK/ERK pathway plays a role in the prolongation of the PGE2-induced hyperalgesia, in rats previously primed with ψεRACK 17. Thus, since we observed similarities in the second messengers involved in the enhanced and prolonged PGE2-induced hyperalgesia in the ψεRACK-induced priming and the priming induced by activated αCaMKII or ryanodine, we investigated if MEK/ERK also has a role in the expression of the plasticity produced in the two latter models. Pretreatment with the specific inhibitor of MEK 1/2 U0126 on the dorsum of the hind paw, but not with vehicle, 10 min before the injection of PGE2 in the same site that previously received intradermal injection of activated αCaMKII (Fig. 5A) or ryanodine (Fig. 5B) significantly attenuated the PGE2-induced hyperalgesia at the 4th h after injection in male and female rats (Fig. 5A, male rats: p < 0.0001; female rats: p = 0.0011; Fig. 5B, male rats: p < 0.0001; female rats: p = 0.0005, when compared to the vehicle groups). This result indicates that the previous treatment with activated αCaMKII or ryanodine in the hind paw induced a switch in the PGE2 signaling pathway, the prolongation of PGE2 hyperalgesia now dependent on the MEK/ERK pathway.
Figure 5. Prolonged phase of PGE2-induced hyperalgesia is dependent on the MEK/ERK pathway in rats previously treated with activated αCaMKII or ryanodine.
Different groups of rats that were treated with intradermal injection of activated αCaMKII (25 ng, panel A) or the ryanodine receptor agonist (1 μg, panel B) on the dorsum of the hind paw two or one week prior, respectively, received PGE2 (100 ng), at the same site, 5 min after the injection of vehicle or U0126 (1 μg). Mechanical nociceptive thresholds were evaluated 30 min and 4 h after PGE2, by the Randall-Selitto paw withdrawal test. We observed, in all cases, significant attenuation of the hyperalgesia induced by PGE2 at the 4th h (***p < 0.001) in the groups pretreated with the U0126 (panel A, male rats: F1,10 = 85.58, p < 0.0001; female rats: F1,10 = 20.37, p = 0.0011; panel B, male rats: F1,10 = 40.34, p < 0.0001; female rats: F1,10 = 25.35, p = 0.0005), when compared to the vehicle groups (two-way repeated measures ANOVA followed by Bonferroni post-test, n = 6 paws per group), indicating a role of the MEK/ERK pathway in the prolongation of PGE2 hyperalgesia in the primed condition.
Discussion
Our group developed a model in rat of the transition from acute to chronic pain, referred to as hyperalgesic priming, in which a prior inflammatory stimulus induces very long-term neuroplasticity in primary afferent nociceptor function 3,37,39,41 that changes the signaling pathway activated by direct-acting inflammatory mediators, such as PGE2, adenosine and serotonin 3, so that the hyperalgesia they produce is markedly prolonged. One of the main signaling molecules responsible for the prolongation of PGE2 hyperalgesia is PKCε 3,39,41.
That the primed state persists for months suggested that it is maintained by a more long-term change in the nociceptor other than simple activation of PKCε, and subsequent studies showed a role of local protein translation in the priming of the peripheral nociceptor 19. Furthermore, this protein translation depends on activation of 3 molecules previously shown to be involved in learning and memory (another form of long-lasting neuroplasticity 20): the cytoplasmic polyadenylation element binding protein (CPEB; a molecule that regulates translation of dormant mRNAs) 42,51, αCaMKII 8,10,12,25,26,54, and ryanodine receptors (which can activate αCaMKII, 43,44,53). In this study, we tested if 3 molecules that participate in the induction of the primed state, PKCε, αCaMKII and the ryanodine receptor 20, also play a role in the prolonged phase of hyperalgesia that is induced by a PGE2 challenge in the primed state.
We previously showed that the prolonged phase of hyperalgesia induced by PGE2 in the primed state in male rats is mediated by a novel addition of a delayed Gi - PLCβ3 and PKCε signaling pathway 15,28,37. In this study we found that PKCε also participates in the PGE2- induced hyperalgesia in male rats primed with ryanodine, confirming that the changes in the nociceptor produced by both stimuli (activated αCaMKII or ryanodine) fit in the definition of hyperalgesic priming.
Another interesting feature regarding the hyperalgesic priming model is that while the activation of PKCε does not induce priming in female rats 29, activation of PKCε does induce priming in ovariectomized female rats similarly to that observed in the male rat, an effect reversed by estrogen replacement 29. These findings support the suggestion that the action of estrogen at the level of PKCε was the limiting step in the cascade of events, in female rats, that lead to protein translation in the terminal of the nociceptor and, ultimately, to the development of hyperalgesic priming. In fact, we have further demonstrated that activation of αCaMKII or the ryanodine receptor induces priming in female as well as male rats 20. Our current experiments showed that the prolongation of PGE2-induced hyperalgesia in female rats primed with αCaMKII or ryanodine is also PKCε-dependent in the late phase (evaluated at the 4th h). Thus, even though PKCε is not involved in the production of priming in females, it plays a role in its expression. Moreover, considering that in male rats PKCε is involved in both the induction of priming and its expression, we tested if αCaMKII activation, which induces priming in both male and female rats, also participates in the prolongation of PGE2 hyperalgesia. Again, the inhibition of αCaMKII by the combination of antisense and the αCaMKII inhibitor CaMINtide significantly attenuated the prolonged PGE2-induced hyperalgesia in rats primed with ψεRACK (male), activated αCaMKII or ryanodine (male and female). Of note, once the effect of the PKCε-I and the αCaMKII ODN-AS/CaMINtide were washed off, the injection of PGE2 again produced prolonged hyperalgesia.
In order to demonstrate that the neuroplastic changes induced by activation of αCaMKII and the ryanodine receptor fulfill all the criteria that characterizes hyperalgesic priming, i.e., PKCε-dependent enhancement and prolongation of the hyperalgesia induced by PGE23,39,41 and protein translation at the terminal of the nociceptor 19, we tested the effect of the protein translation inhibitors cordycepin and rapamycin, previously described to permanently reverse the hyperalgesic priming induced by ψεRACK 19, on the expression of priming produced by activated αCaMKII (female rats) or ryanodine (male and female). We observed, in all cases, significant attenuation of PGE2-induced hyperalgesia at the 4th h. Based on these results, we suggest that the neuroplastic changes in the nociceptor induced by the different stimuli are similar, and probably resultant from the same process. Furthermore, treatment with the protein translation inhibitors permanently reversed the increased sensitivity to PGE2 (data not shown), in line with our previous results 19, suggesting that local synthesis of proteins in the terminal of the nociceptors contributes to the “memory” observed in the primed condition. However, the proteins involved in the maintenance of the hyperalgesic priming remain to be determined.
Finally, it has been described that MAPK can regulate the translation of mRNAs 5,24,30,31,52, including by activation of factors that are affected by cordycepin or rapamycin, such as the mammalian target for rapamycin (mTOR) 4,23,34. Since we have previously shown that these inhibitors of protein translation reversed the ψεRACK-induced priming 19, we considered the possibility that the MEK/ERK pathway could also play a role in the prolongation of PGE2 hyperalgesia. In fact, we have previously shown that inhibition of this pathway attenuates the prolongation of the PGE2-induced hyperalgesia in rats primed with the PKCε activator ψεRACK 17. Therefore, we evaluated if the specific inhibitor of MEK ½, U0126, would also attenuate the increased response to PGE2 in αCaMKII- and ryanodine-primed rats. As expected, the local treatment with U0126 significantly attenuated the prolongation of the PGE2 hyperalgesia, showing another similarity with the priming induced by ψεRACK. Thus, another signaling molecule that does not play a role in the PGE2 effect in naïve rats 2 is now “recruited” by PGE2 in the expression of the primed state.
In conclusion, the present study confirms that a transient activation of PKCε, αCaMKII or the ryanodine receptor triggers a permanent switch in the signaling pathway activated by PGE2, in which second messengers that are not involved in its pronociceptive effect in the normal state, such as PKCε and the MEK/ERK pathway, now contribute to the prolongation of PGE2 hyperalgesia. Moreover, the maintenance of this neuroplasticity produced by activation of αCaMKII or ryanodine receptors is dependent on local protein translation at the terminal of the sensory neuron. Such changes in the phenotype of the nociceptor, expressed as a hyperresponsiveness to inflammatory mediators, may be the underlying mechanism of clinical conditions in which, after recovery from an initiating event (such as work-related ergonomic insults, inflammation or psychological stress), the susceptibility of developing a recurrent painful condition is increased.
Perspectives.
The data provided by this study suggests that direct intervention on specific targets may help to alleviate the expression of chronic hyperalgesic conditions.
Abbreviations
- ODN
oligodeoxynucleotide
- αCaMKII
alpha calmodulin-dependent protein kinase II
- PGE2
prostaglandin E2
- PKCε
protein kinase C epsilon
- SEM
standard error of the mean
Footnotes
Disclosures
This study was funded by the National Institutes of Health (NIH). The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci. 1999;19:2181–6. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aley KO, Martin A, McMahon T, Mok J, Levine JD, Messing RO. Nociceptor sensitization by extracellular signal-regulated kinases. J Neurosci. 2001;21:6933–9. doi: 10.1523/JNEUROSCI.21-17-06933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20:4680–5. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allard EK, Grujic M, Fisone G, Kristensson K. Prion formation correlates with activation of translation-regulating protein 4E-BP and neuronal transcription factor Elk1. Neurobiol Dis. 2013;58:116–22. doi: 10.1016/j.nbd.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Banko JL, Hou L, Klann E. NMDA receptor activation results in PKA- and ERK-dependent Mnk1 activation and increased eIF4E phosphorylation in hippocampal area CA1. J Neurochem. 2004;91:462–70. doi: 10.1111/j.1471-4159.2004.02734.x. [DOI] [PubMed] [Google Scholar]
- 6.Bogen O, Alessandri-Haber N, Chu C, Gear RW, Levine JD. Generation of a pain memory in the primary afferent nociceptor triggered by PKCepsilon activation of CPEB. J Neurosci. 2012;32:2018–26. doi: 10.1523/JNEUROSCI.5138-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borle AB, Snowdowne KW. Measurement of intracellular free calcium in monkey kidney cells with aequorin. Science. 1982;217:252–4. doi: 10.1126/science.6806904. [DOI] [PubMed] [Google Scholar]
- 8.Buard I, Coultrap SJ, Freund RK, Lee YS, Dell’Acqua ML, Silva AJ, Bayer KU. CaMKII “autonomy” is required for initiating but not for maintaining neuronal long-term information storage. J Neurosci. 2010;30:8214–20. doi: 10.1523/JNEUROSCI.1469-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burch RM, Axelrod J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A. 1987;84:6374–8. doi: 10.1073/pnas.84.18.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cammarota M, Bevilaqua LR, Viola H, Kerr DS, Reichmann B, Teixeira V, Bulla M, Izquierdo I, Medina JH. Participation of CaMKII in neuronal plasticity and memory formation. Cell Mol Neurobiol. 2002;22:259–67. doi: 10.1023/A:1020763716886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Churn SB, Sombati S, Jakoi ER, Severt L, DeLorenzo RJ. Inhibition of calcium/calmodulin kinase II alpha subunit expression results in epileptiform activity in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2000;97:5604–9. doi: 10.1073/pnas.080071697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coultrap SJ, Bayer KU. CaMKII regulation in information processing and storage. Trends Neurosci. 2012;35:607–18. doi: 10.1016/j.tins.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeSilva DR, Jones EA, Favata MF, Jaffee BD, Magolda RL, Trzaskos JM, Scherle PA. Inhibition of mitogen-activated protein kinase kinase blocks T cell proliferation but does not induce or prevent anergy. J Immunol. 1998;160:4175–81. [PubMed] [Google Scholar]
- 14.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152:521–5. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dina OA, Khasar SG, Gear RW, Levine JD. Activation of Gi induces mechanical hyperalgesia poststress or inflammation. Neuroscience. 2009;160:501–7. doi: 10.1016/j.neuroscience.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dina OA, Levine JD, Green PG. Enhanced cytokine-induced mechanical hyperalgesia in skeletal muscle produced by a novel mechanism in rats exposed to unpredictable sound stress. Eur J Pain. 2011;15:796–800. doi: 10.1016/j.ejpain.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dina OA, McCarter GC, de Coupade C, Levine JD. Role of the sensory neuron cytoskeleton in second messenger signaling for inflammatory pain. Neuron. 2003;39:613–24. doi: 10.1016/s0896-6273(03)00473-2. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari LF, Bogen O, Alessandri-Haber N, Levine E, Gear RW, Levine JD. Transient decrease in nociceptor GRK2 expression produces long-term enhancement in inflammatory pain. Neuroscience. 2012;222:392–403. doi: 10.1016/j.neuroscience.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrari LF, Bogen O, Chu C, Levine JD. Peripheral administration of translation inhibitors reverses increased hyperalgesia in a model of chronic pain in the rat. J Pain. 2013;14:731–8. doi: 10.1016/j.jpain.2013.01.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari LF, Bogen O, Levine JD. Role of nociceptor alphaCaMKII in transition from acute to chronic pain (hyperalgesic priming) in male and female rats. J Neurosci. 2013;33:11002–11. doi: 10.1523/JNEUROSCI.1785-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrari LF, Bogen O, Levine JD. Nociceptor subpopulations involved in hyperalgesic priming. Neuroscience. 2010;165:896–901. doi: 10.1016/j.neuroscience.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrari LF, Levine E, Levine JD. Role of a novel nociceptor autocrine mechanism in chronic pain. Eur J Neurosci. 2013;37:1705–13. doi: 10.1111/ejn.12145. [DOI] [PubMed] [Google Scholar]
- 23.Fonseca BD, Alain T, Finestone LK, Huang BP, Rolfe M, Jiang T, Yao Z, Hernandez G, Bennett CF, Proud CG. Pharmacological and genetic evaluation of proposed roles of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK), extracellular signal-regulated kinase (ERK), and p90(RSK) in the control of mTORC1 protein signaling by phorbol esters. J Biol Chem. 2011;286:27111–22. doi: 10.1074/jbc.M111.260794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukunaga R, Hunter T. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 1997;16:1921–33. doi: 10.1093/emboj/16.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gleason MR, Higashijima S, Dallman J, Liu K, Mandel G, Fetcho JR. Translocation of CaM kinase II to synaptic sites in vivo. Nat Neurosci. 2003;6:217–8. doi: 10.1038/nn1011. [DOI] [PubMed] [Google Scholar]
- 26.Jama AM, Gabriel J, Al-Nagar AJ, Martin S, Baig SZ, Soleymani H, Chowdhury Z, Beesley P, Torok K. Lobe-specific functions of Ca2+.calmodulin in alphaCa2+.calmodulin-dependent protein kinase II activation. J Biol Chem. 2011;286:12308–16. doi: 10.1074/jbc.M110.157057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson JA, Gray MO, Chen CH, Mochly-Rosen D. A protein kinase C translocation inhibitor as an isozyme-selective antagonist of cardiac function. J Biol Chem. 1996;271:24962–6. doi: 10.1074/jbc.271.40.24962. [DOI] [PubMed] [Google Scholar]
- 28.Joseph EK, Bogen O, Alessandri-Haber N, Levine JD. PLC-beta 3 signals upstream of PKC epsilon in acute and chronic inflammatory hyperalgesia. Pain. 2007;132:67–73. doi: 10.1016/j.pain.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 29.Joseph EK, Parada CA, Levine JD. Hyperalgesic priming in the rat demonstrates marked sexual dimorphism. Pain. 2003;105:143–50. doi: 10.1016/s0304-3959(03)00175-1. [DOI] [PubMed] [Google Scholar]
- 30.Kanhema T, Dagestad G, Panja D, Tiron A, Messaoudi E, Havik B, Ying SW, Nairn AC, Sonenberg N, Bramham CR. Dual regulation of translation initiation and peptide chain elongation during BDNF-induced LTP in vivo: evidence for compartment-specific translation control. J Neurochem. 2006;99:1328–37. doi: 10.1111/j.1471-4159.2006.04158.x. [DOI] [PubMed] [Google Scholar]
- 31.Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–79. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 32.Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–30. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24:253–60. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Vertommen D, Rider MH, Lai YC. Mammalian target of rapamycin-independent S6K1 and 4E-BP1 phosphorylation during contraction in rat skeletal muscle. Cell Signal. 2013;25:1877–86. doi: 10.1016/j.cellsig.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 36.Ouseph AK, Khasar SG, Levine JD. Multiple second messenger systems act sequentially to mediate rolipram-induced prolongation of prostaglandin E2-induced mechanical hyperalgesia in the rat. Neuroscience. 1995;64:769–76. doi: 10.1016/0306-4522(94)00397-n. [DOI] [PubMed] [Google Scholar]
- 37.Parada CA, Reichling DB, Levine JD. Chronic hyperalgesic priming in the rat involves a novel interaction between cAMP and PKCepsilon second messenger pathways. Pain. 2005;113:185–90. doi: 10.1016/j.pain.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 38.Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci. 2003;17:1847–52. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- 39.Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2003;120:219–26. doi: 10.1016/s0306-4522(03)00267-7. [DOI] [PubMed] [Google Scholar]
- 40.Quanhong Z, Ying X, Moxi C, Tao X, Jing W, Xin Z, Li W, Derong C, Xiaoli Z, Wei J. Intrathecal PLC(beta3) oligodeoxynucleotides antisense potentiates acute morphine efficacy and attenuates chronic morphine tolerance. Brain Res. 2012;1472:38–44. doi: 10.1016/j.brainres.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 41.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–8. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richter JD. CPEB: a life in translation. Trends Biochem Sci. 2007;32:279–85. doi: 10.1016/j.tibs.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Shakiryanova D, Klose MK, Zhou Y, Gu T, Deitcher DL, Atwood HL, Hewes RS, Levitan ES. Presynaptic ryanodine receptor-activated calmodulin kinase II increases vesicle mobility and potentiates neuropeptide release. J Neurosci. 2007;27:7799–806. doi: 10.1523/JNEUROSCI.1879-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shakiryanova D, Morimoto T, Zhou C, Chouhan AK, Sigrist SJ, Nose A, Macleod GT, Deitcher DL, Levitan ES. Differential control of presynaptic CaMKII activation and translocation to active zones. J Neurosci. 2011;31:9093–100. doi: 10.1523/JNEUROSCI.0550-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song MJ, Wang YQ, Wu GC. Additive anti-hyperalgesia of electroacupuncture and intrathecal antisense oligodeoxynucleotide to interleukin-1 receptor type I on carrageenan-induced inflammatory pain in rats. Brain Res Bull. 2009;78:335–41. doi: 10.1016/j.brainresbull.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Su L, Wang C, Yu YH, Ren YY, Xie KL, Wang GL. Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain. BMC Neurosci. 2011;12:120. doi: 10.1186/1471-2202-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun JL, Xiao C, Lu B, Zhang J, Yuan XZ, Chen W, Yu LN, Zhang FJ, Chen G, Yan M. CX3CL1/CX3CR1 regulates nerve injury-induced pain hypersensitivity through the ERK5 signaling pathway. J Neurosci Res. 2013;91:545–53. doi: 10.1002/jnr.23168. [DOI] [PubMed] [Google Scholar]
- 48.Taiwo YO, Coderre TJ, Levine JD. The contribution of training to sensitivity in the nociceptive paw-withdrawal test. Brain Res. 1989;487:148–51. doi: 10.1016/0006-8993(89)90950-5. [DOI] [PubMed] [Google Scholar]
- 49.Taiwo YO, Heller PH, Levine JD. Characterization of distinct phospholipases mediating bradykinin and noradrenaline hyperalgesia. Neuroscience. 1990;39:523–31. doi: 10.1016/0306-4522(90)90288-f. [DOI] [PubMed] [Google Scholar]
- 50.Taiwo YO, Levine JD. Prostaglandin effects after elimination of indirect hyperalgesic mechanisms in the skin of the rat. Brain Res. 1989;492:397–9. doi: 10.1016/0006-8993(89)90928-1. [DOI] [PubMed] [Google Scholar]
- 51.Villalba A, Coll O, Gebauer F. Cytoplasmic polyadenylation and translational control. Curr Opin Genet Dev. 2011;21:452–7. doi: 10.1016/j.gde.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Waskiewicz AJ, Johnson JC, Penn B, Mahalingam M, Kimball SR, Cooper JA. Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol Cell Biol. 1999;19:1871–80. doi: 10.1128/mcb.19.3.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong MY, Shakiryanova D, Levitan ES. Presynaptic ryanodine receptor-CamKII signaling is required for activity-dependent capture of transiting vesicles. J Mol Neurosci. 2009;37:146–50. doi: 10.1007/s12031-008-9080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamauchi T. Neuronal Ca2+/calmodulin-dependent protein kinase II--discovery, progress in a quarter of a century, and perspective: implication for learning and memory. Biol Pharm Bull. 2005;28:1342–54. doi: 10.1248/bpb.28.1342. [DOI] [PubMed] [Google Scholar]